Abstract
Bakanae disease caused by Fusarium fujikuroi Nirenberg is reported from almost all the rice growing countries of the world and it has emerged as a major problem in Asian countries. The typical and distinguished symptoms of the disease are elongation and rotting of rice plants. F. fujikuroi produces broad spectrum secondary metabolites, pigments and mycotoxins resulting quantitative and qualitative losses to rice crop. Recent changes in climate and cropping patterns have aggravated this disease. In this article, the information on disease etiology and management has been discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Historical perspective
The bakanae disease of rice is known to be present from 1828 in Japan (Ito and Kimura 1931), but the disease was first described by Hori (1898). He named the pathogen as Fusarium heterosporum Nees. Sawada (1917), identified the perfect stage of the pathogen and named it as Lisea fujikuroi Saw. The name of pathogen was changed later to Gibberella fujikuroi Sawada (Ito and Kimura 1931) and the imperfect stage was changed to Fusarium moniliforme Sheld. Current name of the pathogen is F. fujikuroi (Nirenberg 1976) and the name F. moniliforme is no longer used. Bakanae effect of pathogen on plant was described by Kurosawa (1926), where he observed that pathogen is producing a chemical which may be responsible for the stimulation of elongation of stems and suppression of chlorophyll content in infected plants. This finding attracted the different scientists from the fields of plant Physiology, Biochemistry, etc., to know the chemical responsible for disease development. Yabuta and Hayashi (1939) discovered gibberellins from the bakanae disease pathogen F. moniliforme.
The name bakanae is a Japanese word which refers to ‘bad’ or ‘foolish’ seedlings for the specific elongation symptoms of the disease. The disease is known by different names in different parts of the world. In Japan it is known as otoke nae (male seedling), in China white stalk and Fusariosis, in British Guiana palay lalake (man rice), in French Equatorial foot rot and bakanae, in India foolish plant or foot rot (Singh and Sunder 1997). It is also known as white head disease and root rot also (Saremi et al. 2008).
Geographical distribution of bakanae disease
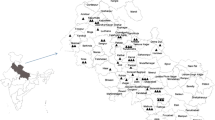
The bakanae disease has been reported from all over the globe in all the rice producing countries like Turkey, Pakistan, Thailand, Japan, European countries, America, African countries, California, Philippines, Nepal, Bangladesh, Cameroon, Nigeria, Vietnam, Indonesia, Malaysia, Sri Lanka, Ivory Coast, Uganda, Brazil, Spain, China, Trinidad, Iran, Venezuela, Mexico, etc. (Surek 1997; Khokhar 1990; Kanjanasoon 1965; Desjardins et al. 2000; Nelson et al. 1993; Cumagun et al. 2011). Karov et al. (2009) reported bakanae disease for first time in Macedonia. The disease is emerging as a major problem in the area of South and South East Asia including India, Nepal, Thailand, Indonesia and Japan due to change in cultivation conditions (Bashyal et al. 2016a; Saremi 2005; Webster and Gunnell 1992; Desjardins et al. 2000; Kini et al. 2002). In India, the disease was reported first time by Thomas (1931). This disease has been reported from different parts of country like Uttar Pradesh (Pavgi and Singh 1964), Bihar, Andhra Pradesh (Vidyasekaran et al. 1967), Assam, Maharashtra (Parate and Lanjewar 1987), Punjab (Bedi and Dhaliwal 1970), West Bengal (Hajra et al. 1994), Tripura (Sarkar 1986), Odisha (Kauraw 1981), etc. In India bakanae disease is more prevalent in basmati growing states and high disease incidence (Fig. 1) was recorded in different districts of Haryana, Punjab and Uttar Pradesh (Bashyal and Aggarwal 2013; Bashyal et al. 2014, 2016a).
Economic importance of disease
The reduction in crop production due to bakanae disease changes under different locations and at same location under different period of time of crop production. In congenial environmental conditions the disease is known to cause a yield reduction of as much as 70% in different corners of the world (Hajra et al. 1994; Singh et al. 1996). Yield loss of 25% has been reported in Bangladesh (Hossain et al. 2007). Desjardins et al. (2000) reported about 40% reduction in crop yield in Nepal. Bakanae disease is responsible for 20–50% reduction in crop production in Japan while 3.7–14.7% yield reduction has been reported in Thailand (Kanjanasoon 1965; Ito and Kimura 1931). The disease is known to cause a yield loss of 10–50% in Pakistan (Bhalli et al. 2001; Ghazanfar et al. 2013; Khokhar and Jaffrey 2002). In India, bakanae disease has been reported to be more prevalent and more severe in varieties of basmati rice (Bashyal et al. 2014; Gupta et al. 2014). The reduction in crop production of 15–25% have been recorded from different states of India (Pavgi and Singh 1964; Rathaiah et al. 1991; Sunder et al. 2014; Pannu et al. 2012). The disease is known to cause both qualitative as well as quantitative losses to crop produce. The pathogen has been reported to be associated with grains of various basmati rice genotypes (Butt et al. 2011; Bashyal and Aggarwal 2013; Bashyal et al. 2016b) and is having a huge impact on quality of rice grains.
Symptoms
Bakanae disease of rice caused by F. fujikuroi is known to produce various kind of symptoms ranging from rotting of seedlings prior to emergence to infection of grains at ripening, seedling blight, crown rot, rotting of root, stunting of plants, etiolation, excessive or abnormal elongation of plants or hypertrophy, sterility and discoloration of grains and production of empty panicles under different climatic conditions around the world (Sun and Snyder 1981; Ou 1985; Webster and Gunnell 1992; Desjardins et al. 2000). The disease is known to appear in main crop as well as ratoon in Japan (Sasaki 1976).The pathogen growth is visible at the coleoptiles just above the soil and also at the joint of lemma and palea in the grains affected by the pathogen (Thomas 1931). The seedlings starts dying 1 week after the sowing in wet nurseries. The infected plants are known to show brown discoloration when the nodal tissues are split opened. Initially the brown discoloration is limited to lower nodes but during later stages the upper nodes also show brown discoloration. Mycelial masses bearing conidia are known to be present inside the hollow internodes. During the high incidence of disease the fungal growth appears on the surface of the nodes and produces pink or white colour on plant surface (Surek and Gumustekin 1994). Thomas (1931) reported adventitious roots development from the lower part of culm as a one of the disease symptom from India. The pathogen forms lesions on the surface of leaves of rice plants (Sasaki 1976). The infected plants are known to exhibit yellowish flag leaves. Some plants may survive up to maturity but they produce tall, lanky and thin tillers. Different types of symptoms are visible in highly susceptible variety Pusa Basmati 1121 in India which includes above ground symptoms as (1) elongated pale yellow seedlings, (2) elongated green plant, (3) elongated and rotted plant, (4) normal and rotted plant, (5) rotting in few tillers, (6) completely rotted plants, (7) elongated plant bearing empty panicles, (8) adventitious root formation (Fig. 2). It produces underground symptoms like (i) rotting and blackening of roots and (ii) adventitious roots (Fig. 3).
