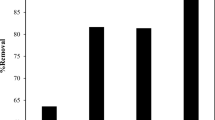Abstract
Novel adsorbent composite beads based on sodium alginate/Activated charcoal (SA/AC) were prepared. The beads were characterized using a variety of instruments, including a Fourier transform infrared spectrometer (FTIR), and scanning electron microscope (SEM). The adsorption capacity of the beads was investigated using batch adsorption studies. This study investigated the influence of several parameters, including the initial pH of the MB solution, the contact time, the initial concentration of the MB, and the temperature of the MB solution. Methylene blue (MB) was effectively adsorbing on sodium alginate/activated charcoal beads after 60 min. The adsorption process was fitted with a pseudo-second-order kinetic model, yielding maximum adsorption capacities of 555 mg/g in mildly alkaline solutions SA/Ac beads were development to be capable of regenerating the MB up to four times without compromising their adsorption efficiency. The SA/AC beads were found to be excellent adsorbents for organic contaminants in wastewater in this investigation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Pollutants can cause eye sickness, cyanosis, vomiting, shortness of breath, burns, and other health problems. Some of the industries that make these pollutants are paper, printing, leather, textiles, electroplating, and cosmetics [1,2,3,4]. They have a huge impact on human health and the environment because the majority of them are toxic and non-degradable. As a result, it's critical to get rid of these harmful chemicals from the water supply. Adsorption technology, the easiest and most cost-effective treatment method among chemical, physical, and biological treatments, is widely used [5]. A common type of direct dye is anionic and water-soluble, making it easy to apply and cost-effective in the textile industry. Viscose dyeing, silk and silk printing, leather dying, the production of organic pigment sediment color, etc. all employ methylene blue, a commercial dye [6,7,8,9]. As a result, scientists are focusing their efforts on developing new technologies for removing MB from contaminated water. To date, several strategies have been used to extract or degrade MB from contaminated water systems, including biological, physical, and chemical approaches [1, 10, 11]. Adsorption, coagulation, biological processes, membrane separation, ion exchange methods, and flocculation techniques are some of the ways used to remove harmful compounds from wastewater. Other approaches include photocatalytic degradation and electrochemical procedures that are used for these purposes [12,13,14,15,16]. Separating hazardous compounds from industrial effluents can be accomplished using any of these methods. However, new solutions are constantly being looked at because current procedures are either ineffective or produce harmful waste. This method of dye separation has become popular because of its superior performance. Adsorbents are readily available, the operation is simple, and reusability and regeneration are cost-effective. Therefore, it is widely utilized around the world. Due to its pilot-scale use, the adsorption approach can be used to offer exact feedback for industrial-scale operations [17,18,19]. A carbon nanoparticle (CNP) is a form of carbon that is non-graphite and can be produced by activating carbon obtained from agricultural wastes, such as coconut shells, bamboo, wood chips, or sawdust. Two steps are involved in the activation process. The raw material is first charred, then activated chemically, thermally, or with steam to drive the highly porous material [20]. The high porosity, huge surface area, and thermal stability of CNP improve its adsorption efficiency by making it a flexible adsorbent. That led to the usefulness of CNP for wastewater treatment. Its high production costs make it prohibitively expensive, which leads to the limits of its use most of the time. As a result, companies are scrambling to find raw materials for making CNP that are less expensive, more readily available, and more efficient [21,22,23]. Various doping approaches, such as the addition of additional functional groups and cross-linking, have been used to improve the efficiency of alginate-beads for MB adsorption. The use of lignocellulosic biomass is suggested as an alternative carbon precursor because it is abundant, renewable, and low in cost [1, 24, 25]. The surfactant-impregnated AC-alginate beads ability to remove MB has not been studied. In light of the above [26, 27]. From these findings and in continuation of our efforts to produce several matrices of biodegradable composites for industrial and environmental applications [1, 28,29,30,31], we have constructed a composite from alginate and CNP in the form of microsphere beads with a highly porous structure for efficient removal of methylene blue as an example of an industrial dye.
