Abstract
Due to increasing environmental awareness, tightening regulations and the need to meet the climate obligations under the Paris Agreement, the production and use of electric vehicles has grown greatly. This growth has two significant impacts on the environment, with the increased depletion of natural resources used for the production of the lithium-ion batteries for these electric vehicles and disposal of end-of-life lithium-ion batteries. In particular, when end-of-life lithium-ion batteries are incorrectly landfilled, pollution to groundwater and soil occurs. Therefore, sustainable recycling technologies must be implemented to construct a cyclic economy for the lithium-ion battery market and help alleviate the severity of these environmental consequences. The majority of current recycling methods involve energy-intensive pyrometallurgy, whereas hydrometallurgy techniques pose a viable alternative with promising advances at lab scale that can adapt with the evolution of new mixed cathode chemistries. As reviewed in this work, a combination of pre-treatment and hydrometallurgical processes was identified as a potential mechanism that could meet this criterion, which focuses on the recovered economic value and cumulative environmental benefits. Furthermore, automation of the pre-treatment process and mechanisms for electrolyte recovery were identified as potential opportunities for future works. Here, we evaluate the opportunities for sustainable recycling technologies for lithium-ion batteries.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The lithium-ion battery market is increasing exponentially, going from $12 billion USD in 2011 to $50 billion USD in 2020 [1]. Estimates now forecast an increase to $77 billion USD by 2024 [1]. Data from the International Energy Agency shows a sixfold increase in lithium-ion battery production between 2016 and 2022 [2] (Fig. 1). Therefore, combined with estimates from Winslow et al., 5.9 Mt of lithium-ion batteries is expected to be produced in the year 2022 alone [3]. This growth is unprecedented and will continue to grow in the future, largely attributed to the rapid propagation of electric vehicles [4].
Global lithium-ion battery production by capacity [2]
The environmental consequences of this growth are profound, with the depletion of natural resources, and the pollution to groundwater and soil when spent lithium-ion batteries are not recycled. Sustainable recycling technologies must be implemented to construct a circular economy for the lithium-ion battery market. Currently, global recycling rates are less than 4% [5], with the majority of these recycling processes using energy-intensive, polluting processes that fail to recover the environmentally harmful and valuable metals contained within.
To address the environmental and social issues that stem from the rapid growth in the lithium-ion battery market, there are several review articles published over the last few years summarising the current status of lithium-ion batteries and developed strategies in pre-treatment and metallurgical processes [3,4,5]. Furthermore, the challenges and limitations of lithium-ion battery recycling are analysed and discussed. The policy and regulation concerning the disposal of lithium-ion batteries are also reviewed. The aim of this review is to provide guidance on the limitations and potential for sustainable lithium-ion battery recycling technologies.
2 Background
2.1 Primary consumers of lithium-ion batteries
Lithium-ion batteries are used in a wide range of applications including electric vehicles, electronic devices and electric micro-mobility. Electric vehicles utilise the largest market share of lithium-ion batteries and offer consumers a sustainable transportation option, with zero exhaust emissions, better efficiency than vehicles with internal combustion engines and a potential for reduced embodied energy when coupled with renewable electricity infrastructure—as in New Zealand, where 82% of electricity generated is from renewable resources [6]. Globally, electric vehicles avoided the consumption of over 600,000 barrels of oil products per day or approximately 35 GL in 2019 [2]. Similarly, the electricity generation to supply the global electric vehicle fleet in 2019 is estimated to have avoided 53 Mt CO2−e of emissions for an equivalent fleet of internal combustion engine vehicles [2]. Although beneficial to meeting global climate obligations, electric vehicles are the primary consumers of lithium-ion batteries globally [4] and pose a serious problem in the generation of lithium-ion battery waste. Insomuch, there is rapid increase in electric vehicle sales, reaching 2.1 million globally in 2019 [2], with estimates of 11 million in 2025, and expanding to 30 million in 2030 [7]. Significantly, the cathode chemistry, shape and packaging used in electric vehicles vary between manufacturers. This makes the processing aspect of lithium-ion battery recycling difficult. A life cycle analysis conducted by Gaines et al. found that up to 4 Mt of spent lithium-ion batteries from electric vehicles could be generated between 2015 and 2040 [8], with a commodity value of approximately $8 billion USD assuming complete recovery [4, 8].
2.2 Structure and composition of lithium-ion batteries
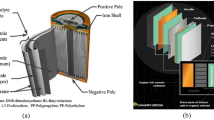
Lithium-ion battery cells consist of negative and positive electrodes, electrolytes and a separator coiled in varying shapes and sizes [4, 9,10,11,12]. The environmentally harmful and valuable metal oxides are found deposited on aluminium current collectors, which make up the positive electrode (cathode during discharge). A variety of cathode compositions—summarised in Table 1 below—result in different types of lithium-ion batteries: lithium cobalt oxide (LCO), lithium iron phosphate (LFP), lithium manganese oxide (LMO), lithium nickel cobalt aluminium oxide (NCA) and lithium nickel manganese cobalt oxide (NMC) [3]. The negative electrode (anode during discharge) is comprised of graphite deposited on a copper current collector [3, 14, 15].
The typically liquid electrolyte is composed of lithium salt dissolved in organic solvent—typically a mixture of ethylene carbonate and dimethyl carbonate [15]. The solvent containing the electrolyte has high permittivity and low viscosity, which provides high electrochemical stability and operability on a wider voltage range [16, 17]. The separator is a porous polypropylene, semi-permeable, membrane that provides an ionic conduction pathway for the electrolyte and prevents short-circuiting [15, 16, 18]. The remainder of the cell is comprised of the casing. Although dependent on the cell type and manufacturer, an indicative percentage contribution of each component in a typical lithium-ion cell is shown below (Fig. 2).
2.3 Mechanisms responsible for lithium-ion battery capacity loss
Over time, the capacity of lithium-ion batteries decreases, and eventually they do not hold enough charge for their intended applications, thereby reaching their end-of-life. The three main processes responsible for this capacity loss and the eventual end-of-life of lithium-ion batteries are as follows: mechanical degradation, formation of a solid electrolyte interface, electrolyte oxidation and lithium plating.
