Abstract
Manganese-rich slag is a raw material for smelting silicon–manganese alloys using an electric furnace. The blast furnace method is the main method for smelting manganese-rich slag. This method has the problems of a long process, large coke consumption, and easy volatilization of metals such as lead and zinc, which affects smelting safety. A new technology for smelting manganese-rich slag with low-manganese high-iron ore by smelting reduction optimization was proposed. This technology has the advantages of a short process, low energy consumption, low carbon emissions, and comprehensive recycling of lead, zinc, and other metals. According to the chemical composition, X-ray diffraction analysis, and particle size analysis of Cote d’Ivoire low-manganese ore, an experiment was carried out on manganese-rich slag by reduction–smelting separation. Combined with the design scheme of the Box–Behnken principle, three experimental factors (temperature, basicity, and carbon content) were selected as the influences to study. The influence that each factor has on the recovery rate of manganese was studied by response surface methodology, and the experimental factors were optimized. The results show that under the conditions of a reduction-smelting temperature of 1402 °C, basicity of R = 0.10, and carbon content of 10 mass%, the recovery rate of manganese is 97%. A verification experiment was carried out under the optimal conditions, and the error was only 1.24%; this proves that the response surface method prediction model is reliable and accurate. This is of great significance for the comprehensive utilization of lean-manganese ore resources.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Manganese is an important metal mineral and is widely used in metallurgy and chemical and defense industries [1,2,3]. It is usually used to produce ferromanganese, nonferrous alloys [4, 5], dry batteries, coatings, cells, fertilizers, and fine chemicals [6]. At present, the annual consumption of manganese continues to grow worldwide. Although the overall distribution of manganese resources in the world is rich, it is uneven [7]. Most of the world's manganese-rich minerals (those that are more than 35% manganese) are in countries such as South Africa, Australia, Brazil, India, and Gabon [8, 9]. Manganese deposits are widely distributed around South Africa [10,11,12], ranking the first in the world [13]; however, there are still some controversies about their metallogenic types [14,15,16]. Brazil produces high-grade manganese ore and high-grade battery manganese ore [17, 18]. Manganese ore reserves in China are relatively small, unevenly distributed, and account for only 6.67% of the total global manganese ore resources [19, 20]. Meanwhile, manganese deposits are small-scale and low-grade, have complex associated components and high mining costs, and result in low utilization rates of manganese resources in China [21,22,23]; thus, manganese ore has become a scarce ore in China. Manganese-rich pyrometallurgical enrichment is supported by the state as an effective way to achieve comprehensive utilization of low-grade manganese ore. Because manganese-rich slag has low Fe and P contents and high Mn and Si contents [24, 25], it can partly replace imported manganese ore that is high in manganese and iron. Also, reducing the amount of added silica can decrease production power consumption and costs [26,27,28]. To promote a low-carbon economy and harmony between humans and nature, preparing manganese-rich slag by reducing low-grade manganese ore is significant and has great practical value [29,30,31,32].
Wu et al. [33] prepared manganese-rich slag from Chinese low-manganese high-iron ore and international lean-manganese ore. The product quality of manganese-rich slag is better. Also, the authors considered that best economic benefits could be obtained when using small-volume blast furnaces to produce high-grade manganese-rich slag with manganese content of 36 mass%. Ming et al. [34] studied the influence of temperature, basicity, and added amount of sintered ore on the smelting process of manganese-rich slag that was produced by the blast furnace method with high-iron, high-phosphorus low-manganese ore as raw materials. The results showed that the added amount of sintered ore increased with an increase in the manganese–iron ratio in the furnace. In the reduction, the basicity of the slag was controlled to be below 0.4 by adding flux, and the highest temperature was controlled to be no more than 150 °C; this effectively inhibited manganese from entering the molten iron. Li et al. [35] reported that when the basicity of slag was 0.3 and the amount of reducing agent was 1.6%, qualified manganese-rich slag can be obtained by roasting at 1000 °C for 45 min and reducing at 1410 °C for 60 min. Good slag–metal separation was achieved, and the recovery rates of manganese and iron were as high as 92.16% and 95.32%, respectively. Gao et al. [36] used pulverized coal as fuel to prepare manganese-rich slag by reduction with high-iron manganese ore. They achieved effective separation of slag and iron, and the recovery rate was high after reaction at 1200 °C for 20 min.