2 Experimental
2.1 Materials
All chemicals such as calcium chloride (CaCl2, 98%), MB Sodium alginate (98%), sodium hydroxide (NaOH, 99%), activated charcoal and hydrochloric acid (37%) having molecular were provided by Sigma Aldrich. As well as sodium hydroxide, hydrochloric acid, and activated charcoal were purchased from Merck, Germany.
2.2 Preparation of Alginate/Activated Charcoal Nanocomposite Beads
Sodium alginate and charcoal nanoparticle composite beads (SA/AC) were prepared by dissolving 2 g sodium alginate in 100 mL distilled water for 3 h. To remove any remaining gas bubbles, the resultant solution was degassed by sonication for 2 h. 0.2 g of active charcoal was added to the alginate solution at room temperature and stirred for 3 h. Using the NRC-5 Pro microencapsulation device (developed and built by our group), we made SA/Ac composite beads by dropping an alginate Active charcoal solution into a magnetically stirred 3% (w/v) CaCl2 solution. The proposed chemical structure of SA/AC is illustrated in Scheme 1.
2.3 Characterizations
Shimadzu FT-IR Spectrophotometer (8007S) spectra were measured from 500 to 4000 cm−1 at 4 cm−1 resolution. SEM was carried out using JEOL JEM-2100 electron microscope equipped with 100 × magnification and 120 kV acceleration voltage was used to obtain an image of the SA and AC/SA beads. Thermal analysis (STA6000, Perkinelmer) was performed at 10 °C/min.
2.4 Adsorption Studies
A stock solution of MB with a concentration of 2000 ppm was diluted with water to produce a series of aqueous solutions with varying concentrations. The following measures were taken in general: SA/Ac nanocomposite beads are prepared in MB solution using appropriate starting concentrations and time intervals. A spectrophotometer (UNICO UV-2000) was used to measure the aliquots at maximum absorbance (668 nm). It is possible to conduct batch adsorptions by adsorbing to SA/Ac composite beads regularly, in a variety of pH solutions, and at regular intervals. The following equation was used to determine the amount of MB dye adsorbed on SA/Ac composite beads at the adsorption equilibrium (in mg/g).
Concentrations of dye at initial and equilibrium (Co and Ce respectively) in SA/Ac nanocomposite bead solution (mg/L), dye solution volume in L (V), and nanocomposite weight (W) (g).
In order to investigate the recyclability of beads, we mixed 10 mg with 20 mL of MB solution using a batch concentration of 100 mg/L and a pH of 8. The beads were then agitated with 0.5 M sodium chloride in C2H5OH (50:50 v/v) solution as an adsorbing reagent. The beads were utilised four times, with Eq. (2).
3 Results and Discussion
3.1 Adsorbent Characterization
3.1.1 SEM
To characterize the surface structure of beads produced, scanning electron microscopy (SEM) was used. At various magnifications, SEM images indicated a high degree of porosity and a number of voids in the morphology. As illustrated in Fig. 1, the surface of the beads was extremely porous, providing additional active sites for MB adsorption. Because the porous structure of alginate active charcoal provides high surface area of 650 m2/g for MB adsorption, its capacity to absorb MB molecules has improved.
3.1.2 FTIR Spectra
The FTIR spectra for SA, and SA/AC beads before and after MB adsorption are shown in Fig. 2. The peak at 3400 cm−1 corresponds to the hydroxyl group (–OH group). The peak shifts to 3300 cm−1 after MB adsorption which represents the H-bonding interaction between AS/AC beads and MB. Also, significant peaks were appeared in the spectra after MB adsorption such as at 1314 cm−1 (C–N stretching), 1407 cm−1 (N–H), and 1592 cm−1 (C=N of aromatic ring).
3.1.3 TGA
In Fig. 3, the thermogravimetric analysis (TGA) and its derivatives are shown for SA and SA/AC beads with a temperature range of 25 to 700 °C and a heating rate of 10 °C/min. Weight loss between 50 and 200 °C is attributed to moisture content. The degradation of SA and SA/AC beads above 200 °C is caused by the thermal degradation of sodium alginate, and the final weight loss is 46 and 49%, respectively. It seems that there is no significant difference in the thermal stability with the presence of AC.
