Abstract
Ion exchange is widely used for the removal of selected ions from water streams. Within oil & gas, one application is boiler feedwater treatment to remove hardness, i.e. calcium and magnesium. Weak acid cation (WAC) resins are typically used and advances in material science, polymer chemistry and manufacturing methods have resulted in new resins being introduced to the market. These new resins can lower operating costs through higher capacity, reduced chemical consumption during regeneration or improved physical properties.
In this research, the performance of a WAC resin used for boiler feedwater treatment in oil & gas operations (resin A) was compared with two new commercial resins (resins B & C).
The results indicated that resin B had the highest operational capacity in comparison to A & C. During regeneration, resin B was the most efficient with 0.43 meq of calcium and magnesium removed from the feed per meq of HCl consumed during regeneration, slightly higher than resins A & C at 0.38 and 0.30 meq/meq respectively. All three resins demonstrated preferential affinity for calcium over magnesium. As breakthrough approached, previously adsorbed magnesium ions were released back to the water resulting in a spike in effluent magnesium that was ≈3× higher than in the feed stream. In full-scale systems, breakthrough can be determined by measuring only the effluent magnesium concentration which can be more sensitive parameter than total hardness and/or calcium.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Ion exchange (IX) technology is fundamentally a reversible chemical reaction where dissolved ions are preferentially adsorbed by a resin and, because electroneutrality must be maintained, the resin releases other ions back to the solution [1]. The use of weak acid cation (WAC) resins for hardness removal, specifically calcium and magnesium, is a classic application of IX technology.
Throughout upstream and downstream oil & gas operations, IX is used in boiler feedwater treatment [2, 3], where effective hardness removal is integral to maximizing energy efficiency and minimizing boiler maintenance and downtime. Steam is used in a variety of ways including:
-
oil extraction (steam-assisted gravity drainage in oil sands)
-
processing (e.g. steam strippers for H2S removal)
-
refining (e.g. hydrocracking for gasoline production)
IX can also be used in the treatment of flowback and produced water from unconventional resources, i.e. shale plays & oil sands [4, 5].
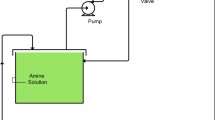
This research focuses on investigation of novel IX resins for oil sands application. Oil sands are naturally occurring mixtures of sand, clay, water and bitumen, an extremely viscous oil that is typically extracted via in situ production or open pit mining. The steam assisted gravity drainage (SAGD) process is used in oil sands which requires drilling of two horizontal wells, one on top of each other, through the oil sands deposit. Steam is injected continuously to the top well and, as the temperature rises, the bitumen becomes more fluid and flows to the lower well. The hot water and bitumen are then pumped to the surface. For steam generation, the SAGD facilities recycle 90% of hot produced water. The additional 10% comes from brackish groundwater which is used as makeup. The brackish groundwater treatment train, prior to steam generations, comprises of various units such as cold lime softeners, after filters and WAC resins as illustrated in Fig. 1. This study focused on evaluation new IX WAC resins for brackish groundwater treatment.
In Qatar, steam is used in liquified natural gas (LNG), gas-to-liquids (GTL) and refinery operations. In addition to boiler feedwater treatment, IX is used as pretreatment for reverse osmosis to reduce inorganic fouling when treating coal seam gas wastewater [6]. Outside of oil & gas, IX is used in various water treatment applications including dissolved organic carbon removal [7], water softening [8, 9], trace metal and nitrate removal [10,11,12] and wastewater treatment to meet effluent discharge [13, 14].
IX resins can be categorized depending on their functional groups as strong acid cation (SAC), strong base anion (SBA), weak acid cation (WAC) and weak base anion (WBA) [1]. SBA and SAC are generally used in low salinity streams for either complete removal of ions or for the removal of trace anions and cations. WBA & WAC resins are mainly applied for high TDS water where performance is less impacted by elevated salinity [1, 15].
The rate of adsorption on IX resins is controlled by diffusion and could be governed primarily by either the static boundary layer around the resin beads, referred to as film diffusion, or by the pores on the resin crosslinked matrix, referred to as particle diffusion [16, 17]. In some cases, both types of diffusion control the rate of adsorption. Since the concentration of the ions in the feed solution is low (<3 meq/L), the rate of adsorption is governed by film diffusion and hence primarily dependent on particle diameter and degree of crosslinking of the resin since those parameter directly influence the boundary layer around the beads [16, 17].