In this experiment, reduction-smelting was used for a low-manganese high-iron ore in Cote d’Ivoire to obtain manganese-rich slag that can be used for the production of a high-quality special manganese–silicon alloy. The research on smelting manganese-rich slag from low-manganese ore has mainly focused on blast furnace smelting or using a submerged arc furnace, and these methods have a small furnace capacity. In the smelting process, lining erosion is generally serious, and the recovery rate and resource utilization rate of manganese are low [37]. At present, research on smelting manganese-rich slag has mostly used the single-factor analysis method without considering the influence of multi-factor interactions on the recovery rate of manganese [38]. This experiment is designed according to the Box–Behnken principle to construct a quadratic polynomial model of the manganese recovery rate with temperature, basicity, and carbon content [39]. The optimal value is verified by experiments, and the optimal process parameters are obtained.
2 Experimental
2.1 Raw material
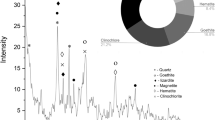
The main raw materials used in this study were low-manganese high-iron ore, coke, and lime. The low-manganese high-iron ore was provided by a company in Cote d’Ivoire, and the coke was provided by a company in Xinjiang, China. The main chemical components of the manganese ore from Cote d'Ivoire are shown in Table 1, where Mn was determined by industrial standard YD2.8.23-91; Fe was determined by YD2.8.12-91; P was determined by YD2.7.39-91; SiO2 was determined by YD3.2.2-91; CaO was determined by YD2.3.24-91; and Al2O3 was determined by YD2.8.1-91. The particle size composition results are shown in Table 2, and the X-ray diffraction results are shown in Fig. 1. As seen in Table 1, the Mn content in the manganese ore was 27.7 mass%, the iron content was 18.1 mass%, and the manganese–iron ratio was 1.53. According to the manganese grade, Mn/Fe ratio, and content of impurity elements, the manganese ore is low-manganese high-iron ore, and the silica content in the ore is high (5.54 mass%). As seen in Table 2, the particle size of the raw material is generally fine, and as seen in Fig. 1, the manganese in the raw materials is mainly in the form of cryptomelane (KMn8O16) and ferromanganese spinel ((Mn0.6Fe0.4)(Mn0.4Fe1.6)O4). Si is mainly in the form of quartz SiO2 and mica ((K,Na)Al2(Si,Al)4O10(OH)2), Fe is mainly in the form of ferromanganese spinel, and Al is mainly in the form of mica.
In this experiment, coke was used as a reducing agent. Industrial analysis of coke is shown in Table 3. The determination method is based on Chinese standard GB/T 2001–2013. As seen in Table 3, the contents of this coke are fixed carbon 84.97 mass%, volatiles 1.61 mass%, ash 13.18 mass%, and moisture 0.24 mass%. This coke has a high FCad value and is low in volatile compounds, ash, and sulfur; thus, the coke is in high quality, and this is beneficial for the reduction of low-manganese high-iron ore.
2.2 Test scheme and research method
The manganese ore and coke were crushed to 8–10 and 5–8 mm, respectively. The influences of various factors on the reduction–smelting process of low-manganese high-iron ore from Cote d’Ivoire were explored. The reduction temperatures were 1300, 1350, 1400, 1450, and 1500 °C. The coke dosages were 5%, 10%, 15%, 20%, and 25%. The basicity values were 0.034, 0.1, 0.2, 0.3, 0.4, and 0.5. The influences of various factors on Mn and Fe recovery in low-manganese high-iron ore were studied. The mixture was dried for 2 h in an oven at 120 °C in different proportions for further use.
A reduction–smelting test of manganese ore was carried out in a high-temperature box furnace. The mixed raw materials were put in a graphite crucible, which was then put in the high-temperature box furnace. The temperature was increased according to a preset temperature curve. After the temperature was increased to the preset temperature, the crucible was kept constant at this temperature for 1 h. Finally, the crucible was removed and cooled naturally to room temperature. After the temperature was reduced, the reaction products were separated to obtain slag and pig iron.
The reduction–smelting product was ground to be less than 0.074 mm, and the main chemical components in the product were analyzed to test whether or not the product met the standard. The determination of manganese was based on Chinese standard GB/T 5686.1-2008, and the determination of iron was based on Chinese standard GB/T 8564.1-2007.