3.2 Application of the Alginate/Activated Charcoal Nanocomposite for Dye Adsorption
3.2.1 Effect of pH on the Adsorption
An adsorbent's and an adsorbate's chemical characteristics are greatly influenced by the pH of the solution in the adsorption process. A range of pH values were assessed in the current study for the adsorption of the MB dye, as shown in Fig. 4. Variation in pH can result in the ionization of the MB molecule, which then interacts with the adsorbent's surface. The MB (cationic dye) has a positive charge and interacts strongly with negatively charged surfaces. The adsorption capacity increased with pH, reaching 94 mg/g at neutral pH. Thus, pH influences adsorption behavior affecting the absorbent's surface quality and dye ionization. In acidic pH ranges, free protons compete with MB molecules for surface adherence. Conversely, an increase in the hydroxyl group, which might give the beads' surface a negative charge, increases the basic area's adsorption capability. This hydroxide ion increases the bead-MB molecule contact. Because pH 8 had the best MB dye adsorption capacity on the beads that were made, it was chosen as the best pH.
3.2.2 Contact Time and Adsorption Kinetics
Figure 5 shows MB dye adsorption capacity of alginate/active charcoal based on contact time. The figure shows that MB adsorption increases from 10 to 180 min of contact time. The results showed that removing MB from beads in 10–60 min was fast. Synthesized beads have more active centers on their surface, which become saturated when dye molecules adsorb. This quick increase suggested significant electrostatic force causing rapid MB cation transport towards the surface of beads. In around 120 min, the MB adsorption reached equilibrium (94 mg/g).
Different kinetic models, such as the Pseudo first and Pseudo second models, were used to determine the adsorption appliance. The linear equation for the Pseudo first kinetic model is as follows:
Because the values of \(\mathrm{log} \left({q}_{e }- {q}_{t}\right)\) and t have a direct relationship, A linear plot's intercept and slope can be used to calculate K1 and qe. The R-squared value and the expected qe value are typically used to choose the better fit (Fig. 6).
K2 is the equilibrium rate constant for the pseudo-second kinetic model, qe is the quantity of MB loaded on beads at equilibrium, and qt is the amount of MB loaded on beads at any time t. The kinetics of pseudo-seconds are linear.
qt (mg/g) is adsorption time, qe (mg/g) is equilibrium adsorption, k1 (min−1) and k2 (g mg−1 min−1) are pseudo first and second model rate constants respectively.
We used pseudo first and second kinetics to analyse the experimental data for MB uptake by manufactured beads. Figure 5 shows simulated charts for the Pseudo first and second models (a, b). Also, Table 1 displays the experimentally determined MB uptake factors by alginate/AC. Compared to the pseudo first model, the pseudo second model yields a better regression coefficient for MB. The estimated adsorption capability (qcal = 204 mg/g) is close to the experimentally obtained adsorption capacity (qexp = 194 mg/g), demonstrating that the second models kinetic accurately captures MB absorption by beads. Adsorption of MB on synthesised beads appears to be dominated through chemisorption.
3.2.3 Adsorption Isotherms
Studies of adsorption isotherms are typically used to illustrate how dye molecules diffuse across the interfaces between solids and liquids. In this study, the adsorption isotherms of nanocomposite have been calculated at various initial levels of MB concentration after reaching saturation at ambient conditions. It has been found that the Langmuir isotherm provides accurate data on homogenous adsorption and predicts the formation of monolayers on surfaces. Freundlich isotherms describe heterogeneous and multilayer surfaces. Isotherm models by Langmuir and Freundlich (Eqs. 5 and 6) are as follows:
MB adsorbed by nanocomposites is qe (mg/g), dye equilibrium concentration is Ce (mg/l), maximum MB is qmax (mg/g), and Langmuir constant is Ks (mg/l).
The Freundlich equation is given by:
In this equation, P refers to adsorption capacity (mg/g) and n is a constant that represents adsorption intensity (dimensionless).
According to Table 2, these isotherm adsorption factors are reported for isotherm models. Langmuir isotherms of MB dye molecules adsorbed on a prepared nanocomposite showed a higher correlation coefficient (R2). The high correlation coefficient indicated in the Langmuir isotherm indicates that identical adsorption sites are directly connected to the adsorbed MB-shaped monolayer. Adsorption efficiency according to the Langmuir isotherm was 555 mg/g. Figure 7 displays the results of the study for varying concentrations from 25 to 1000 mg/L. An increase in MB concentration promotes the force that moves from the solid phase to the aqueous phase, resulting in an increase in MB adsorption to the active sites on the nanocomposite. Table 2 parameters for MB adsorption by SA/Activated charcoal nanocomposites according to different equilibrium models.
