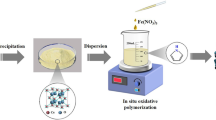
Abstract
Aim of this work is to synthesizing a new highly efficient adsorbent as of magnesium oxide (MgO2) entrapped Polypyrrole (Ppy) nanocomposite preparation for toxic pollutant of fluoride removal form drinking water. The synthesized MgO2/Ppy hybrid nanocomposite shows an extraordinary defluoridation capacity of 4328 mg F− Kg−1 in the room temperature through batch adsorption technique. It was performed to know the effect of various parameters such as contact time, initial concentration, pH, competitor ions and temperature. The structural and morphological changes on the fluoride adsorbent in before and after were analyzed by using XRD, FTIR & SEM techniques. The adsorption isotherm of Freundlich, Langmuir, & Dubnin - Radushkevich isotherms were studied and the fluoride adsorption is well fixed with Langmuir isotherm model. The kinetic model studies were carried out by both diffusion and reaction based models. The mechanism of fluoride on the MgO2/Ppy nanocomposite is mainly influence the interaction of electrostatic adsorption and ion-exchange. The reusability and regeneration studies were performed for the reusability of the nanocomposite.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Fluoride is an essential element for living systems related to the total amount consumed. Fluoride ions to cause significant properties in human through intake of water [1]. The existence of fluoride has favorable effects on the maintenance of teeth and bones at low concentrations, however various adverse problems would be increased such as, neurological damage, bones softening teeth mottling and so on within an excessive exposure [2]. The World Health Organization (WHO) has suggested the maximum permissible concentration of fluoride ion in drinking water between 0.5 &1.5 mg/L [3]. Henceforth, an excessive quantity of fluoride in drinking water requisite is removed using suitable technologies and materials.
Certain technologies have been established for fluoride removal from water such as ion exchange, precipitation, nanofiltration, reverse osmosis, Donnan dialysis electrodialysis, and adsorption [4,5,6,7,8]. Several disadvantages, i.e. production of unwanted chemicals, waste removal issues minimum economic feasibility, high maintenance cost, scaling, fouling, and membrane degradation are associated with some exciting techniques such as precipitation, electrodialysis, Donnan dialysis and membrane process. Therefore, adsorption and ion exchange method are expansively accepted technologies as these are reasonably effective in decreasing to the tolerable limt of fluoride concentration. Adsorption has extra advantage due to low operational maintenance cost, and simple procedure [9]. Abundant, waste, natural and synthetic materials have been tested as an adsorbent for fluoride removal from drinking water. The perfect fluoride removal adsorbent have some favorite characteristics such as low cost, quick uptake, easy regeneration, good adsorption capacity and greater physical characteristics such as inability to block the pores of the filter. Unfortunately, most inorganic adsorbent materials have all of these features. In spite of the fact that nanomaterial has high surface areas, yet they need different adsorbing functional groups. On the conflicting, organic polymers hold a large number of multifunctional groups but still their small specific area due to aggregation, limits their small specific area. The synthesis of hybrid/core–shell nanocomposites as absorbent with both multifunctional groups and the high surface area has recently gained considerable attention in wastewater treatment.
Polypyrrole (Ppy) is a well-known conducting polymer that has fascinated the researchers for various applications due to its facile synthesis, high electrical conductivity, excellent biocompatibility, redox and ion exchange properties [10, 11]. Ppy chains have positively charged nitrogen atoms that may easily become major active sites for the adsorption of anions and also has a anion exchanger behavior due to the presence of exchangeable counter anions doped in its chains [12]. Highly reactive of Magnesium nanoparticles derived adsorbents are well known for wastewater treatment. The majority of the magnesium derived adsorbents were prepared by high temperature hydrolysis [13, 14]. The power type of metal oxides has practical problem for water purification purpose and inconvenience in solid/liquid separation, because of low hydraulic conductivity and leaching of metal/metal oxide along with purified water. To overcome the above problem, more environmental friendly, cost effective and efficient materials need to be developed [15]. The MgO is comparatively low cost material than other metal oxides.
Based on the view, in this work was planned to synthesize magnesium oxide fabricated with Polypyrrole polymer matrix (MgO2/Ppy) for fluoride removal studies. The effects of parameter such as contact time, competitive ions dosage, initial concentration, on the fluoride removal efficiency of MgO2/Ppy were studied. Finally its real field applicability was also tested.
