Abstract
Turbidity is one part of the physical characteristics of wastewater that is highly observed in domestic wastewater. The electrocoagulation process is an effective method by applying only electric current with sacrificial electrodes for the removal of turbidity from domestic wastewater under the consideration of different operating parameters. In this study, current (0.03–0.09 A), pH (3–9), and reaction time (15–45 min) were considered as operating parameters using Al–Fe and Fe–Al electrode combinations. The highest removal efficiency was achieved 91.23% and 96% at current − 0.09 A, pH—9, and reaction time—45 min using Al–Fe and Fe–Al electrode combinations respectively. The mathematical and statistical data were analyzed and also maximum optimization of the experimental investigation using response surface methodology was 91.053% for Al–Fe and 96.68% for Fe–Al electrode combination. The interaction of different operating parameters indicated that, the model was valid. In addition to this, the model was validated based on the percentage absolute error of deviation (AED) < 10% and the regression coefficient (R2) > 0.7. Estimation of the operating cost of electrocoagulation was done for both electrode combinations depending on selected operating parameters that were based on energy consumption, electrode consumption, and cost of chemicals used up during the investigation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Several major problems facing human beings to survive on earth are due to the lack of providing clean water for a large number of communities and also affecting the aesthetic of the environment [1, 2]. Wastewater generating from tanning industry [3], textile industry [4,5,6], pulp and paper industry [7,8,9], pharmaceutical activities [10], car wash service [11, 12], electroplating industry [13], mine industry [14], paint industry [15], automobile garage [16], brewery industry [17], sugar industry [18], hospital [19], food industry [20], etc. are some sources of wastewater that discharged to an environment. Domestic wastewater is one source of wastewater that is formed due to the daily consumption of water by human beings for different purposes and is discharged into an environment [21]. Domestic wastewater consists of black water like wastewater with excreta, urine, and fecal sludge as well as also gray water, which is generated from the kitchen and bathing wastewater [22]. Turbidity of water and wastewater is formed because of the availability of suspended particles, fine organic matters, microorganisms, different forms of sludge, and colloidal particles [1, 23]. Turbidity is one part of the physical characteristics of wastewater that happen due to high cloudiness in wastewater that form a favorable condition for the growth of microorganisms and cause waterborne disease as well as also reduce the aesthetic of an environment [1]. Hence, domestic wastewater treatment using electrocoagulation is important to minimize such problems even if different methods are available.
There are different methods implemented for the treatment of domestic wastewater treatment. Natural coagulants like Moringa oleifera [24], wetland mechanism [25, 26], anaerobic and filtration process [27], rotating biological contactor and membrane separation process [28] are some studies concerned with the treatment of domestic wastewater treatment. However, researchers indicated the electrochemical method is a better alternative systematic treatment for wastewater and water due to versatility, environmental compatibility, the amenability of safety and automation, efficiency in energy, and cost-effectiveness [29]. Electro-reduction, electrocoagulation, and electro-oxidation are the common categories of the electrochemical method for the treatment of water and wastewater [29]. The Electrocoagulation process is based on the principle of electrochemical when there is a production of destabilizing agents that are used to remove different types of pollutants through charge neutralization, adsorption, floatation, and precipitation [30].
The electrocoagulation process was used an electric current induced in the reactor tank with sacrificial electrodes like aluminum and Iron [31]. Two main processes occur when iron anodes are used in an electrolytic system which is indicated in Eqs. ((1)–(4)). Fe2+ is formed at the anode due to the oxidation of iron [32]. Proton reduction in an acidic medium or water reduction in an alkaline medium produces H2 gas at the cathode. When the wastewater is electrolyzed, the pH rises and hydroxyl complexes, both monomeric and polymeric, are formed [32]. pH 3.5–7.0 has a strong tendency to polymerize the complexes [32]. To remove it from the wastewater, it can be removed by coagulation, absorption, co-precipitation, and sweep flocculation, among other methods [32].
On the other hand, the cationic monomeric species such as Al3+ and Al(OH)2+, which at appropriate pH values are transformed first into Al(OH)3 and then polymerized to Aln(OH)3n which indicated in Eqs. ((5)–(8)) [33].
Anode reaction;
Cathode reaction;
Overall reaction;
This occurs when an electric current passes through a metal electrode and releases the metal ion from the anode. Because of their chemical reaction, metal hydroxide and a variety of complex species, depending on pH range, act as a coagulant [34] that is indicated in Eqs. ((5)–(8)).
