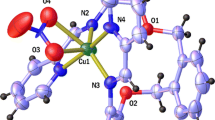
Abstract
An octadentate azodye ligand containing phenolic/ketonic oxygen and azo nitrogen donor atoms when complexed with divalent metal ions forms tetranuclear complexes of composition [M4LCl4(H2O)12] and [\( M_{4}^{/} \) LCl4(H2O)4] where, M = CoII,CuII, NiII, MnII and M/ = Zn II,, CdII and LH4 = 4,4/-bis(2,4/-dihydroxy-5/-acylphenylazo)diphenylsulphone (BDHAPADPS). The metal complexes were characterised by analytical, magnetic moment, conductance, I.R., electronic spectra, thermal, ESI–MS, ESR, XRD, SEM and EDX. DFT calculations of the ligand and metal complexes have been made by GAUSSIAN 03 rev. A.01 programme package. The antibacterial study of the ligand and the complexes has been made using Gram positive and Gram negative bacteria.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Azodyes are synthesized by coupling reaction of aromatic primary amines with aromatic hydroxyl compounds. Due to the presence of active azo (–N=N–) group they show a wide range of applications in various fields. 4,4/-diaminodiphenyl sulfone is an antibiotic used for the treatment of Leprosy [1]. It is also used for the treatment and prevention of pneumocystis pneumonia and toxoplasmosis and for the treatment of various types of skin diseases as acne, dermatitis herpetiformis [2]. Azodye have enormous applications in medical science as a potent antibacterial, antitumor and anti-inflammatory drugs and the pharmacological activity of these compounds is enhanced on complexation with various metal ions. We have reported a number of azodye ligands contaning nitrogen, oxygen and sulfur donor atoms and their mono, bi, tri, tetra, penta, hexa and octameric metal complexes [3,4,5,6]. In the present investigation, we describe here the preparation of one octadentate azodye ligand (Fig. 1) and its six tetrameric Co(II), Ni(II), Cu(II), Mn(II), Zn(II) and Cd(II) complexes.
2 Experimental
2.1 Materials
The metal chlorides used in this work are of SRL grade and 4,4/-diaminodiphenyl sulfone and 2,4-dihydroxyacetophenone are from Sigma Aldrich.
2.2 Synthesis of the Ligand
4,4/-Diaminodiphenyl sulfone (0.01 mol, 0.8 g.) was treated with a mixture of NaNO2 and HCl. The resulting solution was added to an alkaline solution of 2,4-dihydroxyacetophenone (0.02 mol, 1.0 g.) at 0–5 °C, when the azodye 4,4/-bis(2,4/-dihydroxy-5/-acylphenylazo)diphenylsulphone (BDHAPADPS) was formed. LH4 = C28H22N4SO8 (574.54), m.p. > 250 °C. Anal. Calc. (%) C, 58.5; H, 3.86; N, 9.7; S, 5.5; Found (%) C, 58.2; H, 3.5; N, 9.4; S, 5.4, IR (KBr pellet cm−1): 1475 \( \nu \) (C–O); 1607 \( \nu \) (N=N); 1631 \( \nu \) (C=O). 13C {1H} NMR δ: 26.0 (C-1), 103.0, 108.0, 115.0, 129.0, 130.0, 133.0 (Ar–C), 163.0 (C-4), 166.0 (C-6), 203.0 (C-2).

2.3 Synthesis of the Metal Complexes
An ethanolic solution of metal chloride was mixed with a solution of the ligand in ethanol in 4:1 ratio. The resulting solution is heated in a heating mantal to 50–60 °C for about 1 h and the pH is raised to ~ 7 by adding conc. NH4OH drop by drop with stirring. Where upon metal complexes were precipitate out which were filtered out, washed with ethanol followed by ether and dried in vacuum.
[Co4(BDHAPADPS)Cl4(H2O)12] (Dark brown) C28H42Cl4Co4N4O20S (1163.7), m.p. > 250 °C, Anal Calc. (%) C, 28.8; H, 3.6; N, 4.8; S, 2.7; Cl, 12.2; Co, 20.2; Found (%) C, 28.5; H, 3.4; N, 4.6; S, 2.5; Cl, 12.0; Co, 19.8, IR (KBr pellet cm−1): 1435 \( \nu \) (C–O); 1499 \( \nu \) (N=N); 1593 \( \nu \) (C=O); 577 \( \nu \) (M–O); 492 \( \nu \) (M–N).