The recovery rate of manganese in the slag phase was used as the evaluation standard. The recovery rate of manganese can be calculated using the following formula:
where γ is the manganese recovery rate, %; α is the grade of the manganese in the raw materials, %; β is the manganese grade in slag, %; m1 is the ore mass, g; and m0 is the slag mass, g.
3 Experimental results and discussion
3.1 Influence of various factors on smelting of manganese-rich slag by smelting reduction of low-manganese high-iron ore
3.1.1 Effect of temperature on Mn and Fe contents in slag and recovery rate of Mn
When the basicity was 0.034, and the added coke was 15%, the effects of reduction temperature (1300, 1350, 1400, 1450, and 1500 °C) on the contents of Mn and Fe in slag and the recovery rate of Mn in slag were measured. The results are shown in Fig. 2.
In Fig. 2, with carbon content of 15% and natural basicity of 0.034, the mass fraction of Fe in slag decreases significantly with an increase in reduction temperature. Also, the mass fraction of Mn in slag increases continuously with an increase in reduction temperature. This is because the reduction temperature affects the chemical reaction. With an increase in reduction temperature, the chemical activity of the reactants increased, and the reaction rate was accelerated. Also, in Fig. 2, when the reduction temperature was 1400 °C, the recovery rate of manganese reached 91.82%. With an increase in temperature, the recovery rate of manganese in slag decreases sharply. Thus, although the increased temperature is beneficial for the enrichment of manganese in slag, the recovery rate of manganese in slag decreases when the temperature is too high. Therefore, the reduction temperature of 1400 °C was selected in following test.
3.1.2 Effect of basicity on Mn and Fe content and recovery rate of manganese in slag
When the carbon content was 15%, and the reduction temperature was 1400 °C, the effects of basicity (0.034, 0.1, 0.2, 0.3, 0.4, and 0.5) on the Mn and Fe contents in slag and the recovery rate of Mn in slag were measured. The results are shown in Fig. 3.
As seen in Fig. 3, for a reduction temperature of 1400 °C and 15% added coke, the mass fraction of Fe in the slag after reduction decreased gradually with an increase in basicity. When the basicity was further increased above 0.2, the mass fraction of Fe remained unchanged (at 0.4%). As seen in Fig. 3, the recovery rate of manganese in slag had an overall upward trend with an increase in basicity.
3.1.3 Effect of carbon content on Mn and Fe content and recovery rate of manganese in slag
When the reaction temperature was 1400 °C, and the basicity was 0.034, the effects of carbon content (5%, 10%, 15%, 20%, and 25%) on the Mn and Fe contents and the recovery rate of Mn in slag were measured. The results are shown in Fig. 4.
As seen in Fig. 4, when the reaction temperature was 1400 °C and the basicity was natural basicity, the mass fraction of iron in slag first decreased and then increased with an increase in carbon content. When the carbon content was increased from 5% to 15%, the iron content in the slag decreased from 1.6% to 0.5%. This indicates that the iron oxide was reduced more thoroughly when the carbon content was increased. However, with further increases in the carbon content, the iron content in the slag increased from 0.5% to 1.4%. This is because many coke powder residues were distributed in the slag liquid when the coke content was too high; this resulted in a viscous slag liquid, which affected the reduction of iron oxide. In Fig. 4, the recovery rate of manganese in slag first increased and then decreased with an increase in carbon content. When the carbon content was increased from 5% to 15%, the yield of manganese increased. With the further increases in the carbon content, the yield of manganese decreased from 92.13% to 77.43%.
3.2 Preparation of manganese-rich slag by reduction of low-manganese high-iron ore optimized by response surface methodology
3.2.1 Design and analysis of response surface experiment
Using single-factor variable research, three influencing factors (carbon content, reaction temperature, and basicity) were selected, and the ranges of the variables were narrowed. Design Expert software was used to determine the experimental scheme using Box-Behnke theory, and the influence of each factor on the recovery rate of manganese in manganese ore reduction slag was analyzed. The horizontal coding of test factors is shown in Table 4. The design scheme and measured values of the response surface experiment are shown in Table 5.
In this study, the quadratic model was used for multiple regression fitting of the recovery response value of Mn (Table 5). The quadratic polynomial regression equation for Mn recovery in slag is as follows:
In the Design Expert software, the ANOVA module was selected to analyze the variance of the model. The results are shown in Table 6.