Different types of above ground symptoms of bakanae disease observed in rice variety Pusa Basmati 1121 a elongated pale yellow seedlings; b elongated green plant; c elongated and rotted plant; d normal and rotted plant; e elongated plant bearing empty panicles; f completely rotted plant; g rotting in few tillers; h, i adventitious root formation; j white mycelium in diseased plant; k colonization of pathogen in nodal area
The pathogen
The bakanae disease is caused by F. fujikuroi Nirenberg but some other species of Fusarium such as F. proliferatum (Mats.) Nirenberg and Fusarium verticillioides (Sacc.) Nirenberg are also found to be associated with this disease (Amoah et al. 1995; Desjardins et al. 2000). Gibberella fujikuroi Sawada is sexual stage for species of genus Fusarium under the Liseola section (O’Donnell et al. 1998; Leslie and Summerell 2006). The pathogen Gibberella fujikuroi is filamentous fungi which comes under Phylum Ascomycota of Fungi kingdom. The fungi belong to class Sordariomycetes, Hypocreales Order and Nectriaceae family. The pathogen is known to produce ascus and ascospores. The pathogen is known to produce mycelium of white colour. Generally sporodochia are not produced but if present they are pale orange in colour. Asci are produced under perithecia or ascocarps with oval to spherical shape and a size of 250–330 × 220–280 µm. Asci are 4–8 spored piston shaped cylindrical with a size of 90–102 × 7–9 µm. The pathogen is known to have both macro and micro conidiophores bearing macro and micro conidia, respectively. Three to five septate apically tapered and slightly slender macroconidia and 0–1 septate oval or club shaped with flattened base microconidia are produced by the pathogen. Polyphialides produces false heads which after proliferation forms monophialides, and chlamydospores are not formed (Leslie and Summerell 2006).
The disease is known to be caused by species of Gibberella fujikuroi species complex which is divided into 10 different fully fertile mating populations (MP A-J) (Kvas et al. 2009; Bashyal et al. 2012). Three different mating populations (A, C and D) from Gibberella fujikuroi species complex are associated with the bakanae disease in rice. The conidial stage of MP-A is F. verticillioides, of MP-C is F. fujikuroi and conidial stage for MP-D is F. proliferatum (Amoah et al. 1995; Desjardins et al. 2000). Hseieh et al. (1977) first identified Mating population C (MP-C) from the strains of pathogen infecting rice in Taiwan. In Italy also this mating population has been discovered to be associated with the bakanae disease (Amatulli et al. 2010). Two mating populations A and D were isolated from the rice plant infected with bakanae disease from Australia, USA and Africa, whereas, MP-D has been identified from bakanae infected rice of Asia (Amoah et al. 1995; Desjardins et al. 1997). Among all the species associated with the bakanae disease of rice, F. fujikuroi has been found most abundant and most virulent by most of the researchers in their studies all around the world (Nancy 2002; Zainudin and Salleh 2010; Zainudin et al. 2008a, b; Amatulli et al. 2010; Bashyal et al. 2016a). On the other hand, some other Fusarium spp. namely, F. andiyazi, F. moniliforme, F. subglutinans and F. sacchari have been identified to be related with bakanae disease from different rice growing nations by various researchers like India, Bangladesh, Malaysia, Nepal, Pakistan, Italy, Indonesia and Iran (Bashyal and Aggarwal 2013; Quazi et al. 2013; Khokhar 1990; Desjardins et al. 2000; Pra et al. 2010; Saremi and Farrokhi 2004; Wulff et al. 2010; Zainudin et al. 2008a, b).
Three different Fusarium species viz. F. fujikuroi, F. verticillioides and F. Proliferatum were identified to be associated with bakanae disease of rice in India (Bashyal and Aggarwal 2013; Bashyal et al. 2016b). Gibberella fujikuroi species complex were reported to be infecting 1–24% of rice seeds in India (Bashyal and Aggarwal 2013). These Fusarium spp. varied in bakanae disease symptom production and pathogenicity. Bakanae disease elongation symptoms were produced by F. fujikuroi while, crown and stem rot was induced by F. verticillioides (Bashyal and Aggarwal 2013). Bashyal et al. (2016b) studied the single and combined effect of three pathogenic Fusarium spp. F. fujikuroi (Ff), F. proliferatum (Fp) and F. verticillioides (Fv) on bakanae disease severity on susceptible rice genotype Pusa Basmati 1121. In single pathogen inoculation F. fujikuroi was most virulent with disease severity of more than 70% while disease severity was 43% for F. proliferatum and 62% for F. verticillioides. Disease severity was maximum when rice plants were co-inoculated with Fv + Ff (75%) followed by Ff + Fv + Fp (70%), Ff + Fp (63%) and Fv + Fp (48%). Bashyal et al. (2016a) conducted extensive survey for the bakanae disease in different basmati rice growing states of India and observed high disease incidence (up to 20%) in Karnal and Mathura district of Haryana and Uttar Pradesh, respectively. Out of one hundred twenty-six Fusarium spp. isolates collected from symptomatic bakanae diseased rice plants 99.2% isolates were identified as F. fujikuroi. On the basis of virulence isolates were further categorized as highly virulent (28.6%), virulent (34.1%) and moderately virulent (37.3%).