Furthermore, different studies indicate that the electrocoagulation process used to remove turbidity [11, 35, 36], color [7, 36], COD [7], organic matter [11], sulfate [29], nitrate [29], copper [37], oil and grease [36, 38], heavy metal ions [39], Fluoride [40], etc. from water and wastewater. After all experimental investigations, optimization of all parameters and the removal efficiency was done by response surface methodology (RSM). It is a multivariate system and consists of different mathematical and statistical systems established based on the fit of the polynomial model to the data that's required for the statistical prediction that is used for optimization [20]. Response surface Methodology is important for evaluating the interaction effects between different parameters based on the response and for the generation of a large number of information from a small number of experiments in addition to optimization [19]. Response surface methodology is a mathematical model in the form of linear, square, polynomial, and others to the experimental results from the deigned set of experiments and for model verification by techniques of statistics [41].
Central composite designs (CCD) as well as Box–Behnken design (BBD) are the two common types of response surface methodology [42]. On the other hand, CCD, BBD, and Doehlert matrix design (DMD) were used for the optimization of different experimental investigations. Different studies were used CCD [43], BBD [43], and DMD [44] for experimental design. Doehlert matrix design requires a lower number of experiments; In the theoretical approach, Doehlert matrix design is more efficient than central composite design, but this may not be always true because of some experimental constraints that results the theoretical approach may differ in practice [43]. A Box Behnken design (BBD) is a three-level fractional factorial design that is used to determine the nature of the response surface in an experimental zone. The design is a hybrid of a two-level factorial design and an incomplete block design, with a specific amount of variables running through all design combinations in each block, while other elements remain at the central levels [45]. In comparison to the central composite design (CCD), the BBD experiment lacked an embedded factorial design and extreme points, as well as the rotatability value (α) in the experimental design. This is because of it involves fewer experiments while producing comparable findings; the CCD is an important alternative to the full factorial, three-level design. However, CCD is experiment lacked an embedded factorial design and extreme points, as well as the rotatability value (α) in the experimental design whereas, DMD is not rotatable in its design [43]. Moreover, CCD is a commonly used experimental design for second order models and CCD outperforms other approaches in terms of prediction [43].
CCD is the best category of response surface methodology that provides an important prediction of linear and quadratic interaction effects of factors that affect the selected process [8, 41]. The purpose of this study was to evaluate the removal efficiency of turbidity on domestic wastewater using the electrocoagulation process by fixing desired operating parameters. The estimation of the total operating cost of electrocoagulation was studied regarding energy and electrode consumption as well as the cost of chemicals. In addition, this study also evaluates the statistical and mathematical model for validity concerning the interaction of operating parameters.
Aluminum and Iron were preferred as electrodes since they are locally available at a low cost and also aluminum and iron electrodes were used due to multivalent ions having coagulating properties [46]. In this study two iron and aluminum, electrodes were used based on the cost consumed for electrocoagulation. According to [47], as the number of electrode combinations increases the cost needed for the implementation of the electrocoagulation process increases. The study also used, Response surface methodology for determining operating parameters in the process just by fixing the total experiments performed in number.
2 Materials and Methods
2.1 Materials
Real wastewater was collected from Jimma university teachers’ apartment that accommodates approximately 700 people. Initial wastewater has a pH of 7.2 ± 0.5, turbidity of 340 ± 20 NTU, and a temperature of 26 ± 3 °C. An experiment was performed in the Jimma Institute of Technology, Environmental Engineering Laboratory. Electrocoagulation cell, Electrode (Aluminum and Iron), DC power supply (ANDELI: Model WY-1-0, 15v/5A), Magnetic Stirrer (REMI: Model R-24), Magnetic bar stirrer, Copper Wires, Electrical clips, and turbidity meter (HANNA pH meter) are materials used up during the determination of turbidity using electrocoagulation process.
2.2 Experimental Setup and Procedures
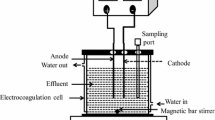
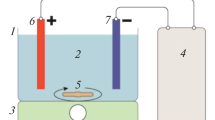
A batch reactor was used during an experiment in the electrocoagulation process which is indicated in Fig. 1. An experimental setup consisted of an electrocoagulation cell or a reactor (glass beaker) that can hold 1.5 L, but the working volume of the wastewater/sample was 1 L for each run of the experiment to minimize the loss of wastewater samples during an investigation. During an experiment sodium hydroxide and sulfuric acid were used for adjusting the pH of a sample. Iron and Aluminum electrodes were used for each experimental run that connected as Al–Fe (anode–cathode) and Fe–Al (anode–cathode).
The surface area for each electrode was 60 cm2 to achieve a good removal percentage of turbidity and the inter-electrode distance between each connected electrode was adjusted to 1.5 cm.
There are different studies concerning electrocoagulation performed at a different distance between electrodes such as [48] at 1 cm and [49] at 2 cm. But according to [50] indicates that the distance between electrodes is between 1 to 5 cm. At the experimental run, electrodes were cleaned by hydrochloric acid followed by distilled water for the removal of an attached particle to the surface of electrodes which may lead to rust and corrode due to the oxidation process of electrodes. A magnetic bar stirrer was placed inside an electrocoagulation cell and an electrocoagulation cell was placed on a magnetic stirrer at 10,000 rpm to obtain a uniform concentration as a sample. Using copper wires and electrical clips, connected electrodes were connected to a DC power supply after all parameters were fixed. Then at the end of each experiment, the wastewater sample was transferred to another empty beaker and settled for 20 min and the supernatant samples of wastewater were used for the measurement.