A solid electrolyte interface forms during charging, wherein both lithium oxide and lithium carbonate form a film on the negative electrode. The formation of this barrier of lithium-ions impedes lithium intercalation, thus increasing the internal resistance [14, 15]. Solid electrolyte interface formation is the leading contributor to capacity loss in most graphite-based LiBs below 3.9 V [8]. Various electrolyte additives can mitigate the formation of the solid electrolyte interface in the short term [8].
Mechanical degradation of electrodes or loss of stack pressure in pouch-type cells is another mechanism for the loss of battery capacity. During the charging and discharging of lithium-ion batteries, lithium-ion intercalation causes strain within the electrodes, resulting in electrode fracturing [15, 20]. Mechanical degradation does not often hinder lithium-ion battery performance but has the potential to reduce capacity and in some cases cause complete failure of the cell. Cell design and correct electrolyte additives can minimise these effects [4].
Electrolyte oxidation can occur on the positive electrode (during charging) if the lithium-ion battery is operated in temperatures exceeding 40 °C [21], which is often achieved under normal operating conditions. Electrolyte oxidation also induces self-discharge and is exacerbated when the lithium-ion battery is at full charge [22]. The film caused by electrolyte oxidation is thickened over several charge and discharge cycles, and it reduces the mobility of lithium-ions, thereby reducing battery capacity.
Lithium plating typically occurs on the surface of the negative electrode (anode during discharge) and is induced by high charging rates and cold temperatures [23]. This occurs when the charging rate exceeds the rate at which the negative electrode can intercalate the lithium. This effectively reduces the available lithium and thus reduces battery capacity and, in some cases, can cause cell-damaging short circuit due to dendrite formation.
2.4 Policy and regulation for lithium-ion battery disposal
A significant driver for the development of lithium-ion recycling is the implementation of environmental regulations. It is these policies which will help guide the lithium-ion battery market towards a circular economy through the implementation of sustainable recycling technologies [22].
New Zealand has a growing recognition of the indigenous Māori people and their values. The concept of ōhanga āmiomio, the circular economy, is reflected in regulation [24]. New Zealand’s Resource Management Act and the Waste Minimisation Act provide definitions for sustainable management and encourage a reduction in the amount of waste generated and disposed of [25]. However, end-of-life lithium-ion batteries are yet to be specifically integrated into New Zealand’s legislation.
China has recently issued regulatory measures on the recycling and reuse of batteries from electric vehicles—specifically including lithium-ion batteries. This was introduced in August 2018; it mandated strict guidelines on maintenance, collection and transport, as well as reuse and recycling technologies [8, 26]. The progressive policy ensures electric vehicle manufacturers are responsible for the collection, sorting, storage and transportation of lithium-ion batteries. It is also stipulated that lithium-ion batteries must have a means of tracking for future recycling purposes. The regulation also promotes initial design forethought for disassembly and recycling with open-source information across the supply chain [8].
The European Union mandates that battery manufacturers are responsible for the collection and recycling of spent lithium-ion batteries [8]. As discussed above, this mandate encourages the planning for lithium-ion battery disposal in the design stages. The mandate required 45% of battery production—inclusive of lithium-ion batteries—to be recovered by 2016, which the majority of members have achieved [27].
At the national level, no policy exists in the USA to address the recycling of lithium-ion batteries. Lithium-ion batteries are not considered dangerous under the United States Environmental Protection Agency Universal Waste Rules and therefore are not covered under the Battery Act, even though they are classified as hazardous substances by the Department of Transportation because of their fire risk [8]. Eight states, in the USA, have waste management regulations and mechanisms for battery disposal, only three of which explicitly incorporate lithium-ion batteries [8, 28].
3 Environmental impacts of lithium-ion batteries
A life cycle analysis conducted by Peters et al. found that it took 330 kWh and 110 kg CO2−e [15, 29] to produce 1 kWh of lithium-ion battery storage. Through sustainable recycling technologies, the environmental impact of manufacturing new lithium-ion batteries can be reduced by minimising the extent of natural resource extraction. Further reduction of the environmental impact of lithium-ion batteries can be achieved if pyrometallurgical methods are avoided, which release toxic flue gases [15].
3.1 Natural resource extraction
Resource extraction is a serious environmental issue and is responsible for the majority of global greenhouse gas emissions and more than 80% of biodiversity loss according to the United Nations Global Resources Outlook for 2019 [30]. Within the lithium-ion battery context, the estimated material demand for the 2.1 million electric vehicles sold in 2019 was approximately 19 kt for cobalt, 17 kt for lithium, 22 kt for manganese and 65 kt for nickel [2]. By 2030, the resource demand for LiBs is set to increase to 180 kt per year for cobalt, lithium to approximately 185 kt per year, manganese to 180 kt per year and nickel to 930 kt per year [2].
Lithium carbonate is used in the manufacturing of lithium-ion batteries. Chile is the largest supplier, followed by China and Russia [18, 31]. The environmental implications of lithium extraction are profound, insomuch as 750 t of brine and 1.9 kt of water is needed, to produce 1 ton of lithium [4, 31]. Thereby, the process of lithium production consumes as much as 65% of Salar de Atacama’s water supply [4]. In comparison, 1 t of lithium can be recovered from 28 t of spent lithium-ion batteries or 260 electric vehicle battery packs [4, 16]. Therefore, the net environmental impact attributed to lithium in lithium-ion batteries can be greatly reduced if constituents of lithium-ion batteries can be sustainably recycled by methods that consume less resources. Although the global lithium reserves are estimated at 62 Mt [28, 32]—based on current lithium consumption—this should be able to meet long-term projected demands up to 2100 [15]; other metal constituents, such as cobalt, do not have a continuous supply chain due to geopolitical and social implications.
Cobalt is a strategic metal, with global identified reserves estimated in the order of 25 Mt, with estimates of 120 Mt of unidentified reserves [28, 32]. Whilst the annual extraction of cobalt is approximately 100 kt [16, 18], the demand for cobalt is expected to increase in the future associated with the increase in electric vehicles [28]. Therefore, recycling materials containing cobalt are particularly important from an environmental and health standpoint as cobalt is classified as a carcinogen, mutagen and reproductive impacting substance [18]. Cobalt reserves are geographically concentrated in the Democratic Republic of Congo and these reserves have experienced significant price variations attributed to their geopolitics. In addition, cobalt from the Democratic Republic of Congo raises both ethical and environmental concerns around their extraction [4, 18]. According to UNICEF, around 40,000 children are involved in cobalt mining in the Democratic Republic of Congo where they make less than $2 USD per day [24].