Bakanae epidemiology
Fusarium fujikuroi inciting bakanae disease in rice is mainly seed borne in nature but the pathogen also survives in debris of plants and soil. The main source of primary inoculum inciting disease is seeds infected with the pathogen (Watanabe 1974; Anderson and Webster 2005) as the inoculum present in soil is reduced quickly after decomposition of host debris in field situations (Ou 1985; Sun 1975; Kanjanasoon 1965). In harsh conditions the pathogen is known to survive in the form of spores on seed coat and as macroconidia or thick walled hyphae in plant debris in the soil (Watanabe 1974; Saremi and Farrokhi 2004; Ou 1987). Sun (1975) discovered that the pathogen can survive in the soil for a period of 100–120 days in the form of macroconidia or thick wall hyphae. Karov et al. (2009) recorded the very low incidence of bakanae disease when the fields with previous records of disease occurrence were planted with clean and healthy seeds. Puyam et al. (2017) also investigated the survival of F. fujikuroi in the soil and concluded that bakanae pathogen is weak soil inhabitant and survival of the pathogen under field conditions reduces with time. Wind and water are the main source for dissemination of conidia. The seeds are infected by the air borne ascospores produced during flowering of the crop or by the contamination of seeds by conidia at the time of seed harvesting, which after germination infect the seedlings (Sun 1975). In seed pathogen was observed in husk, embryo and endosperm with the maximum colonization in husk region. Embryo infection ranged from 2 to 41% in different cultivars. The isolation frequency of G. fujikuroi from infected, untreated rice seeds were 75, 15 and 25% from the hulls, embryo and endosperm, respectively (Manandhar 1999). The fungus is known to become systemic in plants but it is not reported to infect the panicles systemically. The pathogen was observed inside xylem vessels of host plants by Nisikado and Kimura (1941). Conidial germination of the pathogen was maximum in root tissue followed by stem (Sunani et al. 2017).
The best suitable temperature for the infection of pathogen is 27–30 °C while the most suitable temperature for the development of the disease is 35 °C which is best suited for the growth of plant too. The incidence of disease was reduced when the temperature was reduced (Burgess et al. 1996; Saremi and Farrokhi 2004). Mandal and Chaudhuri (1988) observed the reduced population of the pathogen in soil on the application of nitrogenous fertilizers in high dose. More disease was observed in the rice plants which were transplanted as compared to the plants which grown from broadcasting the seeds (Saremi and Farrokhi 2004). Higher disease incidence was recorded in summer crop, dry nurseries under high temperature and high relative humidity conditions. Kanjanasoon (1965) reported that the disease incidence was less in the presoaked seeds as compared to dry seeds.
Genomics of F. fujikuroi
During the last one decade whole genomes of the multiple Fusarium spp. were sequenced and improved the understanding of host–pathogen interaction including virulence/defence mechanism along with the pathways involved for the same (King et al. 2015). Till now thirteen genomes of F. fujikuroi are published from different countries (Bashyal et al. 2017; Niehaus et al. 2017; Chiara et al. 2015; Wiemann et al. 2013; Jeong et al. 2013) as described in Table 1. Based on the genome sequence of F. fujikuroi isolate IMI58289, Wiemann et al. (2013) identified a polyketide synthase gene (PKS19) and another that includes a non-ribosomal peptide synthetase gene (NRPS31) are unique to F. fujikuroi. Niehaus et al. (2017) sequenced the genome of eight F. fujikuroi isolates from different geographic locations and observed the differences in the type of asexual spores (microconidia and/or macroconidia), size of chromosomes and expression profile of secondary metabolite gene clusters. Further, based on secondary metabolite profiling and symptoms produced (rotting and stunting) isolates were characterized as two distinct pathotypes. Bashyal et al. (2017) identified 1194 secretory proteins in the “F250” isolate of F. fujikuroi. Out of 356 carbohydrate active enzymes (CAZymes) genes identified, glycoside hydrolase (GH) families like GH3 and GH5 involved in cellulose and hemicelluloses degradation were predominant. Further, α-galactosidases encoding CAZymes GH67 and GH36 deficient in other plant pathogens, were present in F. fujikuroi. Pectin degrading enzyme families (GH28, GH78, CE8, PL3, PL1 and PL9) involved in root tissue colonization were abundant in F. fujikuroi genome. Phylogenetic analysis conducted based on the whole genome of the 5 isolates (IMI58289, B14, KSU 3368, FGSC 8932 and KSU X-10,626) of different geographic locations indicated that the Indian isolate “F250” is closer to the Taiwan isolate “IMI58289” (Fig. 4). Further, through comparative analysis 12,240 common clusters were identified between the different genomes of F. fujikuroi.
Disease management
Ma et al. (2008) evaluated rice genotypes carrying dwarf and semi dwarf genes under field conditions and identified genotypes carrying sd6 of sdq(t) and d2q genes as resistant while genotypes carrying d1 as a susceptible against the bakanae disease. Lu (1994) identified moderately resistant genotype Qingxi 96 at seedling stage was resistant at adult stage, while, seedling stage resistant genotype Longjiao 86074-6 was moderately resistant at adult stage and some genotypes Zupei 7, Dongrong 84-21, G-6, Sui 89-17 were stable and showing moderately resistant reaction at both the stage indicating the bakanae disease resistance may be growth stage specific. Studies on varietal resistance screening revealed that aromatic germplasm and cultivars are more susceptible to bakanae disease as compared to non-scented rice cultivars (Sunder and Singh 1998; Bashyal et al. 2017; Fiyaz et al. 2014, Pannu et al. 2012; Ghazanfar et al. 2013; Gupta et al. 2014). Currently, the high disease severity was observed in cultivars viz., Pusa Basmati 1121, and Pusa Basmati 1509 in Northern part of India, however, other basmati rice varieties viz., Pusa 2511, CSR 30, Pusa Basmati 1401, Pakistani basmati and Dehradun basmati were also susceptible with 0.5–15% incidence in India under field conditions (Bashyal et al. 2016a, 2017; Gupta et al. 2014). Rice genotypes C 4-64 (green base), Karjat x 13-21, BR 4363-8-11-4-9, BR 1067-84-1-3-2-1, IR 58109-109-1-1-3, BR 1257-31-1-1, HKR 96-561, HKR 96-565, HKR 07-40, HKR 07-53, HKR 08-13, HKR 08-22, HKR 08-21, PAU 3456-46-6-1-1, MAUB 2009-1, PNR 600 and RDN 01-2-10-9 were identified resistant by Sunder and Singh (1998). Fiyaz et al. (2014) identified rice genotypes C101A51, Athad apunnu, IR 58025B, Chandana, PAU 201, Pusa 1342, Panchami and Varun Dhan as highly resistant, whereas, BPT 5204, Himju, Peeli Badam and Suphala as resistant to bakanae disease. Ghazanfar et al. (2013) screened various rice germplasm through and identified KKS-133 and IR-6 as resistant with 19.82% and 18.80% plant infection. Saremi et al. (2008), Saremi and Farrokhi (2004) identified cultivars Shafagh Fajr, Kadous, Shafagh and Sahel as moderately resistant and cultivar Binam as highly resistant against the foot rot disease. Aggarwal et al. (2017) reported Pusa 1342, IR6582 and Calrose as promising genotype against bakanae disease.