2 Experimental section
2.1 Materials and methods
Anhydrous Magnesium chloride (MgCl2), Sodium fluoride (NaF), Sodium hydroxide (NaOH), Ammonia (NH3) and all other chemicals were used as received form Sigma-Aldrich, India. Pyrrole was bought from CDH-Central Drug House, New Delhi. 1000 ppm of fluoride stock solution was prepared by dissolving 2.21 g of sodium fluoride in 1000 mL of double distilled water. All the chemicals are analytical grade and used as without any other purification.
2.2 Synthesis of MgO2 /Ppy nanocomposite adsorbent
2.2.1 Synthesis of MgO2 nanoparticles
The initial precursor of nano MgO2 was synthesized by 20.0 mL of MgCl2 solution was dropped very slowly by using injection in 1000 mL of double distilled water under safe condition (fume hood) with constant mechanical stirring with 1000 rpm. The pure white colored solution was appeared and it was treated with 1:4 ratio of dilute ammonia solution up to the pH of the liquid reached neutral (~6.8). The white colored precipitate obtained and it was completely washed with double distilled water. The obtained precipitated was dried in hot air oven at 80 °C for 24 h. Then the white precipitate was crushed with ball mill and get a uniform sized nano MgO2 powder.
2.2.2 Synthesis of MgO2/Ppy nano composite
The adsorbent nanocomposite was synthesized by chemical oxidative polymerization method using Iron (III) Chloride as an oxidant. Initially 0.5 g of MgO2 nanoparticles was dispersed in 50 mL of DD (double distilled) water and continuously stirred for 1 h. Then 0.5 mL of Pyrrole was dropped by using syringe into the solution and it again stirred for 50 min. 1.35 g of Iron(III) chloride was then added into the above mixture, the mixture was constantly stirred for 6 h and then left unstirred for polymerization to overnight without any disturbance. The resulted MgO2/Ppy nanocomposite was filtered and washed with double distilled water followed by ethanol and then dried in a hot air oven at 80 °C for 12 h. The obtained MgO2/Ppy nanocomposite used for as an adsorbent of fluoride removal.
2.3 Batch adsorption studies
The defluoridation studies were done by batch adsorption method to the effect of contact time, competitive anions, temperature, solution pH, and initial concentration. The adsorption methodology were done in thermostat shaker with 200 rpm at 25 ± 1 °C using 100 mL of 3 ppm F− solution in a conical flasks at neutral pH. The parameters were varied with respective of experiments. The effects of pH value, contact time, adsorption isotherms, coexisting ions and adsorbent dose on the equilibrium adsorption capacity were studied. The adsorbent dosage was kept as 0.5 mg for all the investigates except for the adsorbent dosage study. The 0.1 mol/L NaOH or HCl, were used to adjust the solution pH. The fluoride ion concentration in the solution was calculated as described by the following equation
Where, DC-defluoridation capacity, Ci (mg/L) is the initial fluoride ion concentration, Ce (mg/L) is the equilibrium fluoirde ionconcentration, V is the volume of the fluoride solution in litre and m is the mass of adsorbent in gram.
The concentration of fluoride ion was measured using fluoride ion selective electrode Thermo Orion Bench top multi parameter kit (Model: VERSA STAR92) with ±1 significant digit of relative accuracy, 0.02 mg·L−1 of detection limit, and ± 2% of the reproducibility. The pH measurements were carried out with the same instrument in addition with a pH electrode. The other water quality factors were examined by standard methods [16]. The pH at zero point charge (pHzpc) of the adsorbent was measured by pH drift method [17].
2.4 Instrumentation studies
The solution pH was altered by using an proper amount of hydrochloric acid/sodium hydroxide, monitored with a pH meter (Orange pH 303, India). The adsorbent equilibrated with an orbital incubator shaker (Biotechnics, India). The fourier transform infra red (FTIR) spectra for the adsorbent were obtained using a Brukertensor 27 series model FTIR spectrometer by mixing 0.2 gram of the sorbent with 1.0 gram Potassium bromide and taking readings in the range of 400–4000 cm−1 at a 4 cm-1 resolution, with 16 scans. The powder X-ray diffraction (XRD) spectra of the adsorbent were obtained using monochromatic nickel-filtered copper radiation (40 kV, 30 mA) in a wide angle X-ray diffractometer (XPERT-PRO system with 2 μ angle). The scanning electron microscopy (SEM) micrograph and mapping studies were done using a VEGA 3 TESCON model.