[Ni4(BDHAPADPS)Cl4(H2O)12] (Light green) C28H42Cl4Ni4N4O20S (1162.7), m.p. > 250 °C, Anal Calc. (%) C, 28.8; H, 3.6; N, 4.8; S, 2.7; Cl, 12.2; Ni, 20.1; Found (%) C, 28.5; H, 3.4; N, 4.5 S, 2.6; Cl, 12.0; Ni, 19.8, IR (KBr pellet cm−1): 1434 \( \nu \) (C–O); 1497 \( \nu \) (N=N); 1590 \( \nu \) (C=O); 575 \( \nu \) (M–O); 490 \( \nu \) (M–N).
[Cu2(BDHAPADPS)Cl4(H2O)12] (Green) C28H42Cl4Cu4N4O20S (1182.6), m.p. > 250 °C, Anal Calc. (%) C, 28.4; H, 3.5; N, 4.7; S, 2.7; Cl, 11.9; Cu, 21.4; Found (%) C, 28.2; H, 3.2; N, 4.5; S, 2.4; Cl, 11.7; Cu, 21.2, IR (KBr pellet cm−1): 1436 \( \nu \) (C–O); 1500 \( \nu \) (N=N); 1589 \( \nu \) (C=O); 575 \( \nu \) (M–O); 488 \( \nu \) (M–N).
[Mn4(BDHAPADPS)Cl4(H2O)12] (Purple) C28H42Cl4Mn4N4O20S (1148.2), m.p. > 250 °C, Anal Calc. (%) C, 29.2; H, 3.6; N, 4.8; S, 2.7; Cl, 12.3; Mn, 19.1; Found (%) C, 29.0; H, 3.4; N, 4.5; S, 2.4; Cl, 12.1; Mn, 18.8, IR (KBr pellet cm−1): 1435 \( \nu \) (C–O); 1499 \( \nu \) (N=N); 1590 \( \nu \) (C=O); 570 \( \nu \) (M–O); 485 \( \nu \) (M–N).
[Zn4(BDHAPADPS)Cl4(H2O)4] (Brown) C28H26Cl4Zn4N4O12S (1045.8), m.p. > 250 °C, Anal Calc. (%) C, 32.1; H, 2.5; N, 5.3; S, 3.0; Cl, 13.5; Zn, 25.0; Found (%) C, 31.8; H, 2.2; N, 5.1; S, 2.8; Cl, 13.2; Zn, 24.8, IR (KBr pellet cm−1): 1436 \( \nu \) (C–O); 1498 \( \nu \) (N=N); 1592 \( \nu \) (C=O); 530 \( \nu \) (M–O); 482 \( \nu \) (M–N).
[Cd2(BDHAPADPS)Cl4(H2O)4] (Grey) C28H26Cl4Cd4N4O12S (1234.0), m.p. > 250 °C, Anal Calc. (%) C, 27.2; H, 2.1; N, 4.5; S, 2.5; Cl, 11.4; Cd, 36.4; Found (%) C, 27.1; H, 2.0; N, 4.2; S, 2.3; Cl, 11.2; Cd, 36.1, IR (KBr pellet cm−1): 1435 \( \nu \) (C–O); 1500 \( \nu \) (N=N); 1590 \( \nu \) (C=O); 525 \( \nu \) (M–O); 485 \( \nu \) (M–N).