2.3 Design of Experiment with Response Surface Methodology (RSM)
Response surface methodology design of expert (version 11) was used to determine the mathematical and statistical data based on an experimental investigation. Central composite design (CCD) was used from response surface methodology where pH (3–9), current (0.03–0.09 A), and reaction time (15–45 min) were considered to evaluate the removal percentage of turbidity. Different studies indicated that, high removal percentages of pollutants were achieved from wastewater under different variables range such as [51] reaction time (0–60 min), [52] pH (2–11), and [53] current (0–6 A).
In addition to analysis of statistical data, CCD was used to reduce the number of experiments performed based on the number of variables used for an experiment [41] and center points were indicated in Eq. (9). Similarly, the coded and actual values of the selected variables were indicated in Table 1. Actual variables are the real variables used during an experiment and coded variables that are randomly given by RSM or can be adjusted based on the requirements.
2.4 Analysis of Data
Central composite design (CCD) from response surface methodology (design of expert version 11), was used to determine or fix the number of tests or experiments that going to be investigated by using Eq. (9).
where k is the number of factors and Co is the center point’s run.
In this study three factors were used, namely; pH, current, and reaction time for an electrocoagulation process. According to [1], the removal percentage of turbidity was determined by using Eq. (10) indicated below.
where Co and C are the initial and final turbidity respectively.
Analysis of model validity was checked based on the percentage absolute error of deviation (AED) and the regression coefficient (R2) between experimental and theoretical results. The percentage absolute error of deviation (AED) was determined using Eq. (11).
where Yexp is the experimental responses and Ytheo is the theoretical responses. N is the number of a point at which measurements were carried out.
The assessment of cost is mandatory such that the total operating cost of electrocoagulation was determined based on the cost of energy consumed, electrode consumed and cost of chemical used up during the investigation.
where Cenergy is an energy consumption that was determined using Eq. (13) and Celectrode was an electrode consumption calculated using Eq. (14) as well a and b is an electrical energy price ($/kWhr) and price of electrode materials ($/kg) respectively. The cost of chemicals used up during the process was represented by D.
where Vs, V, I, and t represent the volume of wastewater sample, voltage, current, and electrolysis time respectively.
where n is the number of electrons transferred from Fe and Al (Z = 2 and Z = 3) respectively, F is Faradays constant (96,487 Cmol−1), V is the volume of wastewater (m3), M is the molecular mass of Al and Fe, it is electrolysis time and I represent current applied for electrocoagulation process.
3 Results and Discussion
3.1 Removal Percentage of Turbidity
The percentage or efficiency removal of turbidity by using Al–Fe (anode–cathode) and Fe–Al (anode–cathode) was shown in Tables 2 and 3 respectively that was calculated using Eq. (10). Based on an experimental investigation, the removal percentage of turbidity was determined at different pH, electric current, and reaction times for Al–Fe (anode–cathode) and Fe–Al (anode–cathode that indicated in Tables 2 and 3 respectively. When the pH was 3 and the electric current was 0.03 A, the removal efficiency of turbidity was relatively approach to each other for both combinations. However, at 45 min of reaction time Fe–Al (anode–cathode) removed more turbidity than Al–Fe (anode–cathode). In the same way, when the pH was 6 and the electric current was 0.06A, in this regard Fe–Al (anode–cathode) removed more turbidity than Al–Fe (anode–cathode) at 15, 30, and 45 min of reaction time which was removed up to 72.5%. Similarly, at pH 9 and electric current 0.09 A, Fe–Al (anode–cathode) removed more percentage of turbidity than Al–Fe (anode–cathode). This indicated that, at the gradual increment of pH and electric current, the combination of Fe–Al (anode–cathode) was more effective than Al–Fe (anode–cathode).
However, as indicated in Tables 2 and 3, Al–Fe (anode–cathode) was somewhat more effective than Fe–Al (anode–cathode) especially when the pH was 3 and electric current was 0.06 A as well as at pH 6 and electric current 0.09 A. In this case, around 48% and 72% of turbidity were removed when pH was 3 and electric current is 0.06 A and pH was 6 and electric current was 0.09 A respectively using Al–Fe (anode–cathode). On the other hand, the removal efficiency of turbidity was high using Fe–Al (anode–cathode) than Al–Fe (anode–cathode) when the pH was 9 and electric current was 0.03 A at a reaction time of 15, 30, and 45 min. Generally, the removal percentage of turbidity was varied depending on the value of pH and electric current supplied for both combinations. In Tables 2 and 3 there was a predicted value that indicates the removal percentage of turbidity which was predicted by Response surface methodology (design of expert 11) based on the actual removal (experimental result) efficiency of turbidity.