In the response surface software, Table 6 shows that the P value of the experimental model is 0.0314 lower than 0.05. This indicates that a good simulation is achieved with the model. The mismatch item is 0.6497, and this result is not significant. This indicates that the selected model in the experiment was well-fit and that the scheme design is scientific, reasonable, and statistically significant. As seen in Table 6, for the recovery rate of Mn in slag, the influence of factor A in the first term is highly significant, that of factor C is significant, and that of factor B is not significant. The interaction items AB, BC, and AC are not significant, and the secondary items A2, B2, and C2 are not significant. In summary, the order of influence of each factor on the recovery rate of Mn in slag is as follows: reaction temperature > carbon content > basicity.
The curve of actual values versus predicted values for Mn recovery rate in slag is shown in Fig. 5. As seen, the actual value of Mn recovery rate in slag fluctuates near the predicted value line, and individual data points are distributed in a straight line. This shows that there is a small difference between the actual value and the predicted value of the Box–Behnken test. Thus, the fitting degree is good. Therefore, the theoretical model established in this experiment is reliable and can be used to optimize the smelting parameters and to predict test results.
3.2.2 Response surface analysis of manganese-rich slag grade under interaction
The recovery rate of Mn in the process of preparing manganese-rich slag by the reduction of low-manganese high-iron ore from Cote d’Ivoire under different process conditions was fitted by multiple regression analysis using the response surface method. The contour map and three-dimensional response surface map of the interactive influences of various factors on the recovery rate of Mn were obtained. The map is a visual representation of the influences of the three factors on the recovery rate of Mn. The height of the response surface represents the value of the response value. When the slope of the surface in the direction of a factor is greater, the impact that the factor has on the response value is greater and vice versa [40, 41]. Therefore, a response surface diagram can be directly used to observe the influences of reaction temperature, carbon content, and basicity on the Mn recovery and the changes in the response value.
3.2.2.1 Effects of basicity and carbon content on grade of manganese-rich slag
The response surface of the interaction between basicity and carbon content on the recovery rate of Mn is shown in Fig. 6. As seen in Fig. 6, the overall height of the response surface becomes lower with an increase in temperature, and thus, the reduction and melting of low-manganese high-iron ore are easier at low temperature. As seen in Fig. 6, the slope of the surface in the direction of the carbon content is significantly greater than that in the direction of basicity when the reaction temperature is constant. This indicates that the carbon content has a greater impact on the recovery of manganese than basicity; in fact, the recovery of manganese gradually decreased with an increase in carbon content. When the temperature and carbon content remain unchanged, the recovery of manganese did not change significantly with basicity. In summary, the recovery rate of Mn was a maximum at low temperature and low basicity.
3.2.2.2 Effects of temperature and carbon content on grade of manganese-rich slag
The response surface diagram of the interaction between temperature and carbon content on the recovery rate of Mn is shown in Fig. 7. The height of the response surface does not change significantly with a change in basicity, and this indicates that the influence of basicity on the reduction and melting process of low-manganese high-iron ore is not significant. As found in Fig. 7, when the basicity is constant, the slope of the surface in the temperature direction is greater than that in the carbon distribution direction. This indicates that temperature has a greater impact on the recovery rate of manganese than carbon content; indeed, the recovery rate of manganese decreased gradually with an increase in temperature. As also seen in Fig. 7, at high temperature, the recovery rate of Mn decreased gradually with the increase in carbon content when the basicity was constant; however, at low temperature, the recovery rate of Mn did not change significantly with carbon content.
3.2.2.3 Effects of temperature and basicity on grade of manganese-rich slag
The response surface diagram of the interaction of temperature and basicity on the recovery rate of Mn is shown in Fig. 8. The overall height of the response surface decreases gradually with an increase in carbon content. However, this decrease is not obvious, and this indicates that the influence of carbon content on the recovery rate of Mn is generally insignificant. As seen in Fig. 8, the slope of the surface in the temperature direction is significantly greater than that in the basicity direction when the carbon content is constant. This indicates that temperature has a very significant impact on the recovery rate of Mn. Also, the slope of the surface in the temperature direction is the most obvious when the carbon content is high. This indicates that temperature has a great influence on the recovery rate of Mn when a sufficient reducing agent is used. When the temperature is higher, the recovery rate of Mn is lower, and this indicates that the reduction temperature of manganese ore should not be too high.