2.4 Physical Measurements
The metal contents of the complexes were determined by EDTA complexometric titration method. Micro analysis of C, H and N was performed on a Perkin–Elmer 2400 analyser. The magnetic susceptibility was measured at RT by Gouy method. Conductance of the complexes were measured by using Toshniwal CL 01–06 Conductivity Bridge. FTIR spectra were recorded using 200i FT-IR Microscopy System. Electronic spectra of CoII, NiII and CuII complexes in DMF were recorded on a Hilger-Watt uvispeck spectrophotometer. ESR spectrum of CuII complex was recorded on an E4—spectromete. 1H NMR and 13C NMR spectra were recorded on a Jeol GSX 400 with CDCl3 as solvent and TMS as internal standard. The Electron Spray Ionization–Mass Spectrometric (ESI–MS) analysis was carried out by micrOTOF-Q ║ 10337. X-ray diffraction study of the NiII complex was recorded on a Phillips PW 1130/00 diffractometer with scan axis-Gonio, start position (2θ-10.004) end position (2θ-89.976) anode material CuK—ALPHA 1[Å] = 1.54060 and the generator settings 30 mA, 40 kV. The Scanning Electron Microscope (SEM) of make JEOL instrument model JSM-5410 was used to determine the microstructure of complexes. Energy Dispersive X-ray (EDX) analysis was done by EDX (TESCAN) X-max version 4.1.17.D/Mi 152. TG, DTG and DTA of the CoII complex was recorded on NETZSCH STA 409 C/CD in a nitrogen atmosphere at a heating rate of 10 °C per minute.
2.5 Theoretical (DFT/B3LYP) Studies
The optimized geometry of the azo dye ligand and its CuII and ZnII complexes were studied with Gauss View 5.0.8 [7]. The theoretical calculations were made using GAUSSIAN 03 rev. A.01 programme package [8,9,10,11,12,13,14] hybrid DFT/B3LYP hybrid functional level of theory with LANL2DZ basic set for copper and zinc atoms in gas phase and 6-31 G(d, p) basic set for other atoms.
2.6 Antibacterial Activity
The antibacterial activity of the azodye ligand and five complexes was studied as per the cup-plate method using seven strains of bacteria, two Gram positive and five Gram negative. The solutions were prepared in dimethylsulphoxide (DMSO) at 500 µg/mL. The bacterial strains were inoculated into 100 mL of the sterile nutrient broth and incubated at 37 ± 1 °C for 24 h. The density of the bacterial suspension was standardised by McFarland method. A well of uniform diameter (6 mm) was made on agar plates, after inoculating them separately with the test organisms aseptically. The standard drug and the test compounds were introduced with the help of micropipette and the petriplates were placed in the refrigerator at 8–10 °C for proper diffusion of drug into the media. After 2 h of cold incubation, the petriplates are transferred to incubator and maintained at 37 ± 2 °C for 18–24 h. Then the petriplates are observed for zone of inhibition by using vernier scale. The results are reported by comparing the zone of inhibition shown by the test compounds with standard drug Tetracycline. The results are the mean value of zone of inhibition measured in millimetre.
3 Results and Discussion
Metal complexes were prepared according to the following reaction scheme:
where, M = Co(II), Ni(II), Cu(II), Mn(II), LH4 = BDHAPADPS.
where, M = Zn(II), Cd(II), LH4 = BDHAPADPS.
Elemental analysis data agree well with the following composition of the metal complexes [M4LCl4 (H2O)12] and [\( M_{4}^{/} \) LCl4 (H2O)4] where M = CoII, NiII, CuII MnII; M/ = ZnII, CdII. All the complexes are amorphous in nature and have high melting points. The complexes are insoluble in common organic solvents but soluble in dimethylformamide and dimethyl sulfoxide. The complexes are non-electrolytic in nature as evident from their low conductance values (3.5–5.4 Ω−1 cm2 mol−1) in 10−3M DMF solution.
3.1 IR Spectra
The sharp bands observed at 1607 cm−1 and 1631 cm−1 in the ligand can be ascribed to ν(–N=N–) and ν(C=O) vibrations respectively. In the metal complex these bands are observed at ~ 1499 and ~ 1593 cm−1, respectively indicating coordination of one of the azo nitrogens and carbonyl oxygen atoms to the metal ions [15]. The band appeared at 1475 cm−1 in the ligand, assigned to ν (C-O) vibrations has been shifted to ~ 1435 cm−1 in the metal complexes which indicates the bonding of phenolic oxygen atom to the metal ions. In the metal complexes a broad band appeared at ~ 3389 cm−1 followed by sharp peaks at ~ 840 and ~ 683 cm−1 assignable to –OH stretching, rocking and wagging vibrations, respectively indicating the presence of co-ordinated water molecules [16]. The conclusive evidence of bonding of the ligand to the metal ions is proved by the appearance of bands at ~ 576 cm−1 (M–O) and ~ 492 cm−1 (M–N) [17].