Advances in material science, polymer chemistry and manufacturing methods have resulted in new resins being introduced to the market [18,19,20]. This research compared the performance of two new commercially available WAC resins with the current WAC resin used in boiler feedwater treatment in SAGD process. The manufacturers of the new resins claimed that these resins possess higher sorption capacities and/or required less chemicals for regeneration.
This evaluation focused on the determination of the:
-
Capacity at breakthrough (operational capacity)
-
Capacity at saturation
-
Chemical consumption during regeneration
-
Key resin properties (density, particle size distribution, moisture content).
This work is an independent study of two new generation resins conducted by an end user to qualify and validate the technology for field implementation.
2 Materials and methods
2.1 Bench scale unit
The test unit consisted of an 80 L feed tank and two parallel systems, each with a positive displacement pump, pulsation dampener, pressure transmitter, IX column and fraction collector (Fig. 2). The feed was pumped at constant flow through the IX column and the pressure was constantly monitored to prevent overpressure. During the service cycle, 10 ml fractions of the effluent were automatically collected every hour and analyzed to generate the capacity curves. During the regeneration cycle, 3 ml fractions of the acid and caustic effluent were collected every 5 min and analyzed to determine chemical consumption.
For each resin, the column height was 25 cm which equated to a bed volume (BV) of 22 cm3 (column inside diameter 1.06 cm). The decision to fix the volume of resin for testing rather than the mass of resin was based on the fact that the new resin was being considered as a replacement for the resin already installed in an existing vessel and volume rather than mass would be the governing criterion when rebedding the filter. Since the resins had different densities, the mass of the resin in each column varied.
2.2 Feedwater
A synthetic feed solution was used since >60 l were needed to run parallel breakthrough tests simulating full-scale plant conditions. The synthetic solution mimicked the effluent from a cold lime softener removing calcium and magnesium from brackish groundwater. Calcium and magnesium were the primary inorganic ions responsible for boiler fouling. The composition of the synthetic solution is shown in Table 1. The total hardness of the feed was 57 mg/L as CaCO3 or 1.15 meq/l. Breakthrough was defined as hardness leakage of 1% (effluent hardness =0.012 meq/mL) and saturation was considered when the effluent hardness was 95% of the influent hardness (1.14 meq/L).
2.3 Resin properties
Three macroporous WAC resins from different vendors, designated “A”, “B” & “C” were evaluated. All resins had a crosslinked polyacrylate matrix with carboxylic functional groups; however, there are slight differences between them. Resin A was used as the reference (control) in this study because it is used for calcium and magnesium removal for boiler feedwater treatment in existing SAGD facilities by various operators. Resins B and C were new generation WAC resins which vendors claimed had improved performance. Resin B was claimed to have 30% more capacity than resin A, most likely attributed to a higher degree of crosslinking, thereby increasing run times without significantly affecting the chemical consumption. Resin C was claimed to have a similar capacity to resin A but a lower chemical consumption due to an inert core which limits the ion diffusion within the resin structure requiring less chemical for regeneration.
For the density and particle size analyses, the resins were converted to the calcium form using 1 M CaCl2 for 2 h and rinsing with DI water. For the analysis in the hydrogen form, the resins were in contact with 1 M HCl for 2 h and similarly rinsed. The true and apparent densities as well as the water retention capacity were measured for all the resins in the sodium, calcium and hydrogen forms. For the all measurements, the resins were fully hydrated and excess water removed using a vacuum filtration system.
The true density was determined by adding the resin into a graduated cylinder half-filled with water and measuring the volume of water displaced (ml) and the mass of resin added (g). The apparent resin density was determined by recording the volume of the top of the resin bed in the cylinder and the mass of resin added (g). The void fraction was calculated based on the ratio of the apparent and true densities.
For the water retention capacity, a known mass of the fully hydrated resin was placed in a drying oven at 105 °C for 18 h. The difference in weight before and after drying was used to determine the water retention capacity.
Before conducting the capacity experiments, the resins were converted into the sodium form by putting each resin in contact with 1 M NaOH for 3 h followed by rinsing in deionized (DI) water.