Current lithium-ion battery recycling often centres around the recovery of cobalt, due to older LCO batteries nearing their end-of-life, and the high value of cobalt. This economic incentive is expected to decline over time due to a shifting market, from LCO batteries towards cathodes with reduced cobalt content, such as NMC and NCA [3, 15]. Therefore, when reviewing reuse, recovery and recycling strategies, it is important to consider a range of varying cathode compositions.
The reserves of other transition metals, such as manganese, nickel, copper and aluminium, used in the construction of lithium-ion batteries are better established. The increase of NMC and NCA in the lithium-ion battery market is largely attributed to manufacturers attempting to reduce their reliance on cobalt, although global identified reserves of nickel are estimated to be only 89 Mt [28, 32]. Magnesium compounds are globally widespread and can be extracted from a wide range of sources, the global resource of magnesite is estimated in excess of 12 Gt [28, 32]. Manganese reserves are estimated as large and dispersed and recycling is crucial to prevent leaching if landfilled incorrectly [15]. Others have shown that the sole recycling of manganese from lithium-ion batteries is not profitable [33].
There are currently 2.1 Gt of identified copper resources, with an estimate of 3.5 Gt of unidentified copper resources available [32]. Similarly, the world’s aluminium resources are estimated to be large, in the order of 55–75 Gt [32]. Geopolitical issues surround the access to graphite, which has large global reserves of approximately 800 Mt, but in the year 2018, 70% of this was produced in China [28, 32]; synthetic graphite offers an alternative but remains expensive.
3.2 Disposal consequences
The repercussions of landfilling spent lithium-ion batteries are dire. When lithium-ion batteries are disposed to landfill, acid-producing microorganisms can corrode the aluminium casing, enabling water to leach out toxic metals such as cobalt, nickel and manganese, which compromise the water table, pollute the soils and often cause major safety hazards [15, 24, 34, 35]. Furthermore, when the lithium-ion battery casing is corroded, the electrolytes within the can also react with water, which can release harmful gases such as hydrogen fluoride into the atmosphere. Similarly, when lithium-ion batteries with significant lithium plating are disposed to landfill, the deposited lithium reacts violently with water causing serious explosions and fires [36].
4 Recycling opportunities for lithium-ion batteries
The environmental impacts of lithium-ion batteries outlined previously can be greatly reduced through sustainable recycling technologies and the establishment of a circular economy, wherein new lithium-ion batteries are able to be manufactured from recycled materials. Lithium-ion battery recycling must utilise the 3-R concept of reduce, reuse and recycle. The number of end-of-life lithium-ion batteries can be reduced through second life options, such as complementing renewable infrastructure to absorb peak loading. Spent electric vehicle batteries can provide for this niche, with end-of-life lithium-ion batteries still maintaining up to 80% of their capacity when removed from electric vehicles [15]. Reuse is defined as lithium-ion batteries being reused for their intended purpose. This extends to direct recycling methods, which recover cathode materials as reusable cathode mixtures instead of as individual metals, thus reducing the need for downstream processing. The recycling of lithium-ion batteries is defined as the recovery of material and extraction of metal constituents. Currently, recovery processes can be divided into four main types: pre-treatment, pyrometallurgy, hydrometallurgy and biometallurgy processes, and often recovery methods utilise a combination of these processes. Disassembly is an advancing field, which aims to integrate automation in the pre-treatment step; physical processes consist of the physical separation of the battery components by methods such as crushing and heating. Whilst the chemical processes involve separation of metal ions from the electrode materials. Biological methods are a rapidly advancing field [37]. Table 2 summarises the main recovery methods used to extract metals from lithium-ion battery scrap, albeit these techniques are often combined in industry. The methods vary in economic cost, environmental cost and extraction efficiencies.
4.1 Disassembly and pre-treatment
The intelligent sorting of lithium-ion batteries is crucial for downstream processes. Wang et al. estimated that, based on recycling efficiencies given in literature, 1 ton of LCO batteries could yield up to $8900 USD, whereas 1 ton of LMO batteries would only yield $890 USD [15, 33]. Therefore, under current market rates, LCO lithium-ion batteries must comprise at least 20% of the total lithium-ion battery scrap in order for current recycling plants to be profitable [33]. For this to be implemented, lithium-ion batteries must be sorted by chemistry prior to treatment. Similarly, manufacturers have varying lithium-ion battery designs, which pose serious issues for pre-treatment prior to recycling [4]. Currently, the lithium-ion battery manufacturing industry has no standardisation of lithium-ion batteries, and this is expected to remain an issue into the future.
Lithium-ion batteries must first be discharged to approximately 2.5 V before safe cell opening [39]. A common method to discharge cells prior to cell opening involves the use of salt-saturated solutions such as immersion in sodium chloride solution [36], despite causing structural damage to the cells before complete discharge [40]. Although this method is less time consuming than other methods [41], it can release hydrogen fluoride if the cell casing is compromised allowing the reaction of electrolyte in water. Discharging is not necessary if the atmosphere for the recycling process is inert; this can be achieved through argon. Alternatively, disassembly can be done under passivation, through cooling with liquid nitrogen, which lowers the reactivity of lithium-ions and thereby reduces the risk associated with explosions, fires and toxic emissions [15].
Automation, in the processing and disassembly of waste lithium-ion batteries, offers a solution to the broad range of designs and chemistries. Automation of the disassembly process would significantly reduce the risk to human workers and would reduce cost, potentially making recycling economically viable [15, 42, 43]. However, there are major challenges in automating the disassembly line, namely, various designs of lithium-ion batteries; environmental damage, such as corrosion; and lack of labelling systems to identify varying cathodic compositions. Hermann et al. investigated the state of automation in the industry and proposed a multifaceted process based on industry-specified, technical difficulties [42]. It was found that some aspects were well suited for automation such as dismantling of the individual cells by a prototype jaw system, in which cell health was monitored before dismantling.