3.2 Electronic Spectra and Magnetic Measurements
In the electronic spectrum of CoII complex, four ligand field bands appeared at 8250, 16,535, 20,455 and 32,410 cm−1, assignable to 4T1g(F) → 4T2g(F) (ν1), → 4A2g(F) (ν2) and → 4T1g(P) (ν3) and CT transitions [18, 19], respectively. The ligand field parameters as Dq = 828.5 cm−1, B = 816 cm−1, β35 = 0.840 cm−1, \( {\raise0.7ex\hbox{${\nu_{2} }$} \!\mathord{\left/ {\vphantom {{\nu_{2} } {\nu_{1} }}}\right.\kern-0pt} \!\lower0.7ex\hbox{${\nu_{1} }$}} \) = 2.004 and σ = 19.04% have been calculated which suggest an octahedral geometry for the complex [20, 21]. In the electronic spectrum of NiII complex, four ligand field bands were observed at 10220, 17460, 24890 and 32860 cm−1 assignable to 3A2g(F) → 3T2g(F) (ν1), → 3T1g(F) (ν2), → 3T1g(P) (ν3) and CT transition, respectively [18], The ligand field parameters as Dq = 1022 cm−1, B = 779.3 cm−1, β35 = 0.748 cm−1, \( {\raise0.7ex\hbox{${\nu_{2} }$} \!\mathord{\left/ {\vphantom {{\nu_{2} } {\nu_{1} }}}\right.\kern-0pt} \!\lower0.7ex\hbox{${\nu_{1} }$}} \) = 1.708 and σ = 33.68% have been calculated. Which suggest an octahedral configuration for the complex. The electronic spectrum of the Cu(II) complex shows one broad band at ~ 13,375–14,480 cm−1 with maxima at ~ 14,360 cm−1 assignable to 2Eg → 2T2g transition in support of a distorted-octahedral geometry for the complex [22, 23]. The electronic spectrum of the Mn(II) complex shows four weak intensity bands at 18,920, 21,150, 31,750 and 37,440 cm−1 characteristics of octahedral geometry [24] which may be assigned to 6A1g → 4T1g (4G), → 4Eg, 4A1g (4G), → 4Eg (4D) and 4T1g (4P), respectively.
The magnetic moment values of CoII, NiII, CuII and MnII complexes are found to be 5.0, 3.1, 1.78 and 5.91 B.M., respectively indicating octahedral environment around the metal ions [25, 26] (Table 1). These four complexes are no doubt tetranuclear in nature, they exhibit normal magnetic moments due to absence of M–M interaction among the metal ions.
3.3 1H-NMR Studies
A complex pattern observed at δ 6.41–7.61 ppm corresponds to twelve phenyl protons [27]. The sharp peak at δ 2.63 ppm corresponds to six methyl protons (Graph 1). The signal observed at 3.0 ppm with an integration corresponding to eight protons in case of Zn(II) complex is assigned to four water molecules [28] (Graph 2).
3.4 13C NMR Analysis
As given in the (Graph 3) the 13C NMR signal for the carbon of methyl group (C-1) of the ligand appeared at 26.0 ppm (Table 2). The signal for the carbon atom of the carbonyl group (C-2) appeared at 203.0 ppm. The carbon signals of C–O groups are shown at 163.0 and 166.0 ppm. Aromatic carbons signals appeared in the range 103–133.0 ppm. In the Zn(II) complex the signal due to carbonyl carbon and the carbon bonded to phenolic –OH groups are slightly shifted downfield thereby conforming the formation of coordination bonds between zinc and carbonyl oxygen and phenolic oxygen atoms [29] (Graph 4). Thus 1H and 13C NMR results confirm the proposed structure of the azodye ligand ad its complexes.
3.5 ESR Studies
The E.S.R. spectrum of copper(II) complex has been recorded at X-band at RT. The g1, g2 and g3 values are found at 2.0269, 2.0898 and 2.2548, respectively [30]. The axial symmetry parameter “G” for the complex is found to be 4.363 which indicates that the absence of exchange interaction among magnetically equivalent Cu(II) ions in the unit cell. In order to obtain information about the ground state another useful parameter “R” can be calculated by using equation.