3.2 Effects of Operating Parameters on Turbidity Removal
Process performance is strongly influenced by the pH of the solution. As shown Tables 2 and 3 when pH—3, there was a somewhat lower removal percentage of turbidity was obtained. The moderate removal efficiency was obtained when the pH was increased to 6 in both Al–Fe and Fe–Al combinations. At pH—9, the better removal efficiency of turbidity was obtained even if reaction time and electric current applied were other factors considered in both electrode combinations shown in Tables 2 and 3. As pH increased from 3 to 6 and again from 6 to 9, the removal efficiency of turbidity was increased. This was because of the increase in pH during the EC process is mostly due to the evolution of hydrogen gas at the cathode, and the concentration of hydroxyl ions (OH−) in the solution increases due to electrochemical processes that result in high removal efficiency [54].
This includes the dissolution of electrodes, coagulant species, and the state of other ions in the electrodes. Both the solution and the colloids’ zeta potentials are directly influenced by the pH of a solution [55]. Monomeric hydroxyl species dominate in acidic pH ranges. Al(OH)4 and Fe(OH)4 prevail throughout the solution for Al and Fe electrodes, respectively, at high alkaline conditions, and these electrodes possess poor coagulation activities [55]. The current was also another factor that influences the removal percentage of turbidity from wastewater as indicated in Tables 2 and 3. As the current was increased from 0.03 to 0.06 A and then from 0.06 to 0.09 A the removal efficiency of turbidity was increased for both Al–Fe and Fe–Al combinations. While the current increased it was enhanced for a generation of a considerable amount of Al3+ or Fe2+ ions at the anodic dissolution of an electrode as well as the formation of additional hydrogen bubbles that are used for the separation process [56]. As the current increased, so did the removal efficiency. When the current values were increased the amount of hydroxide flocs formation and the density of the bubbles, which resulted in faster removal of pollutants from the wastewater [32].
This study revealed that, electrocoagulation performance was affected by the reaction time (time of electrolysis). As depicted in Tables 2 and 3, the removal percentage of turbidity was affected by reaction time for both Al–Fe and Fe–Al electrode combinations respectively. In both cases, while the reaction time increased from 15 to 45 min, the removal efficiency of turbidity was increased. This is due to the liberation of more coagulation ions from the sacrificial anode as the current density and electrolysis time increase and the number of generated iron/aluminum ions (coagulants) and their hydroxide flocs in the solution increases as the electrolysis time is increased [57].
As indicated in Table 2, when Al–Fe combined around 91.23% of turbidity was removed in 45 min. Similarly, when Fe–Al combined 96% of turbidity was removed in 45 min as shown in Table 3.
3.3 Statistical Analyses with Response Surface Methodology
The analysis of variance of the regression for pH, reaction time, and an electric current was shown in Tables 4 and 5. This was determined using Response surface methodology (quadratic model) for turbidity removal using Al–Fe (anode–cathode) and Fe–Al (anode–cathode) respectively. For both electrode combinations, the removal percentage of the turbidity model has been significant since the value of P < 0.05 which means the model was significant at a probability level of 95%. According to ANOVA results, some variables were insignificant in both electrode combinations that may be improved through the reduction of a model. The evaluation of experimental outcomes in conjunction with experimental design, the removal percentage of turbidity was done as a function of pH (A), current (B), and reaction time (C) for both Al–Fe and Fe–Al electrode combinations. Design of experts (11) provided the quadratic model regression Eqs. (15) and (16) sgiven below for Al–Fe and Fe–Al electrode combinations respectively.
3.4 Model Validation
An experimental (actual) and predicted values for the removal percentage of turbidity was indicated in Tables 2 and 3. This was more illustrated again in Fig. 2 and Fig. 3 using Al–Fe (anode–cathode) and Fe–Al (anode–cathode) respectively, and the actual and predicted values were plotted which linear regression was and the model was also a good fit. The regression coefficient (R2) or the coefficient of determination is the percentage of the total variability in the dependent variable that the regression equation in the independent variable accounts for. The regression coefficient (R2) was determined for Al–Fe and Fe–Al was calculated as shown in Tables 6 and 7 respectively. In both electrode combinations, the value of regression coefficient (R2) was greater than 0.7 especially the suggested source model for Al–Fe was 0.9485 and for Fe–Al was 0.9222. This indicated that the validity of the model was good.
Similarly, using Eq. (11) the percentage absolute error of deviation (AED) was calculated. The results were indicated that, the AED percentages of Al–Fe and Fe–Al were 1.056% and 0.68% respectively. In both cases, the percentage absolute error of deviation (AED) was less than 10% which shows the model validity was good and fit.