The results of the response surface analysis are consistent with those of the variance analysis (Table 6). Interactions between various factors generally have a significant effect on the recovery rate of Mn. To improve the recovery rate of Mn, the reduction and melting process of manganese ore should be carried out at low temperature, low basicity, and low carbon content.
To obtain the optimum process parameters, the optimum theoretical process parameters were calculated by the optimization module in Design Expert software using a 97% recovery rate of Mn. The theoretical results are a reaction temperature of 1402.36 °C, basicity of 0.10, and carbon content of 10.04%.
Combined with the limitations of the actual test process, the optimal theoretical smelting data was determined to be as follows: a reduction–smelting temperature of 1402 °C, basicity of 0.10, and carbon content of 10%. Three verification tests were carried out under these process parameters, and the average value was calculated. The results are shown in Table 7.
As seen in Table 7, the average recovery rate of Mn was 95.80% with an error of 1.24%. This is basically consistent with the best results obtained by software modeling and optimization. This indicates that the model has high reliability and can provide theoretical guidance for the reduction of low-manganese high-iron ore to prepare manganese-rich slag.
4 Conclusions
-
1.
In this experiment, low-manganese high-iron ore was used as the raw material to produce manganese-rich slag through ore blending optimization, and smelting parameters were optimized using the response surface method. This provides a new idea for the utilization of low-manganese ore resources. It also has important practical significance for improving the utilization rate of increasingly depleted low-manganese ore resources, further improving the process of preparing manganese products in China, and finding new ways to achieve sustainable development in industry.
-
2.
The results of single-factor experiments showed that when the temperature was 1400 °C, the recovery rate of manganese in the slag phase reached a maximum. When the carbon content was in the range of 10%–15%, the mass fraction of manganese in slag and the recovery rate of manganese in slag were larger. Increasing basicity within a certain range is beneficial for enriching manganese in slag. The influence order of various factors on the recovery rate of Mn is: reaction temperature > carbon content > basicity.
-
3.
The optimum theoretical process parameters obtained by the response surface experiments are as follows: a reaction temperature of 1402.36 °C, basicity of 0.10, and carbon content of 10.04%. A verification test was carried out under these smelting conditions; the average recovery rate of manganese was 95.80%, and the error was only 1.24%.
References
Y.B. Zhang, Y. Zhao, Z.X. You, D.X. Duan, G.H. Li, T. Jiang, J. Cent. South Univ. 22 (2015) 2515–2520.
F.F. Wu, H. Zhong, S. Wang, S.F. Lai, J. Cent. South Univ. 21 (2014) 1763–1770.
M.N. El Hazek, T.A. Lasheen, A.S. Helal, Hydrometallurgy 84 (2006) 187–191.
P.W. Harben, J.M. Harris, C. Raleigh, Manganese uses and markets, Industrial Minerals Information Ltd., London, UK, 1998.
A.A. Nayl, I.M. Ismail, H.F. Aly, Int. J. Miner. Process. 100 (2011) 116–123.
R.N. Sahoo, P.K. Naik, S.C. Das, Hydrometallurgy 62 (2001) 157–163.
X.L. Lei, Y.D. Hu, Y.L. Du, F.L. Zhang, D. Wang, China Mining Magazine 24 (2015) No. S1, 19–21.
A. D'Harambure, Y.F. Yang, J. Yang, China Manganese Ind. 39 (2021) No. 4, 1–4.
N.J. Beukes, J. Gutzmer, A.M. Burger, S. Afr. J. Geol. 98 (1995) 430–451.
J. Gutzmer, N.J. Beukes, Ore Geol. Rev. 11 (1996) 405–428.
S. Frost-Killian, S. Master, R.P. Viljoen, M.G.C. Wilson, Episodes 39 (2016) 85–103.
USGS, Mineral Commodity Summaries (2021–01–31)[2021–09–10]. https://minerals.usga.gov/minerals/pubs/commodity/manganese/mcs-2020-manganese.
N.J. Beukes, J. Gutzmer, in: S. Hagemann, C.A. Rosière, J. Gutzmer, N.J. Beukes (Eds.), Reviews in Economic Geology, Vol. 15, Society of Economic Geologists, 2008, pp. 5–47.