3 Test conditions
The static capacity test was conducted by placing a known amount of resin in contact with the feedwater for 72 h. The initial and final calcium and magnesium concentrations were measured to determine the amounts adsorbed.
The columns were packed with 22 cm3 of resin to a height of 25 cm and glass wool was placed at each end of the column to prevent resin loss. The resins were added as a wet slurry and compacted several times to minimize void spaces between particles. Compaction was achieved by applying slight vibration on the column while packing it. To allow for resin expansion, 5 cm freeboard (20% of bed height) was provided. Since the resins were packed in the sodium form, resin expansion was not expected. Table 2 shows the properties of the columns and mass of resin used in each. The column packed with Resin B has the highest void fraction (33.6%) due to the uniformity of the beads (Table 4) and for that reason the mass of resin in the sodium form was lower compared to the other two columns.
The flow was downward at a rate of 7.5 mL/min (20 BV/h) and effluent samples (10 ml each) were collected hourly using an automatic fraction collector. Each column was operated for 7 to 10 days to generate the breakthrough curve. The samples were analyzed for calcium and magnesium by ion chromatography to generate the breakthrough curve.
Breakthrough was defined as a total hardness leakage rate of 1% (effluent contains 1% of influent hardness) and column saturation was defined as hardness passage of 95%. Results were compared with published IX models for data interpretation.
The resins were regenerated using 5% hydrochloric acid (HCl) and 5% sodium hydroxide (NaOH) at a flowrate of 1.1 mL/min (3 BV/h) in the same direction as the feed. The HCl effluent was collected automatically (3 ml fractions every 5 min) and calcium & magnesium were measured to assess regeneration efficiency. The NaOH effluent was similarly sampled and pH was monitored to ensure complete removal of the H+ ions.
3.1 Laboratory analyses
The feed and effluent solutions and particle size distribution were analyzed according to the methods in Table 3.
3.2 Static capacity test: Freundlich adsorption isotherm
The static capacity of the resins was modeled based on the Freundlich isotherm, an empirical equation widely used to predict the adsorption capacity of different materials as a function of the expected effluent concentration [23]. The Freundlich isotherm is shown in Eq. 1 [24].
It can be linearized as shown in Eq. 2
where qe is the resin capacity (mg/g), Ce is the equilibrium concentration in solution (mg/L), KF and \( \raisebox{1ex}{$1$}\!\left/ \!\raisebox{-1ex}{$\mathrm{n}$}\right. \) are Freundlich constants which can be calculated from the intercept and slope on the lnqe vs lnCe plot.
Based on the values of \( \raisebox{1ex}{$1$}\!\left/ \!\raisebox{-1ex}{$\mathrm{n}$}\right. \) the adsorption isotherm could be categorized as favorable (\( \raisebox{1ex}{$1$}\!\left/ \!\raisebox{-1ex}{$\mathrm{n}$}\right. \) <1), linear (\( \raisebox{1ex}{$1$}\!\left/ \!\raisebox{-1ex}{$\mathrm{n}$}\right. \) = 1) or unfavorable (\( \raisebox{1ex}{$1$}\!\left/ \!\raisebox{-1ex}{$\mathrm{n}$}\right. \) >1), depending on the adsorption efficiency of the adsorbent, especially at low equilibrium concentrations [13].
3.3 Breakthrough curve: Thomas model
The column breakthrough performance can be predicted using the model developed by Henry C. Thomas [25, 26] for single solute chromatography. It is one of the most widely applied models to evaluate column performance [15, 27, 28] and can be used to predict adsorption kinetics as well as the adsorption capacity in flow-through systems [27].
The equation above can be linearized as shown in Eq. 4
where C0 is the feed concentration (mg/L), Ce is the column effluent concentration (mg/L), V is the column effluent volume (L), Q is the feed flowrate (L/min), M is the mass of resin in the bed (g), Kth is the kinetic rate constant (L/(mg-min) and qm is the adsorption capacity of the column (mg/g).
The breakthrough data can be fitted by plotting \( \ln \left(\frac{C_0}{C_e}-1\right) \) vs V; Kth and qm can be calculated based on the slope and intercept of the graph.