3.2.4 Effect of Temperature and Thermodynamic Parameters
The temperature also affects the adsorption process. Figure 8 and Table 3 show the results of the study of the variation of the MB's adsorption capacity on A SA/AC as a function of temperature. Results show that the temperature variation influences slightly the adsorption capacity of MB dye on SA/AC, so, the adsorption is endothermic.
Studying temperature effects on MB adsorption on SA/AC, the thermodynamic parameters including ΔH° (enthalpy), ΔS° (entropy) and ΔG° (Gibbs free energy) were evaluated. Based on Van't Hof (Eq. 7), ΔG° was calculated.
Kc is the distribution coefficient constant; R is the general gas constant (8.314 J mol−1 K−1) and T is the absolute temperature (Kelvin). ΔH° and ΔS° values were obtained by plotting Ln Kc vs 1/T and calculating their slopes and intercepts (Table 4).
3.3 Desorption Study
The reuse of adsorbent materials is important for industry. In Fig. 9, the results of four repeated adsorption and desorption cycles were shown for SA/AC. With regenerated nanocomposites, after four regeneration cycles, both samples had a slightly lower adsorption capacity, 93 instead of 98. Overall, regenerated SA/AC is an effective adsorbent for removing cationic dyes.
4 Mechanism of the Adsorption
Scheme 2 show the adsorption mechanism of MB onto the surface of SA/AC adsorbent. The mechanism of the adsorption depends on the presence of different functional groups and charges on both adsorbent and adsorbates. The electrostatic interaction will occur between the cationic groups of MB with the negative groups on the surface of SA/AC groups.
5 Conclusions
This work proved the feasibility of producing novel low-cost and sustainable adsorbent composite materials to obtain greater MB dye removal from wastewater. Sodium alginate and charcoal nanoparticles (SA/AC beads) were successfully produced and analyzed utilizing a variety of analytical techniques. Batch adsorption testing showed a maximum capacity of 555 mg/g at pH 8. Kinetic analysis and isotherm data evaluation suggest that the Langmuir model best fits the experimental data. A dye molecule is transferred from the main solution to the external surface of the beads during the first stage, while dye intraparticle are diffused inside the pores of the beads during the second stage. Beads that have been made do not need to be thrown away after four cycles of adsorption. As a result, they have a high efficiency in removing MB, which is important for long-term and industrial-scale operations.
Data Availability
The research data associated with a paper is available.
References
Abouzeid RE, Khiari R, El-Wakil N, Dufresne A (2019) Current state and new trends in the use of cellulose nanomaterials for wastewater treatment. Biomacromol 20:573–597
Katheresan V, Kansedo J, Lau SY (2018) Efficiency of various recent wastewater dye removal methods: A review. J Environ Chem Eng 6:4676–4697
Hasanpour M, Hatami M (2020) Photocatalytic performance of aerogels for organic dyes removal from wastewaters: review study. J Mol Liq 309:2
Salama A, Abou-Zeid RE, Cruz-Maya I, Guarino V (2020) Soy protein hydrolysate grafted cellulose nanofibrils with bioactive signals for bone repair and regeneration. Carbohydr Polym. https://doi.org/10.1016/j.carbpol.2019.115472
Salama A, Abouzeid R, Leong WS et al (2021) Nanocellulose-based materials for water treatment: adsorption, photocatalytic degradation, disinfection, antifouling, and nanofiltration. Nanomaterials 11:2
Salama A, Abou-Zeid RE (2021) Ionic chitosan/silica nanocomposite as efficient adsorbent for organic dyes. Int J Biol Macromol 188:404–410. https://doi.org/10.1016/j.ijbiomac.2021.08.021