Bakanae disease resistance is identified as monogenic as it was dominant in cv. KS 282 and recessive in cv. IR 6 (Khan et al. 1999). Yang et al. (2006) identified two quantitative trait loci (QTLs) viz., qB1 in chromosome 1 and qB10 in chromosome 10 using japonica/indica double haploid population (Chunjiang 06 and TN1). Both the QTLs showed additive effect with almost 13% phenotypic variation by each. Hur et al. (2015), identified a major QTL, named as qBK1, on the long arm of chromosome 1 using near-isogenic lines (NILs) derived from a cross between the highly resistant indica variety Shingwang and the susceptible japonica variety Ilpum, explaining 65% of the phenotypic. Fiyaz et al. (2016) analyzed the RIL population of Pusa Basmati 1121 and Pusa 1342 for the bakanae disease resistance QTLs using QTL IciMapping software and through Interval mapping (IM) identified four QTLs located on chromosomes 1 (qBK1.1, qBK1.2 and qBK1.3) and chromosome 3 (qBK3.1). Two QTLs detected on chromosome 1 viz. qBK1.2 and qBK1.3 were novel QTLs. The QTL qBK1.2 was designated as a major QTL and was mapped in 0.26 Mb region between RM10153 and RM5336 where almost 55 annotated genes were identified.
Lee et al. (2018) identified QTL qBK1WD in japonica variety Wonseadaesoo accounting for 20.2% of the total phenotypic variation. Further, QTLs qBKWD and qBK1 were combined (QTLs pyramiding) and the effect of two QTLs together on bakanae disease reistance was 80.2%, which was significantly higher than the individual effect of each QTL.
The most common and widely accepted practice for the management of bakanae disease is the seed treatment with fungicides (Gupta et al. 2015; Bashyal et al. 2016c). Fungicidal seed treatment with thiram, benomyl, thiram + benomyl, thiram + carboxim, thiram + carbendazim, thiophanate-methyl, mancozeb, fludioxonil, prochloraz, iprodione + triticonazole, ipconazole, @ 1–2% of seed weight was effective in different countries viz., Taiwan, Japan, Korea, Iran, Turkey, Pakistan, India, Bangladesh, Nepal and Italy (Ou 1987; Tateishi et al. 1998; Bagga and Sharma 2006; Bagga et al. 2007; Karov et al. 2009; Ora et al. 2011). Propiconazole, triflumizole, prochloraz and ipconazole were also effective against benomyl resistant strains (Tateishi et al. 1998; Karov et al. 2009). Seedling dip treatments with benlate, carbendazim and topsin were highly effective against the disease (Javed et al. 1996). Benzimidazole fungicide nursery soil drenching @ 0.2% found to be effective against the bakanae disease of rice (Bhalli et al. 2001). Seedling treatment with carbendazim or benlate, (0.1%) for 6 and 8 h reduced the disease incidence significantly and improved the grain yield also (Bagga and Sharma 2006). Tilt 25 EC @ 0.05% was observed effective, but phytotoxic also as it reduced plant height and grain yield under field conditions. The hot water immersion method (Hayasaka et al. 2001), and combination of antagonistic yeasts and thermotherapy was reported effective against the bakanae disease (Matic et al. 2014). Immersing the rice seeds in distilled water and irradiation with plasma reduced the bakanae disease severity from 18.1 to 7.8% depending on the duration of plasma irradiation (Ochi et al. 2016). Out of eleven fungicides evaluated as seed treatment against the bakanae disease Bavistin, Nativo, Carzeb and Sunphanate at 2.5 g/L concentration completely inhibited the growth of the pathogen and significantly reduced the seedling infection (Hossain et al. 2015). Seed treatment and carbendazim-sand mix application @ 1 g/m2 in nursery beds along with seedling dip for 3 h in 0.1% carbendazim was also observed effective against the disease. Reduction in bakanae disease incidence up to 73.9–35.0% was observed when the foliar spray of carbendazim was given at flowering stage (Sunder et al. 2014). Kumar et al. (2016) observed the foliar spray of Tebuconazole 250 EC reduced the bakanae disease incidence and increased the grain yield.