Electrolyte recovery from lithium-ion batteries has been established at both the lab and industrial scales. Often, in recycling processes, the electrolyte and other organic compounds are discarded, although electrolyte recovery has been shown to be profitable in literature. One method of electrolyte recovery involves the recovery of organic solvent by distillation due to its high boiling point relative to water [44]. Some electrolytes may remain captured within the pores of the electrode material, which could be removed by supercritical CO2 extraction from the processed lithium-ion battery powder [45, 46]. Hazardous gases can be released if the electrolyte and solvent are exposed to the atmosphere [35, 47, 48]. The electrolyte and separator are often placed in an alkaline solution after dismantling to prevent the release of toxic fluoride-based gases [35, 49].
Graphite recovery has been investigated at a lab scale. Anodes are separated from lithium-ion batteries before the graphite is separated from the copper current collector by N-methyl-2-pyrrolidone (NMP) dissolution, acid dissolution or mechanical scraping [50,51,52]. However, NMP remains too expensive for scale-up processes, and alternative dissolution compounds must be investigated [53]. Pre-treated lithium-ion battery powder contains electrode materials, wherein separation of graphite and metals can be achieved through flotation or magnetic separation [15]. The main challenge found in the recovery of graphite is in separating the solid electrolyte interface layer, binder and additives away from the graphite [15, 45, 46].
Mechanical separation is a form of pre-treatment that uses processing techniques to concentrate the metallic-fraction, according to properties such as density, conductivity and magnetic behaviour [54]. The disadvantage of mechanical separation is that not all the compounds in lithium-ion cells are able to be separated [22].
4.2 Pyrometallurgy
Pyrometallurgy involves high-temperature processing to recover metals and other materials. Spent lithium-ion batteries including plastic, electrolyte and other components are decomposed in high-temperature, oxygen-rich furnaces, with temperatures exceeding 1200 °C [55], thereby forming a metal oxide. The metal oxide requires further processing before it can be used in lithium-ion battery manufacturing. Although used widely in industry, this method generates toxic flue gas, which requires downstream treatment to meet environmental regulations. However, pyrometallurgy is adaptable, simplistic and capable of a high processing capacity. Furthermore, it is able to facilitate a mixed lithium-ion battery feed, with minimum pre-treatment needed. Pyrometallurgical recycling has been proven to result in a net increase of greenhouse gas emissions and energy consumption compared to raw material extraction, largely attributed to the combustion of non-metallic-based lithium-ion battery componentry [15].
Lower temperature treatment can also be conducted to aid in the separation of insoluble organic additives and adhesives. This is achieved by heating to approximately 150 °C in a controlled atmosphere. Low-temperature treatment is appealing, as it is effective in removing lithium-ion battery impurities before further processing. However, similar to pyrometallurgical processes, thermal treatment has been linked to high dioxin emissions and chloride compounds. Therefore, downstream air purification is necessary [22].
4.3 Hydrometallurgy
Hydrometallurgy encompasses leaching, solvent extraction and precipitation, utilising a broad range of reagents. A summary of these processes and subsequent reagents is given in Table 3. Compared with pyrometallurgy and biometallurgy processes, hydrometallurgy can achieve higher purity, lower energy consumption and lower gas emissions [61, 62]. Hydrometallurgical recycling processes are appealing because they can be applied to a range of lithium-ion battery chemistries.
The remaining metallic dust and residues from mechanical pre-treatment can be leached by an acidic solution in order to transfer the metals into the bulk aqueous solution [53]. Zhang et al. found that hydrochloric acid was the most effective leaching reagent out of the inorganic acids that were tested [63]. However, the use of hydrochloric acid often results in the evolution of chlorine gas and additional downstream processing is needed to ensure this gas is appropriately managed at an industrial scale. The proposed reaction using hydrochloric acid on LCO residue is described in (1):
Hydrometallurgical acid leaching is promising for large-scale applications due to its low energy consumption and capability of dealing with varying cathode chemistries, whilst allowing the metals to be recovered in high purity [15]. A range of leaching acids have been tested in literature and are summarised in Table 4.
Following the leaching process, in most processes, each metal is then selectively extracted from leachate into an organic phase using solvent extraction [84], before being recovered by precipitation (Fig. 3), crystallisation or reduction [86]. In addition to the more conventional recovery methods, Myoung et al. show that direct precipitation of cobalt oxide from an aqueous leachate was possible using an electrochemical-driven process [87]. Whilst many studies focus on separate separation of the metals using solvent extraction [88], others have investigated co-extraction followed by mixed metal oxide precipitation as a method to directly regenerate the cathode material [58].
Process flow diagram of hydrometallurgy process for metal recovery from lithium-ion batteries [82]
Others have explored the use of deep eutectic solvents to extract Li and Co from spent lithium-ion cells and have reported high extraction efficiencies and simple post-leaching precipitation processes [89,90,91]. The significant advantage of deep eutectic solvents over more conventional acid leachants is the ability to tailor the properties of the solvent for specific applications [92, 93]. Whilst deep eutectic solvents are more expensive that conventional leachants, as research continues in this area, it is expected that the cost will decrease and process efficiency will increase.
4.4 Biometallurgy
Biometallurgy is an effective method for extracting metals from waste lithium-ion batteries. The metabolites excrete organic acids, which make soluble metals from insoluble waste [94]. Both bacteria and fungi are used in biometallurgy, with fungi able to grow over a wider range of pH values and leach at a faster rate [95]. The work of Bahaloo-Horeh et al. [95] used biometallurgy techniques on mixed lithium-ion battery scrap. This was achieved using Aspergillus niger, a fungus known to secrete citric, gluconic, malic and oxalic acids [15, 95]. These processes require long incubation periods (e.g. 2 weeks) with high liquid-solid ratios and are yet to be proven at a large scale [15].
4.5 Current processes
Lithium-ion batteries are being recycled on an international scale. This is not only largely due to environmental pressures but also attributed to the economic benefit of recovering metallic lithium-ion battery constituents. Several companies have developed methods to handle the influx of end-of-life lithium-ion batteries entering the waste stream. A wide range of companies from many countries are currently active in recycling lithium-ion batteries on a range of scales (Fig. 4).