4 Results & discussion
4.1 Resin properties
The particle size distribution results and key resin properties are summarized in Table 4. The particle size distribution showed Gaussian distribution for all the resins. In the sodium form, resin A had larger particles with a median diameter of 869 μm, approximately 35% greater than the median diameter of 646 μm for both resins B & C. In the hydrogen and calcium forms, although median diameters were smaller (≈780 μm), resin A was still ≈40% larger than resins B & C (532 to 574 μm). Larger particles minimize the pressure drop across the column, but are usually associated with lower capacity and slower kinetics compared to smaller particles [16].
An important output from the particle size distribution analyses is the uniformity coefficient (d60/d10), which measures the width of the distribution [29]. Packing a column with a resin that has a wide distribution will cause the small beads to fill the interstitial spaces between the larger beads reducing the void fraction and increasing the pressure drop [30]. The void fraction will be higher for resins with a narrow distribution. From the data shown in Table 4, resin B had the narrowest distribution, while resins A and C showed similar uniformity coefficients. The impact of a uniformity in particle sizes on void fraction is clearly evident in comparing the void fractions for compacted and non-compacted resins (Table 2 vs. Table 4). For resins A & C, each with a wide particle size distribution, compaction reduces the void fraction in Na + from ≈35% down to ≈15%. For resin B, compaction has only marginal impact, i.e. reduces void fraction from ≈39% to ≈34%.
Figure 3 shows microscope images of the hydrated resins, highlighting the uniformity in size of the resin B beads.
The resins showed slightly different true densities in the sodium form which could be related to their adsorption capacities. Compared to the other resins, Resin B has the highest true density which is attributed to more active sites with sodium ions translating to higher adsorption capacity.
All the resins showed smaller particle diameters in the hydrogen and calcium forms compared to sodium, and that is attributed to resin swelling. Swelling occurs when the resin traps a cation with a larger hydrated equivalent ionic volume, expanding its crosslinked structure [31, 32]. The hydrated ionic volume is defined as the volume occupied by an ion when it is surrounded by water and it is proportional to its hydrated radius. When the hydrated volume is divided by the ion charge, it is referred to as hydrated equivalent volume. The hydrated equivalent radii for sodium and calcium is 3.6 and 2.1 Å respectively [33, 34]. Due to that, the resins had larger particle diameter in the sodium form.
Additionally, since sodium forms had higher hydrated equivalent volume, more water was trapped inside the resin compared to the other forms, translating to a higher moisture content. Resins A and B showed a slight change in density when the resins were in the sodium form possibly attributed to the higher moisture content. A change in density was not observed in resin C possibly due to its modified physical structure which limited the amount of water trapped in the resin.
The evaluation was conducted with the resins in the sodium form for two reasons: i) to prevent the release of hydrogen ions which could change the pH of the water and, ii) hydrogen form would not have worked since the bicarbonate was less than the hardness (Table 1). Since the larger particle diameter was observed in the sodium form, that would help in reducing the pressure drop and ensure the resin does not expand beyond the packed volume.
4.2 Static capacity test
Calcium removal results for the static capacity test with synthetic feed showed that resin B had the highest static capacity, especially at low equilibrium concentrations (Ce < 2) (Fig. 4). Resins A and C had comparable static capacities over the range tested; however, the model profile for resin A was linear compared to resin C, translating to higher capacities at lower equilibrium concentrations (Ce < 2). The degree of crosslinking on the resins will have a direct effect on the static capacity since more surface area will be available for diffusion [16]. The results showed resin B possesses higher capacity which is attributed to highest degree of crosslinking in the polymer matrix. Also, due to the smaller particles, resin B has a higher surface area which could have contributed to the higher static capacity. Resin C is similar to resin A, but it contains an inert core which may decrease the active sites and facilitates adsorption only in the outer layer of the resin. Although resin B has higher void fraction (33%) due to uniformity coefficient and comparable sodium form (Table 2), the removal mechanism is mainly attributed to the adsorption at the polymer matrix. The true density of resin B in sodium form is higher than A & C and possess higher active sites to attract hardness ions.
The data was fitted using the Freundlich isotherm since it covered the different types of curves observed during the experiments. The shape of the fitting curve revealed:
-
resin A: linear adsorption; \( \raisebox{1ex}{$1$}\!\left/ \!\raisebox{-1ex}{$\mathrm{n}$}\right. \) ≈ 1
-
resin B: favorable adsorption; \( \raisebox{1ex}{$1$}\!\left/ \!\raisebox{-1ex}{$\mathrm{n}$}\right. \) <1
-
resin C: unfavorable adsorption; \( \raisebox{1ex}{$1$}\!\left/ \!\raisebox{-1ex}{$\mathrm{n}$}\right. \) >1
The fitting parameters are shown in Table 5.