The treatment of rice rhizosphere by the suspension of Bacillus oryzicola (YC7007) @ 2.0 × 107 cfu/ml reduced bakanae severity up to 78% (Hossain et al. 2016). The surfactin-producing Bacillus (SPB) strains NH-217 and NH-100 and purified surfactin from them reduced the bakanae disease incidence up to 80% (Sarwar et al. 2018). Trichoderma asperellum SKT-1 (Eco-hope, Kumiai Chemical Industry Co.) and Talaromyces flavus SAY-Y-94-01 (Tough-block, Idemitsu Kosan Co.) have been registered as biofungicides in Japan for the control of Bakanae disease (Kato et al. 2012). The seed treatment of biocontrol agent Talaromyces sp. isolate (KNB-422) was observed effective against bakanae disease of rice (Tateishi et al. 2006). Talaromyces flavus application reduced bakanae disease severity and incidence by 70–75% in India and increased aboveground biomass, grains/panicle and yield of rice (Rawat et al. 2016; Bashyal et al. 2016c). Integrated management module against the bakanae disease of rice which is helpful to reduce 95% incidence of bakanae disease of rice was developed. Effect of nursery drenching with Carbendazim was evaluated against bakanae disease of rice. Eighteen days old seedlings of rice variety Pusa Basmati 1121 were drenched with different concentration of carbendazim and transplanted after 5 days of treatment. Minimum disease incidence was observed in 0.2% carbendazim. Seeds of each treatment were subjected to residue analysis. Results indicated that the use of 0.2% carbendazim as nursery drenching is safe without any residual effect (Anonymous, 2018).
Future perspective
Bakanae disease is emerging as a potential threat in rice cultivation worldwide including India where it is responsible for the tremendous loss in basmati varieties. New effective sources for bakanae resistance need to be identified for resistance breeding for alternative disease control measures. As highlighted by different studies, F. fujikuroi is highly variable in nature therefore, identification of pathotypes/races and resistance sources against them is of utmost important. Identification of differential hosts will be helpful to characterize the pathogen in specific group. As pathogen is seed borne in nature, development of field based, easy and reliable diagnostics will be helpful to reduce the primary inoculums source. Study on the manipulation of different cultural practices to decrease pathogen population needs to be conducted. Integrated disease management modules needs to be developed and validated under field conditions. Further scope is there to develop and evaluate the effective fungicide/chemical molecule/biopesticide and its need based application against bakanae disease.
References
Aggarwal R, Jain RK, Bashyal BM (2017) Plant pathology research series vol II: Genomics, race profiling and characterization of resistance to pathogens. Indian Agricultural Research Institute, New Delhi-110012, India, pp xiv + 413
Amatulli MT, Spadaro D, Gullino ML, Garibaldi A (2010) Molecular identification of Fusarium spp. associated with bakanae disease of rice in Italy and assessment of their pathogenicity. Plant Pathol 59(5):839–844
Amoah BK, Rezanoor HN, Nicholson P, Mac-Donald MV (1995) Variation in the Fusarium section Liseola: pathogenicity and genetic studies of Fusarium moniliforme Sheldon from different hosts in Ghana. Plant Pathol 44:563–572
Anderson L, Webster R (2005) A comparison of assays for Gibberella fujikuroi and their ability to predict resulting bakanae from rice seed sources in California. Phytopathology 95(S4):6
Anonymous (2018) IARI. Annual report 2017–18, Indian Agricultural Research Institute, New Delhi-110012, India
Bagga PS, Sharma VK (2006) Evaluation of fungicides as seedling treatment for controlling bakanae/foot rot (Fusarium moniliforme) disease in Basmati rice. Indian Phytopath 59:305–308
Bagga PS, Sharma VK, Pannu PPS (2007) Effect of transplanting dates and chemical seed treatments on foot rot disease of basmati rice caused by Fusarium moniliforme. Plant Dis Res 22:60–62
Bashyal BM, Aggarwal R (2013) Molecular identification of Fusarium spp. associated with bakanae disease of rice in India. Indian J Agric Sci 83(1):72–77
Bashyal BM, Aggarwal R, Gupta S, Banerjee S (2012) Ecology and genetic diversity of Fusarium spp. associated with bakanae disease of rice. In: Sinha A, Sharma BK, Srivastava M (eds) Modern Trends in Microbial Biodiversity of Natural Ecosystem. Biotech Books Publisher, New Delhi, p 577
Bashyal BM, Aggarwal R, Banerjee S, Gupta S, Sharma S (2014) Pathogenicity, ecology and genetic diversity of the Fusarium spp. associated with an emerging bakanae disease of rice (Oryza sativa L.) in India. In: Kharwar RN et al (eds) Microbial diversity and biotechnology in food security. Springer, New York, pp 307–314
Bashyal BM, Aggarwal R, Sharma S, Gupta S, Rawat K, Singh D, Krishnan SG (2016a) Occurrence, identification and pathogenicity of Fusarium species associated with bakanae disease of basmati rice in India. Euro J Plant Pathol 144(2):457–466
Bashyal BM, Aggarwal R, Sharma S, Gupta S, Singh UB (2016b) Single and combined effects of three Fusarium species associated with rice seeds on the severity of bakanae disease of rice. J Plant Pathol 98(2):405–412
Bashyal BM, Aggarwal R, Singh VK (2016c) Bakanae: an emerging problem in rice cultivation. Indian Farm 65(11):25–26