Whilst this list is not exhaustive, majority of these companies still use traditional, energy-intensive methods such as pyrometallurgy [16]. However, recent awareness as to the adverse environmental effects of such recycling techniques has prompted a move to reduce this reliance [15]. It should be noted that for the data collected, it is unclear whether the processing capacities indicated are inclusive of other types of batteries, ores or manufacturing scraps.
Many recycling companies, such as the ones above, are continuously expanding to make recycling more economic, efficient and less environmentally impacting [97, 98, 99]. Development and integration of the 3-R model offers a step towards a circular lithium-ion battery economy. However, a shift in perspective is needed to move away from currently dominant, environmentally detrimental, pyrometallurgy methods.
5 Conclusions
The lithium-ion battery market is growing rapidly due to the uptake of electric vehicles. Whilst the use of electric vehicles has many positive benefits to the environment, the production of lithium-ion batteries and landfilling and even current recycling methods have a significant environmental impact. Given the rapid growth of the lithium-ion battery market, there will also be a large increase in end-of-life lithium-ion batteries which need to be managed in the near future. As the manufacturing rate of new lithium-ion batteries greatly surpasses current recycling efforts, new sustainable recycling technologies must be implemented to construct a circular economy for the lithium-ion battery market.
The majority of current recycling methods utilise pyrometallurgy, which is energy-intensive and emits toxic flue gases with the production of poor-quality metal alloys. Comparatively, hydrometallurgy techniques offer a viable alternative with promising advances in lab-scale research, suggesting there is a path towards future industrial-scale recycling processes that can compete with lithium-ion battery production. These processes must be able to adapt with the evolution of new mixed cathode chemistries such as those which reduce or completely remove the use of cobalt. As reviewed in this work, a combination of pre-treatment and hydrometallurgical processes has the potential to recover valuable metals, reduce the energy consumption for recycling and ultimately lower the reliance on raw material extraction. Effective solvent recovery of leached metals from cathode scrap was identified as a limitation in literature, in addition to the recovery of the electrolyte and graphite from end-of-life lithium-ion batteries. The utilisation of automation in the pre-treatment process offers additional benefits, which include reduced costs and higher efficiency of material recovery. The recycling of lithium-ion batteries will require political pressure from economic incentives, legislative requirements and public education.
Data availability
Not applicable.
References
Y. Hu, Y. Yu, K. Huang, L. Wang, Development tendency and future response about the recycling methods of spent lithium-ion batteries based on bibliometrics analysis. J. Energy Storage 27, 101111 (2020)
IEA. Global EV Outlook (Technical Report, 2020) https://www.iea.org/reports/global-ev-outlook-2020. Accessed 10 December 2020
K.M. Winslow, S.J. Laux, T.G. Townsend, A review on the growing concern and potential management strategies of waste lithium-ion batteries. Resour. Conserv. Recycl. 129, 263–277 (2018)
G. Harper, R. Sommerville, E. Kendrick, L. Driscoll, P. Slater, R. Stolkin, A. Walton, P. Christensen, O. Heidrich, S. Lambert, A. Abbott, K. Ryder, L. Gaines, P. Anderson, Recycling lithium-ion batteries from electric vehicles. Nature 575, 75–86 (2019)
Y. Shi, G. Chen, Z. Chen, Effective regeneration of LiCoO2from spent lithium-ion batteries: a direct approach towards high-performance active particles. Green Chem. 20(4), 851–862 (2018)
MBIE. Energy in New Zealand 2020. https://www.mbie.govt.nz/building-and-energy/energy-and-natural-resources/energy-statistics-and-modelling/energy-publications-and-technical-papers/energy-in-new-zealand. Accessed 10 December 2020
Vector. New energy futures paper: batteries (Technical Addendum. 2019). https://blob-static.vector.co.nz/blob/vector/media/vector/vector_new_energy_futures_paper_batteries_technical_addendum.pdf. Accessed 10 December 2020
L. Gaines, K. Richa, J. Spangenberger, MRS Energy & Sustainability 5, e14 (2018)
K. Kaviyarasu, E. Manikandan, J. Kennedy, M. Jayachandran, M. Maaza, Rice Husks As A Sustainable Source Of High Quality Nanostructured Silica For High Performance Li-Ion Battery Requital By Sol-Gel Method – A Review. Adv. Mater. Lett. 7(9), 684–696 (2016)
S. Kim, M. Hankel, W. Cha, G. Singh, J.M. Lee, I.Y. Kim, A. Vinu, Theoretical and experimental investigations of mesoporous C3N5/MoS2 hybrid for lithium and sodium ion batteries. Nano Energy 72, 104702 (2020)
T. Kesavan, T. Partheeban, M. Vivekanantha, N. Prabu, M. Kundu, P. Selvarajan, S. Umapathy, A. Vinu, M. Sasidharan, Design of P-Doped Mesoporous Carbon Nitrides as High-Performance Anode Materials for Li-Ion Battery. ACS Appl. Mater. Interfaces 12(21), 24007–24018 (2020)