3.5 Combination of Parameters
By maintaining one variable at the central level and modifying the other two variables within the chosen design space, the multi-variable regression equations were used to develop the response surface plots. The percentage removal of turbidity was determined by considering operating parameters like; pH, reaction time, and electric current. The interaction operating parameters over turbidity removal was plotted using response surface methodology in Figs. 4 and 5 by using Al–Fe combined as (anode–cathode) and Fe–Al combined as (anode–cathode) respectively. The combined effects of pH and current on percentage removal of turbidity were indicated in Figs. 4 and 5 as pH varying from 3 to 9 and current varying from 0.03 to 0.09A. The gradual increase of current from (0.03–0.06A) and pH from (3–6) resulted in the formation of moderate removal of turbidity. This indicated that, at lower pH and minimum electric current supply, moderate % of turbidity removal was achieved in both electrode combinations due to lower degradation of pollutants in wastewater. Keeping other factors constant, as the current was increased from (0.06–0.09A) and pH from (6–9) enhanced for good removal percentage of turbidity as shown in Figs. 4a and 5a. At this stage, especially when the pH was greater than neutral, high % of turbidity was revealed since more amount of Al3+ and Fe2+ ions were produced at anode which resulted in the degradation of pollutants, and a high concentration of the hydrogen gas bubble was formed at cathode. Similarly, the increase of reaction time from (15–30 min) and then from (30–45 min) concerning the increase of pH resulted in a better removal percentage of turbidity that indicated in Figs. 4b and 5c. Especially, when the reaction time of the electrocoagulation process was at 45 min high removal efficiency was achieved for both electrode combinations with increasing of pH to 9 due to more generation of polymer species of metal (aluminum and iron) with the increase of reaction time as shown in Figs. 4b and 5c. In the same manner in Figs. 4c and 5b, the combined effects of reaction time and current are shown such that there has been good removal efficiency of turbidity just by increasing both factors. Keeping other factors constant and combining reaction time and current enhanced for better removal % of turbidity that was indicated in Figs. 4c and 5c that occurred due to the increment of reaction time, high hydroxyl ions were formed in the solution just by applying a good electric current. This indicated that, the removal percentage of turbidity was increased as the pH, reaction time, and electric current were increased for both Al–Fe (anode–cathode) and Fe–Al (anode–cathode) combinations.
3.6 Optimization with Response Surface Methodology
Determining the optimum value for the removal degree of pollutants under different parameters is the main advantage of response surface methodology using central composite design (CCD).
Based on the CCD, the results were optimized using the regression equation of RSM (design expert 11). In the optimization process: pH (A), current (B), and reaction time (C), were selected within the range and the response turbidity removal efficiency was maximized.
For Al–Fe (anode–cathode), the optimum value of turbidity removal was 91.053% at pH 9, current 0.09 A, and reaction time 45 min at the desirability of 0.998 was selected.
Similarly, for Fe–Al (anode–cathode) 96.68% of turbidity removal was achieved at pH 9, current 0.09 A, and 45 min reaction time at the desirability of 1.0 was selected as an optimum value for turbidity removal percentage and operating parameters.
3.7 Operating Cost
The electrocoagulation process is a necessary technique for wastewater treatment without the use of chemicals, hence calculating the total operating cost of the process is required. The operational cost of electrocoagulation is a significant disadvantage of the technique, particularly for large-scale industrial applications, and just a few studies have been published on this topic [58]. Material, electrical energy expenses, personnel costs, maintenance costs, sludge dewatering, and disposal costs, and fixed costs make up the overall operational cost of the electrocoagulation process [59], that was indicated in Eq. (12). Based on an experimental investigation energy consumption and electrode consumption calculated for Al–Fe and Fe–Al were 20.25 kWh/m3 and 13.5 kWh/m3 and 0.63 × 10–5 kg/m3 and 2 × 10–5 using Eqs. (13) and (14) respectively. This indicated that, using the Al–Fe electrode combination consumed more energy than Fe–Al and lowers electrode consumption. The monthly rate for electrical energy is 0.056$/kWh, according to the Ethiopian Electric Power Agency [60], and the cost of electrode pairs was 1$ and also with a total weight of 40.5 g. Then the price of electrodes per weight of electrodes was 24.69$/kg. On the other hand, the total cost used for different chemicals was 0.5$ and according to [54], and the total cost of labor, sludge dewatering, and disposal were roughly 1$/m3. Hence the total cost used up for the operation of this process was 2.634$/m3 using Al–Fe combination and 2.257$/m3 using Fe–Al electrode combination. Therefore, using Al–Fe electrode combination needs more operating cost compared Fe–Al electrode combination.