The “R” value is found to be less than one (0.3812), Hence the ground state is predominantly d 2x 2–y . The ƛ value of the complex is found to be − 372.18 cm−1. The spin–orbit coupling constant (ƛ) can be calculated from the relation [31].
The decrease of the ƛ value (− 3811 cm−1) from the free ion value (− 830 cm−1) indicates the overlapping of metal ligand orbitals in the metal complex (Graph 5).
3.6 Mass Spectra
In the ESI–MS spectrum of the ligand LH4 displayed, a peak with m/z 577.4 corresponding to the molecular mass of the ligand (Graph 6). A peak with m/z 543.3 was also observed which may be formed due to the loss of two methyl groups. The sharp peak obtained with m/z 511.2 corresponds to the loss of two carbonyl oxygen atoms. The peak with m/z 487.3 is due to loss of both the carbonyl carbon atoms. Other fragmentation peaks pattern cannot be reported because it is beyond the range of the instrument used.
3.7 Powder XRD Studies
The XRD (powder pattern) of the complex [Cd4LCl4(H2O)4] was indexed in X-ray diffractometer. The prominent peaks (Graph 7) of the diffraction pattern were indexed and analysed by the computer programme LSUCRPC [32]. The indexing is confirmed by comparing with observed and calculated 2θ values that are evident from the figure of merit 6.9 as suggested by de Woulff [33]. The observed and calculated 2θ values are also in good agreement. The density (d) of the complex was determined by floatation method in a saturated solution of KBr, NaCl and benzene separately. The number of formula units per unit cell (n) is calculated from the relation
where d = density of the complex, N = Avogadro’s number, V = Volume of the unit cell and M = molecular weight of the complex. The value of ‘n’ is found to be 0.5 that agrees well with the suggested triclinic structure of the complex. The mean particle size of the same complex can be measured from the Debye–Scherrer equation
where D = particle size, K = dimensionless shape factor, ƛ = X-ray wavelength, β = line broadening at half the maximum intensity, θ = diffraction angle. This equation relates the size of the particle in a solid in the broadening of a peak in a diffraction pattern. The particle size of the complex [Cd4LCl4(H2O)4] is found to be 9.7 nm [34].
3.8 SEM and EDX Spectra
The two SEM micrographs of [Co4LCl4(H2O)12] were recorded at 500 nm and 1 µm. It provides the surface morphology of the compounds. The SEM micrographs show rock shaped structure. The energy dispersive X-ray (EDX) analysis of the [Co4LCl4 (H2O)12] complex indicates the presence of Co, Cl, S, O and C as shown in (Fig. 2)
3.9 Thermogravimetric Studies
Thermal behaviour of [Co4LCl4 (H2O)12] was studied by TGA and DTA in the temperature range of ambient temperature to 900 °C (Graph 8). Initially the complex being stable up to 139 °C suffered a mass loss of 11.5% that corresponds to loss of six coordinated water molecules and it is supported by an endothermic peak at 138 °C. Thereafter, the compound looses a mass of 3.4% at 196 °C which corresponds to loss of another two coordinated water molecules from the complex which is supported by the endothermic peak on the DTA curve at 196 °C. Then, the compound gradually starts losing its weight with the rise of temperature and at 316 °C looses 17% of its weight which corresponds to the loss of rest of four coordinated water molecules and it is supported by the endothermic peak at 315 °C. Then the compound looses 11.4% at 461 °C which corresponds to the loss of two coordinated chlorine atoms and part of the ligand and it is supported at 460 °C on the DTA curve. Then the compound looses 10% of the weight at 535 °C which corresponds to loss of two coordinated oxygen atoms from ligand supported by the endothermic peak at 534 °C. Thereafter, the compound gradually losses almost all its masses and at 647 °C it form of CoO. Finally, the thermogram becomes a straight line after 647 °C.