Abou-Zeid RE, Awwad NS, Nabil S et al (2019) Oxidized alginate/gelatin decorated silver nanoparticles as new nanocomposite for dye adsorption. Int J Biol Macromol 141:1280–1286. https://doi.org/10.1016/j.ijbiomac.2019.09.076
Pires JRA, Souza VGL, Fernando AL (2019) Valorization of energy crops as a source for nanocellulose production—Current knowledge and future prospects. Ind Crops Prod 140:111642. https://doi.org/10.1016/j.indcrop.2019.111642
El NA, Abdelwahab O, El-Sikaily A, Khaled A (2009) Removal of direct blue-86 from aqueous solution by new activated carbon developed from orange peel. J Hazard Mater 161:102–110. https://doi.org/10.1016/j.jhazmat.2008.03.060
Gago D, Chagas R, Ferreira LM et al (2020) A novel cellulose-based polymer for efficient removal of methylene blue. Membranes (Basel). https://doi.org/10.3390/membranes10010013
Sirajudheen P, Nikitha MR, Karthikeyan P, Meenakshi S (2020) Perceptive removal of toxic azo dyes from water using magnetic Fe3O4 reinforced graphene oxide–carboxymethyl cellulose recyclable composite: Adsorption investigation of parametric studies and their mechanisms. Surfaces Interfaces. https://doi.org/10.1016/j.surfin.2020.100648
Iqbal J, Shah NS, Sayed M et al (2020) Deep eutectic solvent-mediated synthesis of ceria nanoparticles with the enhanced yield for photocatalytic degradation of flumequine under UV-C. J Water Process Eng. https://doi.org/10.1016/j.jwpe.2019.101012
Rivera-Utrilla J, Sánchez-Polo M, Ferro-García MÁ et al (2013) Pharmaceuticals as emerging contaminants and their removal from water. A review. Chemosphere 93:1268–1287
Iqbal J, Shah NS, Sayed M et al (2020) Synthesis of nitrogen-doped Ceria nanoparticles in deep eutectic solvent for the degradation of sulfamethaxazole under solar irradiation and additional antibacterial activities. Chem Eng J. https://doi.org/10.1016/j.cej.2020.124869
Wan Ngah WS, Teong LC, Hanafiah MAKM (2011) Adsorption of dyes and heavy metal ions by chitosan composites: A review. Carbohydr Polym 83:1446–1456
Li L, Iqbal J, Zhu Y et al (2020) Chitosan/Al2O3-HA nanocomposite beads for efficient removal of estradiol and chrysoidin from aqueous solution. Int J Biol Macromol 145:686–693. https://doi.org/10.1016/j.ijbiomac.2019.12.223
Nasrullah A, Bhat AH, Naeem A et al (2018) High surface area mesoporous activated carbon-alginate beads for efficient removal of methylene blue. Int J Biol Macromol 107:1792–1799. https://doi.org/10.1016/j.ijbiomac.2017.10.045
Naseer A, Jamshaid A, Hamid A et al (2019) Lignin and lignin based materials for the removal of heavy metals from waste water—An overview. Zeitschrift fur Phys Chemie 233:315–345
Saber-Samandari S, Saber-Samandari S, Nezafati N, Yahya K (2014) Efficient removal of lead (II) ions and methylene blue from aqueous solution using chitosan/Fe-hydroxyapatite nanocomposite beads. J Environ Manage 146:481–490. https://doi.org/10.1016/j.jenvman.2014.08.010
Rajabi M, Mahanpoor K, Moradi O (2017) Removal of dye molecules from aqueous solution by carbon nanotubes and carbon nanotube functional groups: Critical review. RSC Adv 7:47083–47090. https://doi.org/10.1039/c7ra09377b
Gul Zaman H, Baloo L, Pendyala R et al (2021) Produced water treatment with conventional adsorbents and MOF as an alternative: A review. Materials (Basel) 14:1–29. https://doi.org/10.3390/ma14247607
Abouzeid RE, Owda ME, Dacrory S (2022) Effective adsorption of cationic methylene blue dye on cellulose nanofiber/graphene oxide/silica nanocomposite: Kinetics and equilibrium. J Appl Polym Sci 139:e52377
Bagheri E, Rahnama H, Hassannia MA et al (2021) Oriented polylactic acid/graphene oxide nanocomposites with high mechanical and thermal properties. J Thermoplast Compos Mater. https://doi.org/10.1177/08927057211038625