P.S. Murphin Kumar, A.H. Al-Muhtaseb, G. Kumar, A. Vinu, W. Cha, et al., Piper longumExtract-Mediated Green Synthesis of Porous Cu2O:Mo Microspheres and Their Superior Performance as Active Anode Material in Lithium-Ion Batteries. ACS Sustain. Chem. Eng. 8(38), 14557–14567 (2020)
I. Buchmann (Battery University: Coalescent design; 2020) https://batteryuniversity.com/learn/article/bu_808c_coulombic_and_energy_efficiency_with_the_battery. Accessed 10 December 2020
M. Aaltonen, C. Peng, B. Wilson, M. Lundström, Leaching of Metals from Spent Lithium-Ion Batteries. Recycling 2, 20 (2017)
T. Or, S.W. Gourley, K. Kaliyappan, A. Yu, Z. Chen, Carbon Energy 2, 6–43 (2020)
J. Heelan, E. Gratz, Z. Zheng, Q. Wang, M. Chen, D. Apelian, Y. Wang, Current and Prospective Li-Ion Battery Recycling and Recovery Processes. JOM 68(10), 2632–2638 (2016)
Y. Matsuda, M. Morita, F. Tachihara, Conductivity of Lithium Salts in the Mixed Systems of High Permittivity Solvents and Low Viscosity Solvents. Bull. Chem. Soc. Jpn. 59(6), 1967–1973 (1986)
A. Chagnes, B. Pospiech, J. Chem, A brief review on hydrometallurgical technologies for recycling spent lithium-ion batteries. Technol. Biotechnol. 88(7), 1191–1199 (2013)
J. Nan, D. Han, X. Zuo, Recovery of metal values from spent lithium-ion batteries with chemical deposition and solvent extraction. J. Power Sources 152, 278–284 (2005)
J. Christensen, J. Newman, Stress generation and fracture in lithium insertion materials. J. Solid State Electrochem. 10, 293–319 (2006)
D. Horn, J. Zimmermann, A. Gassmann, R. Stauber, O. Gutfleisch, Battery recycling: focus on Li-ion batteries. Modern battery engineering: a comprehensive introduction. World Sci., 223 (2019)
J. Ordoñez, E. Gago, A. Girard, Renew. Sust. Energ. Rev. 60, 195–205 (2016)
M.O. Ramoni, H.-C. Zhang, End-of-life (EOL) issues and options for electric vehicle batteries. Clean Techn. Environ. Policy 15(6), 881–891 (2013)
S. King, N.J. Boxall, J. Clean. Prod. 215, 1279–1287 (2019)
MFE. (Legal Frame Work for Waste 2020) https://www.mfe.govt.nz/waste/waste-strategy-and-legislation/legal-framework-waste. Accessed 10 December 2020
J. Zhang, J. Hu, W. Zhang, Y. Chen, C. Wang, J. Clean. Prod. 204, 437–446 (2018)
L. Ahllöf, M. Romare, A. Wu, Mapping of lithium-ion batteries for vehicles: a study of their fate in the Nordic countries (Nordic Council of Ministers; 2019), pp. 54
E. Fan, L. Li, Z. Wang, J. Lin, Y. Huang, Y. Yao, R. Chen, F. Wu, Sustainable Recycling Technology for Li-Ion Batteries and Beyond: Challenges and Future Prospects. Chem. Rev. 120, 7020–7063 (2020)
J.F. Peters, M. Baumann, B. Zimmermann, J. Braun, M. Weil, The environmental impact of Li-Ion batteries and the role of key parameters – A review. Renew. Sust. Energ. Rev. 67, 491–506 (2017)
B. Oberle, S. Bringezu, S. Hatfield-Dodds, S. Hellweg, H. Schandl, J. Clement, et al., Global resources outlook 2019: natural resources for the future we want. https://www.resourcepanel.org/reports/global-resources-outlook. Accessed 10 December 2020
S. Castillo, F. Ansart, C. Laberty-Robert, J. Portal, Advances in the recovering of spent lithium battery compounds. J. Power Sources 112(1), 247–254 (2002)
D. Bernhardt, I. Reilly, Mineral commodity summaries (US Geological Survey, Reston, 2016), pp. 42–43 https://prd-wret.s3-us-west-.amazonaws.com/assets/palladium/production/atoms/files/mcs2019_all.pdf. Accessed 10 December 2020
X. Wang, G. Gaustad, C.W. Babbitt, Waste Manag. 51, 204–213 (2016)
L. Li, J. Ge, R. Chen, F. Wu, S. Chen, X. Zhang, Environmental friendly leaching reagent for cobalt and lithium recovery from spent lithium-ion batteries. Waste Manag. 30(12), 2615–2621 (2010)
J. Nan, D. Han, M. Yang, M. Cui, X. Hou, Recovery of metal values from a mixture of spent lithium-ion batteries and nickel-metal hydride batteries. Hydrometallurgy 84(1-2), 75–80 (2006)
I. Weiguang, Z. Wang, H. Cao, Y. Sun, Y. Zhang, Z. Sun, CS Sustain. Chem. Eng. 6(2), 1504–1521 (2018)
S. Barik, G. Prabaharan, L. Kumar, J. Clean. Prod. 147, 37–43 (2017)
X. Zheng, Z. Zhu, X. Lin, Y. Zhang, Y. He, H. Cao, Z. Sun, A Mini-Review on Metal Recycling from Spent Lithium Ion Batteries. Engineering 4(3), 361–370 (2018)
J. Marshall, D. Gastol, R. Sommerville, B. Middleton, V. Goodship, E. Kendrick, Disassembly of Li Ion Cells—Characterization and Safety Considerations of a Recycling Scheme. Metals 10(6), 773 (2020)
J. Xiao, J. Guo, L. Zhan, Z. Xu, A cleaner approach to the discharge process of spent lithium ion batteries in different solutions. J. Clean. Prod. 255, 120064 (2020)
P. Meshram, B. Pandey, T. Mankhand, Hydrometallurgical processing of spent lithium ion batteries (LIBs) in the presence of a reducing agent with emphasis on kinetics of leaching. Chem. Eng. J. 281, 418–427 (2015)
C. Herrmann, A. Raatz, M. Mennenga, J. Schmitt, S. Andrew, Assessment of automation potentials for the disassembly of automotive lithium ion battery systems. Leveraging Technology for a Sustainable (World: Springer); pp. 149-54 (2012)
L. Li, P. Zheng, T. Yang, R. Sturges, M.W. Ellis, Z. Li, JOM. 71(12), 4457–4464 (2019)