Kinetic parameters such as order of the reaction (n) and activation energy (Ea) can be calculated by Freeman-Carrol method [35]. Given below is the equation for this purpose:
where, RH = rate of heating, W = weight fraction of the reacting material, Ea = activation energy and Z = frequency factor. This equation in the difference form can be written as
When Δ (\( {\raise0.7ex\hbox{$1$} \!\mathord{\left/ {\vphantom {1 T}}\right.\kern-0pt} \!\lower0.7ex\hbox{$T$}} \)) is kept constant, a plot of Δ log RT verses Δ log W gives a linear relationship whose slope and intercept provide the value of n and Ea respectively. The order of decomposition reaction and the activation energy are found to be 0.5 and 8.13 J/mole, respectively. The calculated value of activation energy is found to be low that shows auto-catalytic effect of the metal ion [36]. The Correlation factor (r) is found to be 0.98 and the thermal decomposition of the complex fits well with the experimental results.
3.10 Theoretical Calculation
3.10.1 Geometrical Optimization
The ligand LH4 and [Zn4LCl4(H2O)4] complex (Fig. 3) were optimized by using B3LYP/6-31G (d, p) and B3LYP/LANL2DZ basic sets to get the stable geometry. It is observed that the bond lengths and bond angles mentioned in Table 2 of the ligand have been changed in the metal complex (Table 3) indicating the bonding of ligand donor atoms with the metal ions [37, 38].
The single point energy and dipole moment value of LH4, [Cu4LCl4(H2O)12] and [Zn4LCl4(H2O)4] are calculated using DFT/B3LYP 6-31G (d, p) and DFT/B3LYP LANL2DZ basic sets (Table 4). The single point energy of [Zn4LCl4(H2O)4] is found to be less than the energy of the [Cu4LCl4(H2O)12] which indicates the greater stability of the [Zn4LCl4(H2O)4]. In both basic set the higher dipole moment of [Cu4LCl4(H2O)12] complex than the [Zn4LCl4(H2O)4] complex indicates stronger dipole–dipole interaction in [Cu4LCl4(H2O)12].
3.11 Antibacterial Activity
The ligand and metal complexes have been screened at 500 μg/ml for antibacterial activities and results are summarised in Table 5. The antibacterial activity of the compounds was examined against seven strains of bacteria, two gram positive Staphylococcus aureus, Bacillus and five Gram negative Klebsiella, Salmonella, Pseudomonas Aeruginosa, Vibrio Cholerae and E. coli. The drug Tetracycline is taken as standard to compare the effectiveness of the test compounds. It is observed that the zinc complex with the ligand is sensitive to almost all the bacteria. The zone of inhibition of tetracycline drug with the bacteria is found to be 30 mm each. The manganese and copper complexes are found to be intermediate against the bacteria. The other metal complexes are either less effective or resistant to the bacteria (Graph 9). The increased activity of metal chelate can be explained on the basis of the overtone concept and chelation theory [39]. The calculated partition coefficient (C log P) of the Zn(II) complex (4.134) explains better biological activity as C log P is the major factor in the permeability of the tested compound through the cell membrane and can be directly related to the good biological effect. The Zn(II) complex is more potent than other complexes as indicated from its C log P value.
4 Conclusion
The octadentate ligand 4,4/-bis(2,4/-dihydroxy-5/-acylphenylazo)diphenylsulfone and its six tetrameric complexes were synthesized and their structures have been confirmed by elemental analysis, I.R., electronic, 1H NMR, 13C NMR, SEM, EDX, ESI–MS and ESR spectra. The stability of the complexes was confirmed from the thermal study. The kinetic parameters as activation energy (Ea) and order of reaction (n) are determined from the thermal decomposition data. The XRD study shows that the Cd(II) complex is triclinic in nature. The theoretical study using DFT/B3LYP supports experimental evidences of the bonding sites of the ligand, geometry and the stability of the complexes. The quantum chemical parameters reported here will be helpful to the future investigators working in the field of drug designing. The antibacterial study of ligand and its five metal complexes were studied against two Gram positive and five Gram negative bacteria.