2.5 Statistical tools
The Microcal Origin (Version 8.5) software used for the computations studeis. The suitable and best model of the fit was found out using the standard deviation (sd), and chi-square (χ2) analysis and regression correlation coefficient (r) values.
3 Results and discussion
3.1 Effect of contact time
The fluoride ion removal investigations of the MgO2/Ppy nanocomposite adsorbent were carried out with various interval of time period in the choice of 10–60 min at 303 K with starting fluoride concentration of 10 mg/L with an adsorbent measurement as 50 mg. From the results, the defluoridation capacity of MgO2/Ppy nanocomposite adsorbent was found to be 3836 mg F− /Kg and it attained equilibrium at 50 mins (Fig. 1a). After the time of 50 mins, which was attains the equilibrium stage of the adsorbent by the surface of the MgO2/Ppy nanocomposite adsorbent was maximum filled with fluoride ion [18]. Then, 50 min was fixed as an appropriate contact time with maximum fluoride ion removal efficiency by MgO2/Ppy nanocomposite.
3.2 Effect of dosage
The adsorption limits of MgO2/Ppy nanocomposite adsorbent for fluoride ions reduced with the adsorbent dosage increased from 5 to 30 mg. Fig. 1c shows the fluoride ion adsorption limits versus adsorbent dosage (mass of composite/volume of solution) at pH concentration of neutral (7.0 ± 0.5). The most extreme adsorption limit was found by utilizing 20 mg of MgO2/Ppy nanocomposite adsorbent. The efficiency of fluoride removal is directly proportional to active sites of the adsorbent [19]. The most extreme fluoride removal of 98 ± 1% was acquired utilizing 20 mg of composite. In this study, adding sorbent quantities greater than 20 mg did not effect in any substantial increase in the fluoride removal efficiency. This looks to be due to overlapping that decreased the active sites available in the adsorbent [20].
3.3 Effect of initial concentration
We studied the effect of changing the initial fluoride ion concentration in replicated drinking water on the adsorption efficiency of adsorbent, using values from 6.0 mgL−1 to14 mgL−1 (Fig. 1b). At an initial fluoride ion concentration of 6.0 mgL−1 the DC were 2143 mg F− Kg−1 for the 20 mg of MgO2/Ppy composite at 303 K in pH 7.0 ± 0.5. When the fluoride ion concentration was increased to 8.0 mgL−1, the DC enhanced 2526 mg F−Kg−1, at final 14 mg F− Kg−1 the DC was 3484 mg F−Kg−1 at the same conditions. The fluoride ion removal efficiency was increased with increasing the fluoride ion concentration at same condition. Since, adsorption effificeny was directly proportional to the concentration of fluoride ions in the solution [21].
3.4 Effect of pH
The result of solution pH was inspected for the composite such MgO2/Ppy nanocomposite at various initial pH values of 3.0, 5.0, 7.0, 9.0, and 11.0. Fig. 1d shows the adsorption capabilities of the MgO2/Ppy nano composite was considerably affected by solution pH. In general, the surface of the adsorbent is positively charge due to the lower pHzpc than neutral pH of the adsorbent (pH > pHzpc), and negatively charged means the pHzpc is greater than neutral pH (pH < pHzpc). The pHzpc value of MgO2/Ppy nano composite adsorbent was found to be 5.7 (showed in Fig. 2). The observed pHzpc value of the MgO2/Ppy nanocomposite adsorbent has less positive charge is due to the adsorbent has more electropositive magnesium ion. The positive charge on the surface is easier to attract the fluoride ion from drinking water. The outcome exposes that the maximum defluoridation capacity was taking place at pH 3.0, and the pH was increased from 3.0 to 11.0 defluoridation capacity reduced drastically. When the medium pH was lower, the surface of the MgO2/Ppy nanocomposite was positively charged and protonated, than the fluoride ions were moved to the positively charged surface, hence favoring fluoride adsorption onto the surface results in the increase in defluoridation capacity of the composite. But in the pH ranges of 9.0 and 11.0 shows the adsorption capacity is lower, because the surface of the MgO2/Ppy nanocomposite adsorbent covered with more negatively charged species, so the competition of F− and OH−, fluoride adsorption is decreases [22].