4 Conclusion
Domestic wastewater is generated due to the activities of human beings performed daily and that discharged to an environment without any treatment. However, Electrocoagulation is a simple technology implemented for the treatment of domestic wastewater due to its ease of implementation and effectiveness in the reduction of pollutants, especially turbidity. Al–Fe and Fe–Al is the form of electrode combination at (anode–cathode) and the removal percentage of turbidity was determined under the consideration of pH, current, and reaction time. Both electrode combinations were effective in the removal percentage of turbidity at different operating parameters. Especially through gradual increment of operating parameters the Fe–Al (anode–cathode) was more effective than Al–Fe (anode–cathode) for turbidity removal. Statistical data analysis was implemented to evaluate the validity of the model and optimization of being an important part that was done to maximize the removal efficiency of turbidity by considering operating parameters in intervals using central composite design. The operational cost of electrocoagulation was calculated, and it was obtained that the Al–Fe electrode combination consumes more energy than the Fe–Al electrode combination while using fewer electrodes consumption. Similarly, compared to the Fe–Al electrode combination, the Al–Fe electrode combination has a higher operating cost. Finally, the results of this study revealed that electrocoagulation would be a desirable and efficient technology for eliminating turbidity from household wastewater with low cost under specific operating settings.
References
Altufaily MAM, Abedalaama ZA (2018) Enhancing turbidity removal using electrocoagulation method. J Eng Appl Sci 13(1):97–104
Nair DS, Sreedharan S (2018) Reduction of turbidity by electrocoagulation. Advance in Technology, Engineering and Computing – A Multinational Colloquium, pp 315–321
Bouazza G, Assou M, Chatri EH, Souabi S (2020) Optimization by the response surface methodology of turbidity, Box–Behnken plan. In: 6th international conference on optimization and applications ICOA 2020—Proceedings 2020, p 2
Ozyonar F, Muratcobanoglu H, Gokkus O (2017) Taguchi approach for color removal using electrocoagulation with different electrode connection types. Fresenius Environ Bull 26(12):7600–7607
Sivakumar D, Anand R, Saral A (2018) Textile industry wastewater color removal using Lemna minor L. and Lemna minuta L. Int J Eng Technol 7(3.34 Special Issue 34):160–162
Ozmetin E, Calgan E, Suzen Y, Korkmaz M, Ozmetin C (2017) Optimisation of textile industry wastewater treatment using bigadic zeolite (Clinoptilolite) by response surface methodology. J Environ Prot Ecol 18(3):1127–1136
Azadi Aghdam M, Kariminia HR, Safari S (2016) Removal of lignin, COD, and color from pulp and paper wastewater using electrocoagulation. Desalin Water Treat 57(21):9698–9704
Guvenc SY, Erkan HS, Varank G, Bilgili MS, Engin GO (2017) Optimization of paper mill industry wastewater treatment by electrocoagulation and electro-Fenton processes using response surface methodology. Water Sci Technol 76(8):2015–2031
Kumar D, Sharma C (2019) Reduction of chlorophenols and sludge management from paper industry wastewater using electrocoagulation process. Sep Sci Technol 0(0):1–11
Eri IR, Hadi W, Slamet A (2018) Clarification of pharmaceutical wastewater with Moringa oleifera: optimization through response surface methodology. J Ecol Eng 19(3):126–134
Mohammadi MJ, Salari J, Takdastan A, Farhadi M, Javanmardi P, Yari AR et al (2018) Removal of turbidity and organic matter from car wash wastewater by electrocoagulation process. Desalin Water Treat 2017(68):122–128
Gomes AJ, Das KK, Jame SA, Cocke DL (2016) Treatment of truck wash water using electrocoagulation. Desalin Water Treat 57(54):25991–26002
Naghdali Z, Sahebi S, Ghanbari R, Mousazadeh M, Jamali HA (2019) Chromium removal and water recycling from electroplating wastewater through direct osmosis: modeling and optimization by response surface methodology. Environ Health Eng Manag 6(2):113–120
Dinu LR, Badescu VR, Vasile GG, Cristea I, Serban EA, Oncu V et al (2019) Fe–Al recovery from mine water treatment residuals and product testing for wastewater treatment—phosphate and turbidity removal. In: International symposium on the environment industries 2019 (SIMI 2019), pp 56–62
Kakoi B, Kaluli JW, Ndiba P, Thiong’o G (2017) Optimization of Maerua Decumbent bio-coagulant in paint industry wastewater treatment with response surface methodology. J Clean Prod 164:1124–1134