K. He, Z.-Y. Zhang, L. Alai, F.-S. Zhang, J. Hazard, Mater. 375, 43–51 (2019)
M. Grützke, X. Mönnighoff, F. Horsthemke, V. Kraft, M. Winter, S. Nowak, Extraction of lithium-ion battery electrolytes with liquid and supercritical carbon dioxide and additional solvents. RSC Adv. 5(54), 43209–43217 (2015)
S. Nowak, M. Winter, The Role of Sub- and Supercritical CO2 as “Processing Solvent” for the Recycling and Sample Preparation of Lithium Ion Battery Electrolytes. Molecules 22(3), 403 (2017)
A. Sonoc, J. Jeswiet, V.K. Soo, Opportunities to Improve Recycling of Automotive Lithium Ion Batteries. Procedia CIRP 29, 752–757 (2015)
P. Ribière, S. Grugeon, M. Morcrette, S. Boyanov, S. Laruelle, G. Marlair, Investigation on the fire-induced hazards of Li-ion battery cells by fire calorimetry. Energy Environ. Sci. 5(1), 5271–5280 (2012)
M.M. Archuleta, Toxicity of materials used in the manufacture of lithium batteries. J. Power Sources 54(1), 138–142 (1995)
Y. He, T. Zhang, F. Wang, G. Zhang, W. Zhang, J. Wang, J. Clea, Prod. 143, 319–325 (2017)
F. Wang, T. Zhang, Y. He, Y. Zhao, S. Wang, G. Zhang, et al., J. Clean. Prod. 185, 646–652 (2018)
J. Yu, Y. He, Z. Ge, H. Li, W. Xie, S. Wang, A promising physical method for recovery of LiCoO 2 and graphite from spent lithium-ion batteries: Grinding flotation. Sep. Purif. Technol. 190, 45–52 (2018)
J. Xu, H. Thomas, R.W. Francis, K.R. Lum, J. Wang, B. Liang, A review of processes and technologies for the recycling of lithium-ion secondary batteries. J. Power Sources 177(2), 512–527 (2008)
L. Li, E. Fan, Y. Guan, X. Zhang, Q. Xue, L. Wei, F. Wu, R. Chen, Sustainable Recovery of Cathode Materials from Spent Lithium-Ion Batteries Using Lactic Acid Leaching System. ACS Sustain. Chem. Eng. 5(6), 5224–5233 (2017)
S. Amarakoon, J. Smith, B. Segal, EPA (2013). https://archive.epa.gov/epa/sites/production/files/2014-01/documents/lithium_batteries_lca.pdf. Accessed 10 December 2020 \
X. Zheng, W. Gao, X. Zhang, M. He, X. Lin, H. Cao, Y. Zhang, Z. Sun, Spent lithium-ion battery recycling – Reductive ammonia leaching of metals from cathode scrap by sodium sulphite. Waste Manag. 60, 680–688 (2017)
S. Virolainen, M.F. Fini, A. Laitinen, T. Sainio, Solvent extraction fractionation of Li-ion battery leachate containing Li, Ni, and Co. Sep. Purif. Technol. 179, 274–282 (2017)
Y. Yang, S. Xu, Y. He, Lithium recycling and cathode material regeneration from acid leach liquor of spent lithium-ion battery via facile co-extraction and co-precipitation processes. Waste Manag. 64, 219–227 (2017)
X. Chen, B. Fan, L. Xu, T. Zhou, J. Kong, J. Clean. Prod. 112, 3562–3570 (2016)
E.G. Pinna, M.C. Ruiz, M.W. Ojeda, M.H. Rodriguez, Cathodes of spent Li-ion batteries: Dissolution with phosphoric acid and recovery of lithium and cobalt from leach liquors. Hydrometallurgy 167, 66–71 (2017)
M. Joulié, R. Laucournet, E. Billy, Hydrometallurgical process for the recovery of high value metals from spent lithium nickel cobalt aluminum oxide based lithium-ion batteries. J. Power Sources 247, 551–555 (2014)
Y. Xin, X. Guo, S. Chen, J. Wang, F. Wu, B. Xin, J. Clean. Prod. 116, 249–258 (2016)
P. Zhang, T. Yokoyama, O. Itabashi, T.M. Suzuki, K. Inoue, Hydrometallurgical process for recovery of metal values from spent lithium-ion secondary batteries. Hydrometallurgy 47(2-3), 259–271 (1998)
L.-P. He, S.-Y. Sun, Y.-Y. Mu, X.-F. Song, J.-G. Yu, Recovery of Lithium, Nickel, Cobalt, and Manganese from Spent Lithium-Ion Batteries Usingl-Tartaric Acid as a Leachant. ACS Sustain. Chem. Eng. 5(1), 714–721 (2017)
W. Lv, Z. Wang, H. Cao, X. Zheng, W. Jin, Y. Zhang, Z. Sun, A sustainable process for metal recycling from spent lithium-ion batteries using ammonium chloride. Waste Manag. 79, 545–553 (2018)
X. Zhang, L. Li, E. Fan, Q. Xue, Y. Bian, F. Wu, R. Chen, Toward sustainable and systematic recycling of spent rechargeable batteries. Chem. Soc. Rev. 47(19), 7239–7302 (2018)
P. Liu, L. Xiao, Y. Chen, Y. Tang, J. Wu, H. Chen, Recovering valuable metals from LiNixCoyMn1-x-yO2 cathode materials of spent lithium ion batteries via a combination of reduction roasting and stepwise leaching. J. Alloys Compd. 783, 743–752 (2019)
R. Sattar, S. Ilyas, H.N. Bhatti, A. Ghaffar, Resource recovery of critically-rare metals by hydrometallurgical recycling of spent lithium ion batteries. Sep. Purif. Technol. 209, 725–733 (2019)
L. Sun, K. Qiu, J. Hazard, Mater. 194, 378–384 (2011)
S.G. Zhu, W.-Z. He, G.-M. Li, Z. Xu, X.-J. Zhang, J.-W. Huang, Recovery of Co and Li from spent lithium-ion batteries by combination method of acid leaching and chemical precipitation. Trans. Nonferrous Metals Soc. China 22(9), 2274–2281 (2012)
B. Swain, J. Jeong, J.-C. Lee, G.-H. Lee, J.-S. Sohn, Hydrometallurgical process for recovery of cobalt from waste cathodic active material generated during manufacturing of lithium ion batteries. J. Power Sources 167(2), 536–544 (2007)
J. Kang, G. Senanayake, J. Sohn, S.M. Shin, Recovery of cobalt sulfate from spent lithium ion batteries by reductive leaching and solvent extraction with Cyanex 272. Hydrometallurgy 100(3-4), 168–171 (2010)