Bashyal BM, Rawat K, Sharma S, Kulshrestha D, Singh AK, Dubey H, Solanki AKU, Sharma TR, Aggarwal R (2017) Whole genome sequencing of Fusarium fujikuroi provides insight into the role of secretory proteins and cell wall degrading enzymes in causing bakanae disease of rice. Frontiers Plant Sci. https://doi.org/10.3389/fpls.2017.02013
Bedi PS, Dhaliwal DS (1970) Spermatosphere of rice variety Native I from different states of India. Indian Phytopathol 23(4):708–710
Bhalli JA, Aurangzeb M, Ilyas MB (2001) Chemical control of bakanae disease of rice caused by Fusarium moniliforme. Online J Biol Sci 1:483–484
Burgess LW, Backhouse D, Swan LJ, Esdaile RJ (1996) Control of Fusarium crown rot of wheat by late stubble burning and rotation with sorghum. Aust Plant Pathol 25(4):229–233
Butt AR, Yaseen SI, Javaid A (2011) Seed-borne mycoflora of stored rice grains and its chemical control. J Anim Plant Sci 21(2):193–196
Chiara M, Fanelli F, Mule G, Logrieco AF, Pesole G, Leslie JF et al (2015) Genome sequencing of multiple isolates highlights sub-telomeric genomic diversity within Fusarium fujikuroi. Genome Biol Evol 7:3062–3069. https://doi.org/10.1093/gbe/evv198
Cumagun CJR, Arcillas E, Gergon E (2011) UP-PCR analysis of the seedborne pathogen Fusarium fujikuroi causing bakanae disease in rice. Int J Agric Biol 13(6):1029–1032
Desjardins AE, Plattner RD, Nelson PE (1997) Production of fumonisin B (inf1) and moniliformin by Gibberella fujikuroi from rice from various geographic Areas. Appl Environ Microbiol 63(5):1838–1842
Desjardins AE, Manandhar HK, Plattner RD, Manandhar GG, Poling SM, Maragos CM (2000) Fusarium species from Nepalese rice and production of mycotoxins and gibberellic acid by selected species. Appl Environ Microbiol 66(3):1020–1025
Fiyaz RA, Krishnan SG, Rajashekara H, Yadav AK, Bashyal BM, Bhowmick PK et al (2014) Development of high throughput screening protocol and identification of novel sources of resistance against bakanae disease in rice (Oryza sativa L.). Indian J Genet 74:414–422
Fiyaz RA, Yadav AK, Krishnan SG, Ellur RK, Bashyal BM, Grover N et al (2016) Mapping quantitative trait loci responsible for resistance to bakanae disease in rice. Rice 9:45. https://doi.org/10.1186/s12284-016-0117-2
Ghazanfar MU, Javed N, Wakil W, Iqbal M (2013) Screening of some fine and coarse rice varieties against bakanae disease. J Agric Res 51:41–49
Gupta AK, Singh Y, Jain AK, Singh D (2014) Prevalence and incidence of bakanae disease of rice in Northern India. J Agric Search 1(4):233–237
Gupta AK, Solanki IS, Bashyal BM, Singh Y, Srivastava K (2015) Bakanae of rice-an emerging disease in Asia. J Anim Plant Sci 25:1499–1514
Hajra KK, Ganguly LK, Khatua DC (1994) Bakanae disease of rice in West Bengal. J Mycopathol Res 32:95–99
Hayasaka T, Ishiguro K, Shibutani K, Namai T (2001) Seed disinfection using hot water immersion to control several seed-borne diseases of rice plants. J Phytopathol 67:26–32. https://doi.org/10.3186/jjphytopath.67.26
Hori S (1898) Some observations on bakanae disease of the rice plant. Mem Agric Res Sta (Tokyo) 12:110–119
Hossain MA, Latif MA, Kabir MS, Kamal MM, Mian MS, Akter S, Sharma NR (2007) Dissemination of integrated disease management practices through farmers’ participatory field trial. A report on Agricultural Technology Transfer (ATT) Project. Bangladesh Agricultural Research Council, New Airport road, Dhaka-1215, 27
Hossain MS, Ayub Ali M, Mollah MIU, Khan MAI, Sajjadul Islam AKM (2015) Evaluation of fungicides for the control of bakanae disease of rice caused by Fusarium moniliforme (Sheldon). Bangladesh Rice J 19(1):49–55
Hossain MT, Khan A, Chung EJ, Rashid MHO, Chung YR (2016) Biological control of rice bakanae by an endophytic Bacillus oryzicola YC7007. Plant Pathol J 32(3):228–241. https://doi.org/10.5423/ppj.oa.10.2015.0218
Hseieh WH, Smith SN, Snyder WC (1977) Mating groups in Fusarium moniliforme. Phytopathol 67:1041–1043
Hur YJ, Lee SB, Kim TH, Kwon T, Lee JH, Shin DJ, Park SK, Hwang UH, Cho JH, Yoon YN, Yeo US, Song YC, Kwak DY, Nam MH, Park DS (2015) Mapping of qBK1, a major QTL for bakanae disease resistance in rice. Mol Breed 35:78. https://doi.org/10.1007/s11032-015-0281-x
Ito S, Kimura J (1931) Studies on the bakanae disease of the rice plant. Rep Hokkaido Natl Agric Exp Stn 27:1–95
Javed MS, Ayoub M, Mahmood A, Ahmad M (1996) Chemical control of bakanae disease of rice by nursery dip treatment method. Pakistan J Sci 48:90–91
Jeong H, Lee S, Choi GJ, Lee T, Yun SH (2013) Draft genome sequence of Fusarium fujikuroi B14, the causal agent of the bakanae disease of rice. Genome Announc. https://doi.org/10.1128/genomea.00035-13
Kanjanasoon P (1965) Studies on the bakanae disease of rice in Thailand. Doc Agr Thesis Tokyo University, Japan
Karov IK, Mitrev K, Sasa Emilija DK (2009) Gibberella fujikuroi (Sawada) Wollenweber- the new parasitical fungus on rice in the republic of Macedonia. Proc Nat Sci Matica Srpska Novi Sad 116:175–182
Kato A, Miyake T, Nishigata K, Tateishi H, Teraoka T, Arie T (2012) Use of fluorescent proteins to visualize interactions between the bakanae disease pathogen Gibberella fujikuroi and the biocontrol agent Talaromyces sp. KNB-422. J Gen Plant Pathol 78:54–61
Kauraw LP (1981) Effect of length of treatment and fungicide concentration on seed germination and incidence of foot rot disease of rice. Int Rice Res Newslett 6(6):15
Khan TZ, Gill MA, Khan MG (1999) Inheritance studies for bakanae disease of rice. Pak J Phytopathol 11:181–182
Khokhar LK (1990) Bakanae and foot rot of rice in Punjab, Pakistan National Agricultural Research Centre, Crop Diseases Research Institute, Islamabad (Pakistan). IRRN 15:30