Dardeer HM, Mahgoub MY, Abouzeid RE et al (2022) Novel pseudopolyrotaxane composite based on biopolymers: Synthesis, characterization and application in water treatment. Environ Nanotechnol Monit Manag. https://doi.org/10.1016/j.enmm.2021.100639
Hassan E, Hassan M, Abou-zeid R et al (2017) Use of bacterial cellulose and crosslinked cellulose nanofibers membranes for removal of oil from oil-in-water emulsions. Polymers (Basel). https://doi.org/10.3390/polym9090388
Naseer A, Hamid A, Ghauri M et al (2020) Lignin/alginate/hydroxyapatite composite beads for the efficient removal of copper and nickel ions from aqueous solutions. Desalin Water Treat 184:199–213. https://doi.org/10.5004/dwt.2020.25356
Rajkumar M, Meenakshisundaram N, Rajendran V (2011) Development of nanocomposites based on hydroxyapatite/sodium alginate: Synthesis and characterisation. Mater Charact 62:469–479. https://doi.org/10.1016/j.matchar.2011.02.008
Wahba MI, Hassan ME, Ali KA (2021) Chitosan-glutaraldehyde activated carrageenan-alginate beads for β-D-galactosidase covalent immobilisation. Biocatal Biotransformation 39:138–151. https://doi.org/10.1080/10242422.2020.1832476
Ali AO, Abdalla MS, Shahein YE et al (2021) Grafted carrageenan: alginate gel beads for catalase enzyme covalent immobilization. 3 Biotech. https://doi.org/10.1007/s13205-021-02875-9
Assem Y, Abou-Zeid RE, Ali KA, Kamel S (2019) Synthesis of acrylate-modified cellulose via raft polymerization and its application as efficient metal ions adsorbent. Egypt J Chem 62:85–96. https://doi.org/10.21608/ejchem.2018.4005.1351
Abou-Zeid RE, Dacrory S, Ali KA, Kamel S (2018) Novel method of preparation of tricarboxylic cellulose nanofiber for efficient removal of heavy metal ions from aqueous solution. Int J Biol Macromol 119:207–214. https://doi.org/10.1016/j.ijbiomac.2018.07.127
Liu L, Wan Y, Xie Y et al (2012) The removal of dye from aqueous solution using alginate-halloysite nanotube beads. Chem Eng J 187:210–216. https://doi.org/10.1016/j.cej.2012.01.136
Rocher V, Bee A, Siaugue JM, Cabuil V (2010) Dye removal from aqueous solution by magnetic alginate beads crosslinked with epichlorohydrin. J Hazard Mater 178:434–439. https://doi.org/10.1016/j.jhazmat.2010.01.100
Hachi M, Chergui A, Selatnia A, Cabana H (2016) Valorization of the spent biomass of pleurotus mutilus immobilized as calcium alginate biobeads for methylene blue biosorption. Environ Process 3:413–430. https://doi.org/10.1007/s40710-016-0157-z
Li Y, Du Q, Liu T et al (2013) Methylene blue adsorption on graphene oxide/calcium alginate composites. Carbohydr Polym 95:501–507. https://doi.org/10.1016/j.carbpol.2013.01.094
Ma T, Chang PR, Zheng P et al (2014) Fabrication of ultra-light graphene-based gels and their adsorption of methylene blue. Chem Eng J 240:595–600. https://doi.org/10.1016/j.cej.2013.10.077
Acknowledgements
In this work, the authors would like to express their gratitude to the National Research Centre for technical assistance.
Funding
No funding is available.
Author information
Authors and Affiliations
Contributions
The authors of this article and their contribution to this work are equals contributions.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest. Ramzi Khiari is Guest Editor of the Special Issue "Lignocellulose at multiscale: preparation characterization".
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Abouzeid, R., Khiari, R. & Ali, K.A. Activated Charcoal/Alginate Nanocomposite Beads for Efficient Adsorption of the Cationic Dye Methylene Blue: Kinetics and Equilibrium. Chemistry Africa 6, 2369–2379 (2023). https://doi.org/10.1007/s42250-022-00560-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42250-022-00560-9