3.5 Effect of foreign-ions
The common foreign ions such as chloride, sulphate, nitrate, & bicarbonate ions are occur in water in addition with fluoride ions, and they may encounter with fluoride for the active sites of the adsorbent during adsorption. To calculate the defluoridation capacity of MgO2/Ppy nanocomposite in the existence of other ions with 10 mgL−1 initial fluoride ion solution was shifted individually into 200 mg·L−1 initial concentrations of chloride, sulphate, nitrate and bicarbonate ions. The impact of the various foreign ions on fluoride adsorption of the composite at pH 7 is shown in Fig. 1d. It was detected that all of the foreign ions affected a little with the fluoride ion adsorption onto the MgO2/Ppy nanocomposite, but bicarbonate and sulphate caused substantial result on the defluoridation capacity of the MgO2/Ppy nanocomposite. The weakening in the defluoridation capacity of the MgO2/Ppy nanocomposite in the presence of sulphate and bicarbonate ions is primarily due to the increase in the solution pH which decreases the active sites on the adsorbent surface for fluoride adsorption of the composite [23]. The defluoridation capacity of the various competitive ions shows in Fig. 3.
3.6 Chemical characterization
3.6.1 FTIR analysis
The FTIR spectrum of fluoride ion before and after adsorbed MgO2/Ppy nanocomposite as shown in Fig. 4. In the spectrum, the band at 1142 cm−1 and 1086 cm−1 is due to the hydroxyl group bending vibration in metal oxide (Mg-OH). The band appeared in the range of 743–480 cm−1 is due to the metal oxide bonding (Mg-O-Mg) [24]. The peak appeared in 2436 cm−1 in the spectrum, which shows the Mg-F composition. The peaks at 1543 and, 1023 cm−1 were assigned to vibrations of C-N, C-H respectively. The broad and sharp peak at 3445 cm−1 suggested that the stretching peak of N-H group in Polypyrrole [25] and the intensity of the N-H group peak was decreased due to the fluoride adsorption and formation of fluoride adsorbed MgO2/Ppy nanocomposite.
3.6.2 SEM analysis
SEM micrographs of MgO2/Ppy nanocomposite of before and after fluoride adsorption were investigated and presented in Fig. 5. Before, the adsorption the adsorbent has the regular spherical shape with uniform size and after the fluoride removal the adsorbent shows the white color powder like particles are dispersed on the surface of the global regular spheres, which is represented as a fluoride ion and which indicated by green color arrows in the image. The elemental mapping studies of the adsorbent was confirms the presence of elements in the before and after adsorption of fluoride by MgO2/Ppy nanocomposite adsorbent.
3.6.3 XRD analysis
The XRD pattern of MgO2/PPy nanocomposite adsorbent (Fig. 6) shows no distinct peaks for magnesium oxide which suggests its amorphous Mg(OH)2 structure. The XRD spectra of MgO2/PPy nanocomposite (Fig. 5a), showed a broad amorphous diffraction peak in the range of 15degreee < 2θ <30degree which is the characteristic peak for PPy monopolymer due to the scattering of PPy chains at interplanar spacing. Moreover, no noteworthy alteration noticed in XRD patterns of MgO2/PPy nanocomposite after F− adsorption (Fig. 5b) suggests that the F− interaction with the nanocomposite has not affected its crystalline structure.
3.7 Adsorption isotherms
The fluoride adsorption limit of MgO2/Ppy nanocomposite was assessed by using distinct isotherms specifically Freundlich [26], Langmuir [27] and Dubnin–Radushkevich [28] isotherms. The estimations of Freundlich isotherm constants 1/n and kF were found out from the intercept and slope of the plot for log Ce Vs log qe (Table 1). The n values are lying in between 1 to 10, and the values of 1/n are lying in between 0 to l, it shows that the system and conditions were suitable for adsorption fluoride. The higher r value showed the well fit of Langmuir isotherm compared with other two isotherms. The Langmuir adsorption isotherm model constants b and Q° were calculated from the intercept and slope of the plot for Ce versus Ce/qe and the outcomes given in Table 1. The estimations of Q° value was found to rise with the expansion in temperature, which showed adsorption limit expanded with ascend in temperature. The higher r value shows the applicability of Langmuir adsorption isotherm. The possibility of the adsorption isotherm can be tried by ascertaining the dimensionless constant partition factor/equilibrium parameter (RL).