Khokhar LK, Jaffrey AH (2002) Identification of sources of resistance against bakanae and foot rot disease in rice. Pak J Agric Res 17(2):176–177
King R, Urban M, Hammond MCU, Pak KH, Hammond KE (2015) The completed genome sequence of the pathogenic ascomycete fungus Fusarium graminearum. BMC Genom 16:544. https://doi.org/10.1186/s12864-015-1756-1
Kini KR, Let V, Mathur SB (2002) Genetic variation in Fusarium moniliforme isolated from seeds of different host species from Burkina Faso based on random amplified polymorphic DNA analysis. J Phytopathol 150:209–212
Kumar P, Sunder S, Singh R, Kumar A (2016) Management of foot rot and bakanae of rice through fungicides. Indian Phytopath 69(2):124–127
Kurosawa E (1926) Experimental studies on the nature of the substance secreted by the bakanae fungus. Nat Hist Soc Formosa 16:213–227
Kvas M, Marasas WFO, Wingfield BD, Wingfield MJ, Steenkamp ET (2009) Diversity and evolution of Fusarium species in the Gibberella fujikuroi complex. Fungal Divers 34:1–21
Lee SB, Hur YJ, Cho JH et al (2018) Molecular mapping of qBK1 WD, a major QTL for bakanae disease resistance in rice. Rice 11:3. https://doi.org/10.1186/s12284-017-0197-7
Leslie JF, Summerell BA (2006) The Fusarium laboratory manual. Blackwell Publishing, Oxford, p 388
Lu B (1994) Preliminary report on evaluation of resistance to rice bakanae disease in rice germplasms. Plant Prot 20(3):20–21
Ma L, Ji Z, Bao J, Zhu X, Li X, Zhuang J, Xia Y (2008) Responses of rice genotypes carrying different dwarf genes to Fusarium moniliforme and gibberellic acid. Plant Prod Sci 11(1):134–138
Manandhar J (1999) Fusarium moniliforme in rice seeds: its infection, isolation and longevity. ZPflkrankh Pflschutz 106:598–607
Mandal DN, Chaudhuri S (1988) Survivality of Fusarium moniliforme Sheld. under different moisture regimes and soil conditions. Int J Trop Plant Dis 6(2):201–206
Matić S, Spadaro D, Garibaldi A, Gullino ML (2014) Antagonistic yeasts and thermotherapy as seed treatments to control Fusarium fujikuroi on rice. Biol Control 73:59–67
Nancy AE (2002) Foolish seedlings and DELLA regulators: the functions of rice SLR1 and Arabidopsis RGL1 in GA signal transduction. Plant Cell 14:1–6
Nelson PE, Desjardins AE, Plattner RD (1993) Fumonisins, mycotoxins produced by Fusarium species: biology, chemistry and significance. Ann Rev Phytopathol 31:233–252
Niehaus EM, Kim HK, MuÈnsterkoÈtter M, Janevska S, Arndt B, Kalinina SA et al (2017) Comparative genomics of geographically distant Fusarium fujikuroi isolates revealed two distinct pathotypes correlating with secondary metabolite profiles. PLoS Pathogen 13(10):e1006670. https://doi.org/10.1371/journal.ppat.1006670
Nirenberg H (1976) Untersuchungen uber die morphologische und biologische differenzierung in Fusarium-Sektion Liseola. Mitt. Biol Bundesansi. Land-Forstwirtsch. Berlin—Dahlem 169:1–117
Nisikado Y, Kimura K (1941) A contribution to the pathological anatomy of rice plants affected by Gibberella fujikuroi (Saw.). Wr I Ber Ohara Inst Landw Forsch 8:421–426
O’Donnell K, Cigelnik E, Nirenberg HI (1998) Molecular systematics and phylogeography of the Gibberella fujikuroi species complex. Mycologia 90:465–493
Ochi A, Konishi H, Ando S, Sato K, Yokoyama K, Tsushima S et al (2016) Management of bakanae and bacterial seedling blight diseases in nurseries by irradiating rice seeds with atmospheric plasma. Plant Pathol 66(1):67–76. https://doi.org/10.1111/ppa.12555
Ora N, Faruq AN, Islam MT, Akhtar N, Rahman MM (2011) Detection and identification of seed borne pathogens from some cultivated hybrid rice varieties in Bangladesh. Middle East J Sci Res 10:482–488
Ou SH (1985) Bakanae disease and foot rot. Rice diseases survey. Commonwealth Mycological Institute, Kew, pp 262–272
Ou SH (1987) Rice Diseases. CAB International, Commonwealth Mycological Institute, Kew, p 256
Pannu PPS, Kaur J, Singh G, Kaur J (2012) Survival of Fusarium moniliforme causing foot rot of rice and its virulence on different genotypes of rice and basmati rice. (Abstr.). Indian Phytopathol 65(5):149–209
Parate DK, Lanjewar RD (1987) Studies on seed mycoflora of two rice cultivars grown in rice tract of Vidarbha. PKV Res J 11(1):47–50
Pavgi MS, Singh J (1964) Bakanae and foot rot of rice in Uttar Pradesh, India. Plant Dis Reptr 48:340–342
Pra Dal, Tonti MS, Pancaldi D, Nipoti P, Alberti I (2010) First report of Fusarium andiyazi associated with rice bakanae in Italy. Pl Dis 94:1070
Puyam A, Pannu PPS, Kaur J, Sethi S (2017) Investigations on survival and host range of Fusarium moniliforme Sheld causing foot rot disease of rice. Environ Econ 35(3B):2071–2075
Quazi SAJ, Meon S, Jaafar H, Ahmad ZABM (2013) Characterization of Fusarium proliferatum through species specific primers and its virulence on rice seeds. Int J Agric Biol 15:649–656
Rathaiah Y, Das GR, Singh KHU (1991) Estimation of yield loss and chemical control of bakanae disease of rice. Oryza 28:509–512
Rawat K, Bashyal BM, Sharma S, Aggarwal R (2016) Talaromyces flavus: a potential biocontrol agent against the bakanae disease of rice. In: Indian phytopathological society 6th international conference on plant, pathogens and people’ (challenges in plant pathology to benefit humankind) conducted by Plant Pathology held from 23–27 Feb, 2016 at IARI, New Delhi-110012, pp 469
Saremi H (2005) Fusarium, biology, ecology and taxonomy. Ferdossy Mashhad University, Iran, Jihad Daneshgahi, p 152