References
Dapsone (2015) The American Society of Health-System Pharmacists. Retrieved Jan 12
Zhu YI, Stiller MJ et al (2001) Dapsone and sulfones in dermatology: overview and update. J Am Acad Dermatol 45(3):420–434
Mahapatra BB, Mishra RR, Sarangi AK (2016) Bull Chem Soc Ethiop 30(1):87–100. https://doi.org/10.4314/bcse.v30i1.8
Mahapatra BB, Mishra RR, Sarangi AK (2016) J Saudi Chem Soc 20:635–643. https://doi.org/10.1016/j.jscs.2013.07.002
Mahapatra BB, Mishra RR, Sarangi AK (2013) J Chem. Article ID 653540. https://doi.org/10.1155/2013/653540
Mahapatra BB, Chaulia SN, Sarangi AK, Dehury SN, Panda JR (2015) J Mol Struct. https://doi.org/10.1016/j.molstruc.2015.01.030
GaussView 5.0 (2009) Gaussian Inc., Wallingford, CT, USA
Becke AD (1993) J Chem Phys 98(7):5648–5652
Lee CT, Yang WT, Parr RG (1988) Phys Rev B 37:785–789
Becke AD (1988) Phys Rev A 88:1053–1062
Hay PJ, Wadt WR (1984) J Chem Phys 82(1):270–276
Hay PJ, Wadt WR (1985) J Chem Phys 82:284–298
Hay PJ, Wadt WR (1985) J Chem Phys 82:299–310
Frish MJ et al (2004) GAUSIAN 03, Revision E.01. Gaussian Inc., Pittsburgh, PA
Mishra LK, Keshari BN (1981) Indian J Chem Sect A 28:883–887
King RB, Bisnette MB (1966) Inorg Chem 5:300–306
Nakamoto K (1978) Infrared and Raman spectra of inorganic and coordination compounds. John Wiley, NY, p 239
Ferraro JR (1971) Low frequency vibration of inorganic and co-ordination compound. Plenum press, New York, p 321
Atkins PJ, Shriver DF (1999) Inorganic chemistry, 3rd edn. W.H. Freeman and CO, New York
Lever ABP (1984) Inorganic electronic spectroscopy. Elsevier, Amsterdam, p 554
Magee R, Gordan L (1963) Talanta 10:967–971
Yamada S (1966) Coordination Chem Rev 1:415–418
Procter IM, Hathaway BJ, Nicholls P (1968) J Chem Soc A 1678–1683
Tavman A, Gürbüz D, Çinarli A, Tan SB (2015) Bull Chem Soc Ethiop 29(1):63–74
Williams DH, Fleming I (1994) Spectroscopic methods in organic chemistry, 4th edn. Tata Mc Graw Hill, p 1
Jacob W, Mukherjee R (2006) Inorg Chim Acta 359:4565–4573
Dash UN (1995) Analytical chemistry: theory and practice, 1st edn. Sultan Chand & Sons, pp 143–173
Salman RD, Farrant RD, Lindon JC (1991) Spectrosc Lett 24(9):1071–1078
Alaghar AMA (2014) Mol Struct 1072:103–113
Hathway BJ, Billing DE (1970) Coord Chem Rev 5:143–149
Dutta RL, Syamal A (1982) Elements of magnetochemistry. S. Chand & Company Ltd., p 96
Visser JW (1969) J Appl Crystal 2:89–95
de Woulff PM (1968) J. Appl. Crystal 1:108–113
Patterson A (1939) Phys Rev 56(10):978–985
Freeman ES, Carroll B (1958) J Phys Chem 62(4):394–397
Iimura A, Inoue Y, Yasumori I (1983) Bull Chem Soc Jpn 56:2203–2209
Yousef TA, Rakha TH, El Ayaan U, Abu El Reash GM (2012) J Mol Struct 1007:146–157
Yousef TA, Abu El-Reash GM, Rakha TH, El-Ayaan U (2011) Spectrochim Acta 83(1):271–278
Mohanan K, Devi SN (2006) Russian J Coord Chem 32:600–609
Acknowledgements
The authors are thankful to I.I.P Dehradun, India for providing spectral analysis, SEM, EDX, XRD and thermogravimetric analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sarangi, A.K., Mahapatra, B.B. & Sethy, S.K. Synthesis and Characterization of Tetranuclear Metal Complexes with an Octadentate Azodye Ligand. Chemistry Africa 1, 17–28 (2018). https://doi.org/10.1007/s42250-018-0002-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42250-018-0002-z