The linear plots of ln qe vs. ε2 shows the suitability of D − R isotherm. The values of D-R isotherm parameters like KDR, Xm, and E are given in Table 1. The range of E value from 1.0–8.0 kJ·mol−1 indicating physical adsorption (physisorption) and from 9.0–16.0 kJ·mol−1for chemical adsorption (chemisorption). The result of Langmuir and D-R isotherm was recommended that the mechanism of fluoride ion adsorption by the adsorbent overcome the chemisorption compared to physisorption. The acquired E values are 8.7261, 9.2507 and 9.9031 kJ·mol−1 for 303, 313, and 323 K, respectively, which indicates the defluoridation mechanism of MgO2/Ppy nanocomposite is physical in nature. The best fit isotherm was distinguished by utilizing the error analysis and non-linear data information. In view of the above values the suitable isotherm fit was ordered as: Langmuir> Freundlich >D–R isotherm for fluoride sorption by MgO2/Ppy nanocomposite and thus fluoride ion adsorption by MgO2/Ppy nanocomposite takes after Langmuir isotherm which demonstrates the homogenous adsorption. The graphical plots of Langmuir, Freundlcih and Dubnin-Raduskevich isotherms are shown in Fig. 7a, b, and c respectively.
3.8 Thermodynamics parameters
The thermodynamic parameter, such as standard entropy change (∆S°), standard enthalpy change (∆H°) and standard free energy change (∆G°) are temperature dependent and furthermore connected with the adsorption feasibility [29, 30]. The Khan and Singh method [16] is used for to calculate the values and results are presented in Table 2. The randomness and endothermic nature of fluoride adsorption on MgO2/Ppy nanocomposite was confirmed by the positive values of ∆S° and ∆H° respectively. The spontaneous nature of fluoride adsorption on MgO2/Ppy nanocomposite was confirmed by the negative values of ∆G° at different temperatures from 303 Kto323 K [31].
3.9 Fluoride removal mechanism
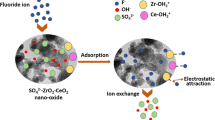
The adsorbent MgO2/Ppy nanocomposite follows basic mechanism for the fluoride removal process, which depends on the solution pH medium. In acidic pH the hydroxyl groups of the adsorbent and protonated amine groups in Polypyrrole have electrostatic interaction with negatively charged fluoride ion. At neutral pH the ion exchange mechanism will follows by the adsorbent because of the hydroxyl group of the Magnesium moiety have exchange the hydroxyl ion with the fluoride ion in the solution. Finally the adsorbent MgO2/Ppy nanocomposite follows both ion exchange and electrostatic attraction mechanism in fluoride removal process.
3.10 Field study
The field condition applicability of the MgO2/Ppy nanocomposite was examined the by collecting the fluoride enriched from the village (Vellaimalaipatti, Usilampatti Taluk, Madurai District (latitude 10°0ˈN and attitude 77°47ˈE)). The 0.1 g of the nano adsorbent and 50 ml of fluoride water sample were shaken with constant time at room temperature, and the results are given in Table 3. The fluoride ion concentration observed in the field water sample is 3.82 mg·L−1, which is higher than the permisible value of WHO recommendation. After treatment with MgO2/Ppy nanocomposite, the fluoride concentration comes below the permisible level in the field water. In field water the concentration of other ions are higher than the fluoirde ion, the concentration of other ions also reduced is the intresting think. Hence, the adsorbent MgO2/Ppy nanocomposite can be effectively used for water purification purpose. The removal of fluoride by the MgO2/Ppy nano composite adsorbent was followed via the ion exchange mechanism. From the field study results shows that the adsorbent was removed 79% of fluoride. As well as other co-ions was also removed by this adsorbent through ion exchange mechanism. Since, the field water comes in the permissible water standard level of advised by WHO.