The capacity test also indicated that the resins had preferential adsorption for calcium compared to magnesium due to selectivity [1]. During the test, the concentration of calcium decreased by more than 40% at the highest resin mass, while the concentration of magnesium decreased only marginally, i.e. <8%. This preferential adsorption could be attributed to the hydrated equivalent volume of the adsorbed ion; IX resins typically have preferential adsorption for the smaller hydrated ion [32]. Although the unhydrated magnesium ion is smaller than the calcium ion, in hydrated form, it is reversed and the calcium is the smaller ion [32].
Because resins are typically operated with the feed passing through a column in plug flow, the results of the batch test for static capacity have little practical value. Since the test objective was to compare the performance of new resins under operating conditions, the remainder of the study focused on column breakthrough experiments to assess the impact of the hydrodynamic conditions and adsorption kinetics on resin performance. Due to the variety of isotherms obtained, kinetics could play an important role in performance.
4.3 Column tests
4.3.1 Operational capacity
Operational capacity is the key performance parameter for resin evaluation. It is a measure of amount of hardness that can be removed before the leakage of hardness in the effluent is considered excessive and resin regeneration is required. From a practical perspective, it reflects the “run time”, or the number of bed volumes of water that can be processed between regeneration cycles. Operational capacity can be reported on a mass basis or volumetric basis and total hardness can be expressed either as mg of CaCO3 or milli-equivalents (meq). This threshold leakage value is dependent upon site conditions. For the purposes of this investigation, 1% hardness leakage was set as the breakthrough criterion, i.e. when the effluent hardness reached 0.012 meq/L. In this investigation, operational capacity is reported based on meq of calcium and magnesium removed per mL of compacted resin volume.
In the column tests, resin B had the longest run time of 1677 BV and this equated to an operational capacity of 2.0 meq/mL, 17% greater than resin A (1432 BV, 1.7 meq/mL) and 42% greater than resin C (1186 BV, 1.4 meq/mL). Combined calcium and magnesium concentrations (as meq/L) as a function of bed volumes processed for each resin is presented in Fig. 5. Adsorptions for individual ions and the operational & saturation capacities are presented in Table 6. Of particular note is the fact that the magnesium removed at saturation was less than that at breakthrough. This magnesium desorption phenomenon is discussed in further detail in subsequent paragraphs.
The operational capacity of each resin was also determined on a mass basis as resin is typically sold on a per kg basis. But rather than using the true density of the resin, the density of the compacted resin when installed in a column was used (Na + form, Table 2 previously). Results showed that resin B also had a higher capacity by mass compared to resins A & C. At breakthrough, resin B had a capacity of 2.3 meq/g, 48% greater than resin A (1.6 meq/g) and 72% greater than resin C (1.4 meq/g). The differences in capacities between the mass and volume methods were attributed to differences in packing densities / void fractions of the columns. The column with resins A and C had lower void fractions (higher packing density) compared to resin B, as shown in Table 2. The highest void fraction was in the column packed with resin B is attributed to its uniform particle size distribution.
To ensure consistency in the water quality, the main anions and cations were measured in the feed and effluent streams; results are shown in Table 7:
4.3.2 Saturation capacity
All resins have a saturation capacity where no more hardness will be adsorbed. For the purpose of this study, resin saturation was deemed to have occurred when the effluent hardness reached 95% of the influent hardness. In comparing resins, saturation capacity serves to indicate the total number of exchange sites available on the resin. It has little practical significance because the bed would be regenerated at breakthrough and never reach saturation conditions.
Figures 6 & 7 show the calcium and magnesium concentrations respectively in the effluent for the three resins as a function of the number of bed volumes processed up to saturation.
Magnesium desorption
The results showed that all three resins had a preferential affinity for calcium over magnesium. As the exchange sites on the resin started filling up, the magnesium ions that had previously been removed were released back into the water and the exchange sites replaced with calcium ions. This resulted in the elevated magnesium levels and a spike in effluent magnesium concentration ≈3× higher than the feed stream. Even though the magnesium spike was observed well after breakthrough, some release of the magnesium was observed prior to breakthrough. This preferential affinity for calcium was consistent with the static capacity test results.