Manilal AM, HarinarayananNampoothiri MG, Soloman PA (2017) Removal of oil and grease from automobile garage wastewater using electrocoagulation. IOP Conf Ser Mater Sci Eng 206(1):0–10
Okolo BI, Nnaji PC, Oke EO, Adekunle KF, Ume CS, Onukwuli OD (2018) Optimizing bio-coagulants for brewery wastewater treatment using response surface methodology. Niger J Technol 36(4):1104
Sahu O, Mazumdar B, Chaudhari PK (2019) Electrochemical treatment of sugar industry wastewater: process optimization by response surface methodology. Int J Environ Sci Technol 16(3):1527–1540
Veli S, Arslan A, Bingöl D (2016) Application of response surface methodology to electrocoagulation treatment of hospital wastewater. Clean: Soil, Air, Water 44(11):1516–1522
Yolmeh M, Jafari SM (2017) Applications of response surface methodology in the food industry processes. Food Bioprocess Technol 10(3):413–433
Utari AW, Herdiansyah H (2020) Using filtration as a technology to remove pollutants in domestic wastewater. IOP Conf Ser Mater Sci Eng 725:1–7
Tripathi VK, Warwade P (2018) Influence of semi-arid climate on characterization of domestic wastewater 281–292
Posavčić H, Halkijević I, Vuković Ž (2019) Application of electrocoagulation for water conditioning. Environ Eng 6(2):59–70
Vunain E, Mike P, Mpeketula G, Monjerezi M, Etale A (2019) Evaluation of coagulating efficiency and water borne pathogens reduction capacity of Moringa oleifera seed powder for treatment of domestic wastewater from Zomba, Malawi. J Environ Chem Eng 7:103118
Sathe SM, Munavalli GR (2019) Domestic wastewater treatment by modified bio-rack wetland system. J Water Process Eng 28:240–249
Ma Y, Zhai Y, Zheng X, He S, Zhao M (2019) Rural domestic wastewater treatment in constructed ditch wetlands: effects of influent flow ratio distribution. J Clean Prod 225:350–358
Lohani SP, Khanal SN, Bakke R (2020) A simple anaerobic and filtration combined system for domestic wastewater treatment. Water Energy Nexus 3:41–45
Waqas S, Roil M, Man ZB, Klaysom C (2020) An integrated rotating biological contactor and membrane separation process for domestic wastewater treatment. Alex Eng J 59:4257–4265
Jo EY, Park SM, Yeo IS, Cha JD, Lee JY, Kim YH et al (2016) A study on the removal of sulfate and nitrate from the wet scrubber wastewater using electrocoagulation. Desalin Water Treat 57(17):7833–7840
Damaraju M, Bhattacharyya D, Kurilla KK (2017) Removal of recalcitrant carbon from an industrial wastewater using electrocoagulation. Int J Civ Eng 15(4):697–703
El Amrety M, Mosaad M, Allaa El Din M (2020) Removal of Chlorpyrifos from aqueous solution using electrocoagulation. Bull Fac Eng Mansoura Univ 43(1):1–6
YaziciGuvenc S, Dincer K, Varank G (2019) Performance of electrocoagulation and electro-Fenton processes for treatment of nanofiltration concentrate of biologically stabilized landfill leachate. J Water Process Eng 31:100863
Bener S, Bulca Ö, Palas B, Tekin G, Atalay S, Ersöz G (2019) Electrocoagulation process for the treatment of real textile wastewater: effect of operative conditions on the organic carbon removal and kinetic study. Process Saf Environ Prot 129:47–54
Nyangi MJ (2021) Remediation of arsenic from water using iron and aluminum electrodes in electrocoagulation technology: adsorption isotherm and kinetic studies. Chem Afr 4:943–954
Kanta S, Majumder C, Saha P (2018) Removal of turbidity and E. coli from surface water by electrocoagulation and study of its economic feasibility. J Indian Chem Soc 95(3):1–6
Yusoff MS, Azwan AM, Zamri MFMA, Aziz HA (2017) Removal of colour, turbidity, oil and grease for slaughterhouse wastewater using electrocoagulation method. AIP Conf Proc 1892:040012
Kessentini I, Mousser H, Zouari S, Bargui M (2019) Removal of copper from aqueous solution using electrocoagulation: importance of stirring effect. Surf Eng Appl Electrochem 55(2):210–218
Manilal AM, Soloman PA, Basha CA (2020) Removal of oil and grease from produced water using electrocoagulation. J Hazard Toxic Radioact Waste 24(1):1–13
Al-Qodah Z, Al-Shannag M (2017) Heavy metal ions removal from wastewater using electrocoagulation processes: a comprehensive review. Sep Sci Technol 52(17):2649–2676
Nyangi MJ, Chebude Y, Kilulya KF, Salim CJ (2021) Comparative study on adsorption isotherm and kinetics of defluoridation using aluminum and iron electrodes in electrocoagulation. Chem Afr 4:391–398
Karimifard S, Alavi Moghaddam MR (2018) Application of response surface methodology in physicochemical removal of dyes from wastewater: a critical review. Sci Total Environ 640–641:772–797