S.M. Shin, N.H. Kim, J.S. Sohn, D.H. Yang, Y.H. Kim, Development of a metal recovery process from Li-ion battery wastes. Hydrometallurgy 79(3-4), 172–181 (2005)
D.P. Mantuano, G. Dorella, R.C.A. Elias, M.B. Mansur, Analysis of a hydrometallurgical route to recover base metals from spent rechargeable batteries by liquid–liquid extraction with Cyanex 272. J. Power Sources 159(2), 1510–1518 (2006)
G. Dorella, M.B. Mansur, A study of the separation of cobalt from spent Li-ion battery residues. J. Power Sources 170(1), 210–215 (2007)
J. Li, X. Li, Q. Hu, Z. Wang, J. Zheng, L. Wu, L. Zhang, Study of extraction and purification of Ni, Co and Mn from spent battery material. Hydrometallurgy 99(1-2), 7–12 (2009)
L. Li, Y. Bian, X. Zhang, Y. Guan, E. Fan, F. Wu, R. Chen, Process for recycling mixed-cathode materials from spent lithium-ion batteries and kinetics of leaching. Waste Manag. 71, 362–371 (2018)
B. Musariri, G. Akdogan, C. Dorfling, S. Bradshaw, Evaluating organic acids as alternative leaching reagents for metal recovery from lithium ion batteries. Min. Eng. 137, 108–117 (2019)
X. Chen, B. Xu, T. Zhou, D. Liu, H. Hu, S. Fan, Separation and recovery of metal values from leaching liquor of mixed-type of spent lithium-ion batteries. Sep. Purif. Technol. 144, 197–205 (2015)
W. Gao, J. Song, H. Cao, X. Lin, X. Zhang, X. Zheng, Y. Zhang, Z. Sun, Selective recovery of valuable metals from spent lithium-ion batteries – Process development and kinetics evaluation. J. Clean. Prod. 178, 833–845 (2018)
C.K. Lee, K.I. Rhee, Preparation of LiCoO2 from spent lithium-ion batteries. J. Power Sources 109(1), 17–21 (2002)
L. Li, R. Chen, F. Sun, F. Wu, J. Liu, Preparation of LiCoO2 films from spent lithium-ion batteries by a combined recycling process. Hydrometallurgy 108(3-4), 220–225 (2011)
L. Zhuang, C. Sun, T. Zhou, H. Li, A. Dai, Recovery of valuable metals from LiNi0.5Co0.2Mn0.3O2 cathode materials of spent Li-ion batteries using mild mixed acid as leachant. Waste Manag. 85, 175–185 (2019)
J. Guan, Y. Li, Y. Guo, R. Su, G. Gao, H. Song, H. Yuan, B. Liang, Z. Guo, Mechanochemical Process Enhanced Cobalt and Lithium Recycling from Wasted Lithium-Ion Batteries. ACS Sustain. Chem. Eng. 5(1), 1026–1032 (2017)
C. Peng, F. Liu, Z. Wang, B.P. Wilson, M. Lundström, Selective extraction of lithium (Li) and preparation of battery grade lithium carbonate (Li2CO3) from spent Li-ion batteries in nitrate system. J. Power Sources 415, 179–188 (2019)
L. Brückner, J. Frank, T. Elwert, Industrial Recycling of Lithium-Ion Batteries—A Critical Review of Metallurgical Process Routes. Metals 10, 1107 (2020)
J. Myoung, Y. Jung, J. Lee, Y. Tak, J. Power Sources 112(2), 639–642 (2002)
Y. Yang, S. Lei, S. Song, W. Sun, L. Wang, Stepwise recycling of valuable metals from Ni-rich cathode material of spent lithium-ion batteries. Waste Manag. 102, 131–138 (2020)
M.K. Tran, M.T.F. Rodrigues, K. Kato, G. Babu, P.M. Ajayan, Deep eutectic solvents for cathode recycling of Li-ion batteries. Nat. Energy 4, 339–345 (2019)
S. Wang, Z. Zhang, Z. Lu, Z. Xu, Green Chem. 22, 4473–4482 (2020)
M.J. Roldán-Ruiz, M.L. Ferrer, M.C. Gutiérrez, F. del Monte, Highly Efficient p-Toluenesulfonic Acid-Based Deep-Eutectic Solvents for Cathode Recycling of Li-Ion Batteries. ACS Sustain. Chem. Eng. 8, 5437–5445 (2020)
A. Alhadid, L. Mokrushina, M. Minceva, Design of Deep Eutectic Systems: A Simple Approach for Preselecting Eutectic Mixture Constituents. Molecules 25, 1077 (2020)
E.L. Smith, A.P. Abbott, K.S. Ryder, Deep Eutectic Solvents (DESs) and Their Applications. Chem. Rev. 114, 11060–11082 (2014)
D. Mishra, D.J. Kim, D. Ralph, J.-G. Ahn, Y.H. Rhee, Bioleaching of metals from spent lithium ion secondary batteries using Acidithiobacillus ferrooxidans. Waste Manag. 28(2), 333–338 (2008)
N. Bahaloo-Horeh, S.M. Mousavi, Enhanced recovery of valuable metals from spent lithium-ion batteries through optimization of organic acids produced by Aspergillus niger. Waste Manag. 60, 666–679 (2017)
H. Pinegar, Y.R. Smith, Recycling of End-of-Life Lithium Ion Batteries, Part I: Commercial Processes. J. Sustainable Metallurgy 5, 402–416 (2019)
R.C. Wang, Y.C. Lin, S.H. Wu, A novel recovery process of metal values from the cathode active materials of the lithium-ion secondary batteries. Hydrometallurgy 99(3-4), 194–201 (2009)
S. Dhiman, B. Gupta, J. Clean. Prod. 225, 820–832 (2019)
W. Schulz, L. Bray, Solvent Extraction Recovery of Byproduct137Cs and90Sr from HNO3Solutions—A Technology Review and Assessment. Sep. Sci. Technol. 22(2-3), 191–214 (1987)
Acknowledgments
Dr. Vivian Fang and Dr. Peter Murmu are acknowledged for helpful discussions and assistance with figures.
Funding
This work was funded by the MacDiarmid Institute for Advanced Materials and Nanotechnology.
Author information
Authors and Affiliations
Contributions
The manuscript was written through the contributions of all authors.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Kader, Z.A., Marshall, A. & Kennedy, J. A review on sustainable recycling technologies for lithium-ion batteries. emergent mater. 4, 725–735 (2021). https://doi.org/10.1007/s42247-021-00201-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42247-021-00201-w