Equations 5 and 6 showed the interaction of the WAC resin (sodium form) with calcium and magnesium ions:
Since the resins showed preferential adsorption for calcium compared to magnesium, magnesium retained in the resin gets displaced by calcium ions, as shown in Eq. 7, explaining the increase of magnesium in the effluent.
To validate the above results, the breakthrough curve was modeled using the Thomas model (Fig. 6). The Thomas model can be applied to compare the adsorption capacity (qm) and process kinetics (Kth) [26] for single solute adsorption applications. In these experiments though, the feed comprised two solutes (calcium and magnesium). As a result, there is a slight discrepancy in the data fitting, especially at higher effluent calcium concentrations.
Based on the qm fitting parameter (Table 5), resin B had the highest capacity followed by resin A and then C, consistent with the column run time and static capacity tests. The data also showed that resin C has the fastest kinetics, based on the kinetic constant, Kth (Table 8), followed by resin A and then B. This is consistent with the length of the mass transfer zone which is related to the process kinetics, the shorter the mass transfer zone (MTZ), the faster the adsorption kinetics. Figure 8 shows the amount of calcium and magnesium adsorbed in the column at breakthrough and saturation based on the feed volume treated.
Mass transfer zone
One important parameter for the evaluation of ion exchange resins is the mass transfer zone (MTZ), defined as the portion of the column where adsorption is taking place [15, 35, 36]. The length of the MTZ depends on the resin properties, kinetics and affinity between the ions being removed and the resin. It is a calculation related to the difference in capacity between breakthrough and saturation and is reflected in the slope of the line on the effluent calcium concentration plot (Fig. 6); the steeper the slope, the shorter the MTZ. Results showed that resin B had the longest MTZ (11.6 cm) followed by resin A (10.9 cm) and then resin C (9.4 cm). Longer MTZ means more unused resin at breakthrough but also translates to slower breakthrough allowing more time for detection. Resin C had the shortest MTZ translating to a better utilization of the resin but also the fastest breakthrough.
4.3.3 Regeneration
Resins can be regenerated many times following standard protocols provided by the manufacturer. In this study, 5% HCl was used to desorb the ions in the columns. Figure 8 shows the meq of calcium and magnesium adsorbed onto the resin after saturation and the meq of 5% HCl needed to desorb them. Since the amount of acid required is proportional to the amount of calcium and magnesium in the column, the normalized acid consumption was calculated by dividing the meq of acid needed by the total meq of calcium and magnesium retained by the resin. As seen in Fig. 8, resin B required 2.3 meq of HCl per meq of adsorbed which is the lowest ratio compared to resins A and C which required 2.6 and 3.0 meq HCl per meq adsorbate respectively.
Based on the results, the resin with the fastest adsorption kinetics (resin C) required the highest amount of HCl per meq of calcium and magnesium while the resin with the slowest adsorption kinetics (resin B) required the lowest. That is attributed to the interaction between the adsorbate and the resin. The higher the affinity, the faster the adsorption kinetics but more acid is needed for regeneration due to the strong bond between the adsorbate and adsorbent [37].
Once the resin was regenerated with HCl, it was converted into sodium form using 5% NaOH. This conversion would prevent the release of hydrogen ions when the feedwater goes through the column and would reduce the pressure drop due to the higher bead size [35, 37]. The pH of the NaOH effluent was monitored and results showed similar NaOH consumption for the three resins, approximately 5 BV were needed for complete conversion. The present investigation was conducted as proof of concept and to compare new resins with the existing one, the regeneration efficiency would be addressed in detail during pilot study evaluation.
4.4 Application
4.4.1 Resin selection
Based on the results shown above, replacing resin A with resin B would increase the run time between regenerations by ≈17% from 1430 to 1680 BV. This would translate into less down time and likely reduce rinse water requirements. Although the total hardness removed is 17% higher with resin B, the total chemical consumption per regeneration is estimated to increase by only 7% because the normalized chemical consumption for resin B is about 10% lower than for resin A. From a capital cost perspective, the vendors claim that rebedding with their products would be competitive, i.e. no premium over current resins.
Additionally, for new designs, using resin B instead of resin A could translate to smaller vessels minimizing the process footprint while maintaining similar performance.