3.11 Regeneration studies
The fluoride adsorbed MgO2/Ppy nanocomposite was desorbed by using the regenerant dilute sodium hydroxide solution. About 0.1 g of the fluoride adsorbed MgO2/Ppy nanocomposite was treated with dilute sodium hydroxide solution (50 mL) with various concentrations ranging from 0.02 to 0.1 M for 1 h. From Fig. 8, at 0.1 M solution the maximum desorption of 92% was achieved. After regeneration, the nanocomposite was separated by filtration, and washed with DD water, and dried in hot air oven and a fresh fluoride solution was added before each run. The desorption efficiency of MgO2/Ppy nanocomposite was found to be 93.0, 84.0, 69.2 56.0 and 44.1% respectively for five cycles.
4 Conclusion
The conclusion of the work is the defluoridation capacity of MgO2/Ppy nanocomposite. The maximum fluoride adsorption was attained at 50 mins of conduct time and 20 mg of the MgO2/Ppy nanocomposite dose. The pH of the solution was raised from 3.0 to 11.0 than the defluoridation capacity of the MgO2/Ppy nanocomposite was drastically reduced. Except bicarbonate and sulphate ions only influences the adsorption capacity of the MgO2/Ppy nanocomposite. The Langmuir adsorption isotherm was well fitted than D-R and Freundlich isotherm models. The MgO2/Ppy nanocomposite possesses a higher DC (4328 mgF−/Kg) because of the MgO2/Ppy nanocomposite removes the fluoride ion by electrostatic adsorption and ion exchange mechanism. The thermodynamic parameters values indicate the fluoride removal is endothermic and spontaneous nature. The field trial results of MgO2/Ppy nanocomposite show that it can be effectively employed for defluoridation and it gives the ides for the development household defluoridation technology unit in future.
References
S. Oguz, J. Hazard, Mater. 117, 227–233 (2005)
Y. Wang, E.J. Reardon, Activation and regeneration of a soil sorbent for defluoridation of drinking water. Appl. Geochem. 16, 531–539 (2001)
WHO, Guidelines for drinking-water quality first addendum to third edition, Volume 1 recommendations, (2006)
M.S. Onyango, Y. Kojima, O. Aoyi, E.C. Bernardo, H. Matsuda, Adsorption equilibrium modeling and solution chemistry dependence of fluoride removal from water by trivalent-cation-exchanged zeolite F-9. J. Colloid Interface Sci. 279, 341–350 (2004)
G. Singh, J. Majumdar, Water Environ. Res. 71, 36–42 (1998)
S.K. Adhikary, U.K. Tipnis, W.P. Harkare, K.P. Govindan, Defluoridation during desalination of brackish water by electrodialysis. Desalination 71, 301–312 (1989)
R. Simons, Trace element removal from ash dam waters by nanofiltration and diffusion dialysis. Desalination 89, 325–341 (1993)
M. Rajan, G. Alagumuthu, Study of fluoride affinity by zirconium impregnated walnut shell carbon in aqueous phase: kinetic and isotherm evaluation. J. Chem. 2013, Article ID 235048, 8 pages (2013)
I. Ali, V.K. Gupta, Advances in water treatment by adsorption technology. Nat. Protoc. 1, 2661–2667 (2007)
J. Chen, J. Feng, W. Yan, Influence of metal oxides on the adsorption characteristics of PPy/metal oxides for Methylene Blue. J. Colloid Interface Sci. 475, 26–35 (2016)
M.L. Zhang, H.Y. Zhang, D. Xu, L. Han, D.X. Niu, B.H. Tian, J.A. Zhang, L.Y. Zhang, W.S. Wu, Removal of ammonium from aqueous solutions using zeolite synthesized from fly ash by a fusion method. Desalination 271, 111–121 (2011)
M. Karthikeyan, K.K. Satheeshkumar, K.P. Elango, Removal of fluoride ions from aqueous solution by conducting polypyrrole. J. Hazard. Mater. 167, 300–305 (2009)