Saremi H, Farrokhi F (2004) Study on bakanae disease of rice and evaluation of cultivars in Gilan and Zanjan provinces. In: Iran Proceedings Fourth International Iran and Russia conference, pp. 358–364
Saremi H, Ammarellou A, Marefat A, Okhovvat SM (2008) Binam a rice cultivar, resistant for root rot disease on rice caused by Fusarium moniliforme in Northwest, Iran. Int J Bot 4:383–389
Sarkar BB (1986) Controlling bakanae and foot rot diseases with seed treatments. Int Rice Res Newslett 11(3):18
Sarwar A, Brader G, Corretto E, Aleti G, Ullah MA et al (2018) Correction: qualitative analysis of biosurfactants from Bacillus species exhibiting antifungal activity. PLoS One 13(7):e0201624. https://doi.org/10.1371/journal.pone.0201624
Sasaki T (1976) Elongation of ratoon in rice plants inoculated with Fusarium moniliforme Sheldon. Ibid 42:606–608
Sawada K (1917) Beitrageuber Formosas-Pilze no. 14. Trans Nat Hist Soc Formosa 3:31–133
Singh R, Sunder S (1997) Foot rot and bakanae of rice: retrospect’s and prospects. Int J Trop Plant Dis 15:153–176
Singh NI, Devi RKT, Singh LNK (1996) Withering of growing shoot of rice caused by Fusarium moniliforme. Plant Dis Res 11(1):99–100
Sun SK (1975) The diseases cycle of rice bakanae disease in Taiwan. Proc Natl Sci Counc Republic Chin 8:245–256
Sun SK, Snyder WC (1981) The bakanae disease of the rice plant. In: Nelson PE, Toussoun TA, Cook RJ (eds) Fusarium diseases, biology and taxonomy. The Pennsylvania State University Press, University Park, pp 104–113
Sunani SK, Bashyal BM, Aggarwal R, Prakash G (2017) Conidial germination of Fusarium fujikuroi causing bakanae disease of rice. Environ Ecol 35(4):2790–2794
Sunder S, Singh A (1998) Screening of rice genotypes for resistance to bakanae disease. Indian Phytopathol 51:299–300
Sunder S, Singh R, Dodan DS (2014) Management of bakanae disease of rice caused by Fusarium moniliforme. Indian J Agric Sci 84(2):224–228
Surek H (1997) Rice diseases in Turkey. In: Chataigner J (ed) Maladies du riz en région méditerranéenne et les possibilités d'amélioration de sa résistance. CIHEAM, Montpellier, pp 45–47
Surek H, Gumustekin H (1994) Research activities on controlling rice bakanae and foot rot disease (Fusarium moniliforme) in Turkey. In: FAO MedNet Rice: breeding and biotechnology groups: proc. workshops Montpellier: CIHEAM. Cahiers Options Mediterran 8, pp 27–30
Tateishi H, Saishoh T, Suzuki T, Chida T (1998) Antifungal properties of the seed disinfectant ipconazole and its protection against bakanae and other diseases of rice. Ann Phytopathol Soc Jpn 64(5):443–450
Tateishi H, Sakuma Y, Miyake T (2006) Isolation and screening of antagonistic fungi against rice seedling diseases and their efficacy (Abstract in Japanese). Jpn J Phytopathol 72:265–266
Thomas KM (1931) A new paddy disease in Madras. Madras Agric J 19:34–36
Vidyasekaran P, Muthaswamy G, Suuramanian C (1967) Role of seed borne microflora in paddy seed spoilage. Indian Phytopathol 19:333–341
Watanabe Y (1974) The possibility of soil transmission in bakanae disease and the contamination of seed with causal fungus during the hastening process of seed germination. Bull Tokai Kinki Nat Agric Exp St 27:35–41
Webster RK, Gunnell PS (1992) Compendium of Rice Diseases. The APS Press St, Paul, p 62
Wiemann P, Sieber CM, Von Bargen KW, Studt L, Niehaus EM, Espino JJ, Bergner SV (2013) Deciphering the cryptic genome: genome-wide analyses of the rice pathogen Fusarium fujikuroi reveal complex regulation of secondary metabolism and novel metabolites. PLoS Pathog 9(6):e1003475
Wulff ED, Sorensen JL, Lubeck M, Nelson KF, Thrane U, Torp J (2010) Fusarium spp. associated with rice bakanae: ecology, genetic diversity, pathogenicity and toxigenicity. Environ Microbiol 12:649–657
Yabuta T, Hayashi T (1939) Biochemiral studies on bakanae fungus of rice. Part III. Studies on physiological action of gibberellin on the plant. J Agric Cliem Soc Jpn 15:403–414
Yang CD, Guo LB, Li XM, Ji ZJ, Ma LY, Qian Q (2006) Analysis of QTLs for resistance to rice bakanae disease. Chin J Rice Sci 20(6):657–659
Zainudin NAIM, Salleh B (2010) Variability of Fusarium species associated with bakanae disease of rice based on virulence, vegetative and biological compatibility. Sydowia 62:89–104
Zainudin NAIM, Razak AA, Salleh B (2008a) Bakanae disease of rice in Malaysia and Indonesia: etiology of the causal agent based on morphological, physiological and pathogenicity characteristics. J Plant Protect Res 48:475–485
Zainudin NAIM, Razak AA, Salleh B (2008b) Secondary metabolite profiles and mating populations of Fusarium species in section Liseola associated with bakanae disease of rice. Malays J Microbiol 4:6–13
Acknowledgements
I thank Indian Phytopathological Society for awarding M. K. Patel memorial lecture award and Director and Joint Director Research, Head, Division of Plant Pathology, ICAR-Indian Agricultural Research Institute for the providing facilities. I acknowledge Dr. Rashmi Aggarwal, Dr. A.K. Singh, Dr. Gopalakrishnan, Dr. S. C. Dubey, Dr. Dinesh Singh for the guidance and Dr. Sapna Sharma, Kirti, Mr. Sunil kumar Sunani, Mr. Jagdish Yadav, Mr. Dhiraj Singh for their help in conducting experiments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bashyal, B.M. Etiology of an emerging disease: bakanae of rice. Indian Phytopathology 71, 485–494 (2018). https://doi.org/10.1007/s42360-018-0091-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42360-018-0091-2