4.4.2 Assessing when breakthrough is imminent
The challenge facing all operators is knowing when to remove an IX column from service and regenerate it. If one does it too early, one wastes chemical and water; if one waits too long, the hardness leakage can contribute to boiler tube fouling. In practice, one way to track bed condition is to monitor the effluent hardness closely as breakthrough approaches. One practical outcome of this research is that monitoring the effluent for magnesium (rather than total hardness) is a better indicator of imminent breakthrough. When the resin approaches breakthrough, the magnesium level in the treated effluent will increase more significantly than the increase in total hardness and that can alert an operator that breakthrough is imminent. At breakthrough, the experimental data indicate that magnesium accounts for 75% of the total hardness in the effluent.
5 Summary & Conclusions
In this investigation, the performance of a weak acid cation resin currently used for hardness removal from boiler feedwater (resin A) was compared with two new commercial resins (resins B & C). The performance was evaluated in parallel columns with a synthetic feed mimicking actual feed from an oilfield operation. The key performance indicator was “operational capacity at breakthrough” with breakthrough defined as “total milli-equivalents of effluent magnesium and calcium =1% of feed (0.012 meq/L)”. Run times ranged from 7 to 10 days and the total feedwater treated per column ranged from 2000 to 3500 bed volumes.
Resin B had the highest operational capacity and, in addition, also had the highest regeneration chemical efficiency of 0.43 (lowest normalized chemical consumption). Resin B can be expected, based on void fraction, to have the lowest pumping energy cost. If resin B were used to replace existing resin A in boiler feedwater treatment in oil & gas operations, longer run times and savings in operating cost are projected, assuming resin fouling (due to oil, iron, suspended solids) is comparable. The effects of fouling on performance were not evaluated during this study.
The results in this study are considered preliminary; performance testing in the field is required to assess the effects of resin fouling, e.g. by oil or iron, on long-term performance. Similarly, the effects of differences in resin properties on bed hydraulics & pressure drop should be further investigated.
The main conclusions were:
-
Column breakthrough tests indicated that resin B had the highest operational capacity (2.0 meq/mL), equal to 1677 bed volumes (BV) being treated before breakthrough. The operational capacities for resins A and C were 1.7 and 1.3 meq/L or 1432 and 1186 BV respectively.
-
Removing resin A from an IX vessel and rebedding with an equivalent volume of resin B would increase the run time between regeneration cycles by 17%, very significant from an operation perspective. For new designs, using resin B instead of resin A could translate to smaller vessels minimizing the process footprint while maintaining similar performance.
-
All three resins demonstrated preferential affinity for calcium over magnesium. As the resins approached breakthrough, magnesium ions that had previously been removed from the feed were released back to the water and calcium ions adsorbed onto the resin. This resulted in the elevated magnesium levels and a spike in effluent magnesium that was ≈3× higher than the feed stream.
-
In full-scale systems, effluent magnesium is considered a better indicator than total hardness that breakthrough is imminent.
-
Resin B has the slowest adsorption kinetics which translated into the highest unused portion of the IX column and the longest mass transfer zone. Resin C had the fastest adsorption kinetics and the shortest mass transfer zone.
-
During regeneration, resin B was the most efficient with 0.43 meq of calcium and magnesium removed from the feed per meq of HCl consumed during regeneration, slightly higher than resins A & C at 0.38 and 0.30 meq/meq respectively.
-
Resin B, with the most uniform particle size distribution and significantly higher void fraction, would be expected to have the lowest pressure drop and lowest pumping energy requirements of the three resins.
-
Static capacity test revealed that resin B had the highest adsorption capacity with a favorable isotherm based on Freundlich model. Resins A and C had lower capacities with linear and unfavorable isotherms respectively.