A.C. Zettlemoyer, E.A. Zettlemoyer, W.C. Walker, Active Magnesia. II. Adsorption of Fluoride from Aqueous Solution. J. Am. Chem. Soc. 69, 1312–1315 (1947)
P. Venkateswarlu, D.N. Rao, Indian J. Med. Res. 41, 473–477 (1953)
S.M. Rao, P. Mamatha, Curr. Sci. 87, 942–947 (2004)
M.V.L. Ramon, F. Stoeckli, C.M. Castilla, F.C. Marin, On the characterization of acidic and basic surface sites on carbons by various techniques. Carbon 37, 1215–1221 (1999)
A.A. Khan, R.P. Singh, Adsorption thermodynamics of carbofuran on Sn (IV) arsenosilicate in H+, Na+ and Ca2+ forms. Colloids Surf. 24, 33–42 (1987)
A. Nagaraj, K.K. Sadasivuni, M. Rajan, Investigation of lanthanum impregnated cellulose, derived from biomass, as an adsorbent for the removal of fluoride from drinking water. Carbohydr. Polym. 176, 402–410 (2017)
L. Liu, Z. Cui, Q. Ma, W. Cui, X. Zhang, One-step synthesis of magnetic iron–aluminum oxide/graphene oxide nanoparticles as a selective adsorbent for fluoride removal from aqueous solution. RSC Adv. 6, 10783–10791 (2016)
K. Pandi, In SituFabrication of Magnetic Iron Oxide over Nano-hydroxyapatite Gelatin Eco-polymeric Composite for Defluoridation Studies. N. Viswanathan J Chem Eng Data. 61, 571–578 (2016)
G. Alagumuthu, M. Rajan, Equilibrium and kinetics of adsorption of fluoride onto zirconium impregnated cashew nut shell carbon. Chem. Eng. J. 158, 451–457 (2010)
S. Gao, R. Sun, Z. Wei, H. Zhao, H. Li, F. Hu, Size-dependent defluoridation properties of synthetic hydroxyapatite. J. Fluor. Chem. 130, 550–556 (2009)
N. Chen, Z. Zhang, C. Feng, N. Sugiura, M. Li, R. Chen, Fluoride removal from water by granular ceramic adsorption. J. Colloid Interface Sci. 348, 579–584 (2010)
Z. Li, S. Deng, X. Zhang, W. Zhou, J. Huang, G. Yu, Removal of fluoride from water using titanium-based adsorbents. Front. Environ. Sci. Eng. China 4, 414–420 (2010)
M. Bhaumik, S. Agarwal, V.K. Gupta, A. Maity, Enhanced removal of Cr(VI) from aqueous solutions using polypyrrole wrapped oxidized MWCNTs nanocomposites adsorbent. J. Colloid Interface Sci. 470, 257–267 (2016)
H.M.F. Freundlich, Over the adsorption in solution. J. Phys. Chem. 57, 385–470 (1906)
I. Langmuir, The constitution and fundamental properties of solids and liquids. Part I. solids. J. Am. Chem. Soc. 38, 2221–2295 (1916)
S. Karahan, M. Yurdakoc, Y. Seki, K. Yurdakoc, Removal of boron from aqueous solution by clays and modified clays. J. Colloid Interface Sci. 293, 36–42 (2006)
Saha, S. Chowdhury, Thermodynamics In Tech. 349–364 (2011)
V.J. Inglezakis, A.A. Zorpas, Heat of adsorption, adsorption energy and activation energy in adsorption and ion exchange systems. Desalin. Water Treat. 39, 149–157 (2012)
M. Mohapatra, D. Hariprasad, L. Mohapatra, S. Anand, B.K. Mishra, Mg-doped nano ferrihydrite—A new adsorbent for fluoride removal from aqueous solutions. Appl. Surf. Sci. 258, 4228–4236 (2012)
Acknowledgements
M. Rajan is grateful to the UGC-University Grants Commission, India, for providing financial support under the schemes of “UGC-MRP Grants”(Ref: F. No. 43-187/2014 (SR)) and Department of Science and Technology, Science and Engineering Research Board (DST-SERB) (Ref: YSS/2015/001532; New Delhi, India) and also acknowledges the PURSE program for the purchase of SEM and FT-IR.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nagaraj, A., Govindaraj, D. & Rajan, M. Magnesium oxide entrapped Polypyrrole hybrid nanocomposite as an efficient selective scavenger for fluoride ion in drinking water. emergent mater. 1, 25–33 (2018). https://doi.org/10.1007/s42247-018-0001-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42247-018-0001-5