Asaithambi P, Aziz ARA, Daud WMABW (2016) Integrated ozone—electrocoagulation process for the removal of pollutant from industrial effluent: optimization through response surface methodology. Chem Eng Process Process Intensif 105:92–102
Zolgharnein J, Shahmoradi A, Ghasemi JB (2013) Comparative study of Box–Behnken, central composite, and Doehlert matrix for multivariate optimization of Pb (II) adsorption onto Robinia tree leaves. J Chemometr 27:12–20
Selmane Bel Hadj Hmida E, Abderrazak H, Ounissi T, Djebali K (2020) Experimental design and response surface methodologies use for the treatment of leachates by electrocoagulation process. Chem Africa 3:821–829
Keskin Gündoğdu T, Deniz I, Çalışkan G, Şahin ES, Azbar N (2016) Experimental design methods for bioengineering applications. Crit Rev Biotechnol 36(2):368–388
Garcia-Segura S, Eiband MMSG, de Melo JV, Martínez-Huitle CA (2017) Electrocoagulation and advanced electrocoagulation processes: a general review about the fundamentals, emerging applications and its association with other technologies. J Electroanal Chem 801:267–299
Gusa RF, Afriani F, Tiandho Y, Sunanda W (2020) Effect of electrode numbers in electrocoagulation of Batik Cual wastewater : analysis on water quality and energy used. IOP Conf Ser Earth Environ Sci. https://doi.org/10.1088/1755-1315/599/1/012061
Nasr M, Ateia M, Hassan K (2016) Artificial intelligence for greywater treatment using electrocoagulation process. Sep Sci Technol 51(1):96–105
Mores R, Treichel H, Zakrzevski CA, Kunz A, Steffens J, Dallago RM (2016) Remove of phosphorous and turbidity of swine wastewater using electrocoagulation under continuous flow. Sep Purif Technol 171:112–117
Sharma A, Mane SJ (2017) Removal of solids from hospital wastewater using electrocoagulation. IJESC 7(6)
Emerick T, Vieira JL, Silveira MHL, João JJ (2020) Ultrasound-assisted electrocoagulation process applied to the treatment and reuse of swine slaughterhouse wastewater. J Environ Chem Eng 8(6):104308
Asaithambi P, Govindarajan R, Yesuf MB, Selvakumar P, Alemayehu E (2020) Enhanced treatment of landfill leachate wastewater using sono (US)—ozone (O3)—electrocoagulation (EC) process: role of process parameters on color, COD and electrical energy consumpti … enhanced treatment of landfill leachate wastewater using. Process Saf Environ Prot 142:212–218
Naje AS, Naser GF, Al-Zubaidi HA (2021) Environmental assessment of kinetics behavior of electrocoagulation process for industrial wastewater treatment. Mater Today Proc. https://doi.org/10.1016/j.matpr.2021.05.691
Ebba M, Asaithambi P, Alemayehu E (2021) Investigation on operating parameters and cost using an electrocoagulation process for wastewater treatment. Appl Water Sci 11(11):1–9
Keyikoglu R, Can OT, Aygun A, Tek A (2019) Comparison of the effects of various supporting electrolytes on the treatment of a dye solution by electrocoagulation process. Colloid Interface Sci Commun 33:100210
Asaithambi P, Beyene D, Raman A, Aziz A, Alemayehu E (2018) Removal of pollutants with determination of power consumption from landfill leachate wastewater using an electrocoagulation process : optimization using response surface methodology (RSM). Appl Water Sci 8(2):1–2
Abbasi S, Mirghorayshi M, Zinadini S, Zinatizadeh AA (2020) A novel single continuous electrocoagulation process for treatment of licorice processing wastewater: optimization of operating factors using RSM. Process Saf Environ Prot 134:323–332
Ozyonar F, Karagozoglu B, Ozyonar F, Karagozoglu B (2011) Operating cost analysis and treatment of domestic wastewater by electrocoagulation using aluminum electrodes. Pol J Environ Stud 20:173–179
Moussa DT, El-naas MH, Nasser M, Al-marri MJ (2016) A comprehensive review of electrocoagulation for water treatment : potentials and challenges. J Environ Manag 186(Pt 1):24–41
Atalo T. Ethiopia’s Electric tarrif Comparision (2021). https://urldefense.com/v3/https://energy4sustainablefuture.blogspot.com/2014/05/electricitytariff__;!!NLFGqXoFfo8MMQ!61N5Bhxz3O3_Sto6ILsDdBdJJds9FRqig_JNjs9n6H9CfdnchpzvxMyhYE_SGog7XiKwJQZmxd0$ethiopia_4788.html. Accessed 29 Sept 2021
Acknowledgements
The Authors thank the Jimma University, Jimma Institute of Technology, Department of Water Supply and Environmental Engineering, Environmental Engineering Laboratory for their support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest.
Rights and permissions
About this article
Cite this article
Bote, M.E., Desta, W.M. Removal of Turbidity from Domestic Wastewater Using Electrocoagulation: Optimization with Response Surface Methodology. Chemistry Africa 5, 123–134 (2022). https://doi.org/10.1007/s42250-021-00303-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42250-021-00303-2