References
D.A. Clifford, Water Qual. Treat. 4, 561 (1999)
C.J. Brown, C.J. Brown, M. Sheedy, M. Sheedy, System 2 (2002)
R.J. Jan, T.G. Reed Jr., SPE Prod. Engineeering (1992)
Q. Jiang, J. Rentschler, R. Perrone, K. Liu, J. Membr. Sci. 431, 55 (2013)
S. Adham, A. Hussain, J. Minier-Matar, A. Janson, R. Sharma, Desalination 440, 2 (2018)
G.J. Millar, S.J. Couperthwaite, C.D. Moodliar, Renew. Sust. Energ. Rev. 57, 669 (2016)
Y. Hu, T.H. Boyer, Water Res. 115, 40 (2017)
J.N. Apell, T.H. Boyer, Water Res. 44, 2419 (2010)
M. Arias-Paic, K.M. Cawley, S. Byg, F.L. Rosario-Ortiz, Water Res. 88, 981 (2016)
J.A. Korak, R. Huggins, M. Arias-Paic, Water Res. 118, 141 (2017)
A.M. Bergquist, J.K. Choe, T.J. Strathmann, C.J. Werth, Water Res. 96, 177 (2016)
S. Ebrahimi, D.J. Roberts, Water Res. 88, 766 (2016)
K.A. Landry, P. Sun, C.H. Huang, T.H. Boyer, Water Res. 68, 510 (2015)
W. Beita-Sandí, T. Karanfil, Water Res. 124, 20 (2017)
M.D. LeVan, G. Carta, C.M. Yon, Energy 16, 17 (1997)
D. Reichenberg, J. Am. Chem. Soc. 75, 589 (1953)
G.E. Boyd, A.W. Adamson, L.S. Myers Jr., J. Am. Chem. Soc. 69, 2836 (1947)
I.M. Abrams, J.R. Millar, React. Funct. Polym. 35, 7 (1997)
Q. Li, L. Fu, Z. Wang, A. Li, C. Shuang, C. Gao, J. Clean. Prod. 165, 801 (2017)
Y. Sun, P. Zuo, J. Luo, R.P. Singh, J. Environ. Sci. 54, 40 (2017)
G.M. Hale, M.R. Querry, Appl. Opt. 12, 555 (1973)
S.N. Kasarova, N.G. Sultanova, C.D. Ivanov, I.D. Nikolov, Opt. Mater. (Amst) 29, 1481 (2007)
H. M. F. Freundlich and others, J. Phys. Chem 57, 1100 (1906)
G.J. Millar, G.L. Miller, S.J. Couperthwaite, S. Papworth, Sep. Purif. Technol. 163, 79 (2016)
H.C. Thomas, J. Am. Chem. Soc. 66, 1664 (1944)
H.C. Thomas, Ann. N. Y. Acad. Sci. 49, 161 (1948)
R.S. Juang, H.C. Kao, W. Chen, Sep. Purif. Technol. 49, 36 (2006)
M. Kalaruban, P. Loganathan, W.G. Shim, J. Kandasamy, G. Naidu, T.V. Nguyen, S. Vigneswaran, Sep. Purif. Technol. 158, 62 (2016)
S. Belaïd, G. Boiteux, P. Cassagnau, Rheol. Acta 52, 75 (2013)
DOW Liquid Separations, March (2006)
K.W. Pepper, D. Reichenberg, D.K. Hale, J. Chem. Soc. 3129 (1952)
H.P. Gregor, F. Gutoff, J. Bregman, J. Colloid Sci. 6, 245 (1951)
H. Ashjian, Q. N. Le, D. O. Marler, J. Shim, and S. S. Wong, (1991)
Y. Marcus, Chem. Rev. 88, 1475 (1988)
L.P. Mazur, T.A. Pozdniakova, D.A. Mayer, R.A.R. Boaventura, V.J.P. Vilar, Water Res. 90, 354 (2016)
J.R. Couper, W.R. Penney, J.R. Fair, S.M. Walas, J.R. Couper, W.R. Penney, J.R. Fair, S.M. Walas, Chem. Process Equip, 529–559 (2012)
V.J. Inglezakis, A. Zorpas, in Ion Exch. Technol. I Theory Mater, ed. by I. DR, M. Luqman. (Springer, Netherlands, 2012), pp. 121–161
Acknowledgements
The research team would like to acknowledge Samir Gharfeh, Aida Rafat and Nabin Upadhyay for their feedback and contributions to the project, as well as the resin manufacturers for providing the resins used on this study.
This evaluation is a contribution to science and does not constitute an endorsement of any particular vendor’s resins.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Janson, A., Minier-Matar, J., Al-Shamari, E. et al. Evaluation of new ion exchange resins for hardness removal from boiler feedwater. emergent mater. 1, 77–87 (2018). https://doi.org/10.1007/s42247-018-0006-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42247-018-0006-0