V.N. Kuleshov, Lithol. Miner. Resour. 46 (2011) 546.
V.N. Kuleshov, E.A. Zhegallo, E.L. Shkol'nik, Dokl. Earth Sci. 441 (2011) 1611–1615.
T. De Putter, G. Ruffet, J. Yans, F. Mees, Ore Geol. Rev. 71 (2015) 350–362.
V. Kuleshov, Isotope geochemistry: the origin and formation of manganese rocks and ores, Elsevier Inc., Amsterdam, Netherlands, 2016.
C.A. Moreira, M. Rezende Borges, G.M. Lira Vieira, W. Malagutti Filho, M.A. Fernándes Montanheiro, Geofís. Intl. 53 (2014) 199–210.
J. Xiang, J. Chen, L. Bagas, S. Li, H. Wei, B. Chen, Ore Geol. Rev. 116 (2020) 103261.
D.L. Qin, N.X. Chen, China Manganese Ind. 39 (2021) No. 4, 10–12 + 21.
Y. Cong, Q.J. Dong, K.Y. Xiao, J.P. Chen, Y.B. Gao, J.N. Yin, Earth Sci. Front., 25 (2018) No. 3, 118–137.
Z.N. Liu, X.Y. Zhang, H. Xu, Q.S. Wang, M. Chen, China Mining Magazine 24 (2015) No. 8, 8–15.
Z.N. Liu, H. Xu, Q.S. Wang, M. Chen, Resour. Ind. 17 (2015) No. 6, 38–43.
L. Gao, Z. Liu, M. Chu, R. Wang, Z. Wang, C. Feng, Sep. Sci. Technol. 54 (2019) 195–206.
K. Li, J. Chen, J. Peng, M. Omran, G. Chen, J. Clean. Prod. 260 (2020) 121074.
S. He, C. Liao, X. Wang, J. Wang, Mining Metallurgy & Exploration 37 (2020) 433–442.
V. Singh, N. Biswas, T. Chakravarty, S.M. Rao, P.K. Banerjee, J. Metall. Mater. Sci. 55 (2013) 59–65.
V. Tathavadkar, V. Singh, P.K. Mishra, P. Mallick, B.D. Nanda, Ironmak. Steelmak. 37 (2010) 103–111.
Y.L. Liang, J.B. Lei, Ferro-Alloys 52 (2021) No. 1, 6–12.
C.Z. Zhang, P. Zhou, China Manganese Industry 38 (2020) No. 1, 1–3.
J. Tong, Ferro-Alloys 35 (2004) No. 1, 37–39.
H. He, China Manganese Industry 35 (2017) No. 1, 23–24.
X.D. Wu, X.Q. Ming, G.H. Huang, N.X. Chen, B.L. Huang, W.S. Lu, China Manganese Industry 33 (2015) No. 3, 34–36+39.
X.Q. Ming, X.D. Wu, G.H. Huang, China Manganese Ind. 34 (2016) No. 3, 131–133.
Z.J. Li, Q. Zhao, H.Q. Wan, Ferro-Alloys 42 (2011) No. 3, 21–24.
J.J. Gao, X.Y. Wan, Y.H. Qi, J. Zhang, F. Wang, China Metall. 26 (2016) No. 10, 54–58.
P. Zhao, Process for coal-based direct reduction and magnetic separation of high-iron manganese ore, Central South University, Changsha, China, 2012.
Y.B. Zhang, Z.X. You, G.H. Li, T. Jiang, Hydrometallurgy 133 (2013) 126–132.
S.P. Yang, S.W. Cao, F. He, J. Iron Steel Res. 31 (2019) 897–903.
S.P. Yang, J.F. Zhou, S.Q. Guo, P.H. Zhang, M. Wang, Nonferrous Metals Eng. 7 (2017) No. 5, 48–53.
J. Zuo, L.J. Yan, X.Q. Yang, Y. Cheng, J. Univ. Sci. Technol. Beijing 32 (2010) 1045–1052.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, Sp., Li, Jh., Gao, Wb. et al. Optimization of manganese-rich slag extraction from low-manganese ore smelting by response surface methodology. J. Iron Steel Res. Int. 29, 1573–1582 (2022). https://doi.org/10.1007/s42243-022-00781-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42243-022-00781-9