Abstract
A novel technology, modified roasting in CO–CO2 mixed gas and magnetic separation, was presented to recover iron from copper slag. The effects of various parameters such as dosage of flux (CaO), gas flowrate of CO and CO2, roasting temperature, roasting time, particle size of modified slag and magnetic flux density on the oxidized modification and magnetic separation were investigated by comparison of the X-ray diffraction patterns and iron recovery ratio. The optimum conditions for recovering iron by oxidizing roasting and magnetic separation are as follows: calcium oxide content of 25 wt.%, mixed gas flow rates of CO2 and CO of 180 and 20 mL/min, oxidizing roasting at 1323 K for 2 h, grinding the modified slag to 38.5–25.0 μm and magnetic separation at 170 mT. The mineralogical and microstructural characteristics of modified slag revealed that the iron-bearing minerals in the copper slag were oxidized, the generated magnetite grew into large particles, and the silicate in copper slag was combined with calcium oxide to form calcium silicate. Finally, the iron-bearing concentrate with an iron grade of 54.79% and iron recovery ratio of 80.14% was effectively obtained.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Pyrometallurgy is still the dominant technology for current copper extraction in the global copper production industry. Copper slag is a complex silicate mineral that is produced during matte smelting and converting steps of pyrometallurgical production of copper. It has been estimated that about 2.2 t of slag is generated for every ton of copper production and in each year, approximately 40 million ton of slag is generated from world copper production, and more than 1/3 of copper slag is produced in China [1, 2]. Dumping and disposal are traditional means of treating copper slag, which not only occupies a large amount of land, but also cannot make rational use of secondary resources of copper slag and even causes a great degree of pollution [3]. Copper slag was used as a raw material for building materials such as cement, concrete, glass and ceramic tiles, as well as for grinding and paving materials due to its excellent physical and mechanical properties [4].
Copper slag produced in the smelting process contains a large number of valuable metals such as copper, zinc, cobalt, nickel and precious metals such as gold and silver [5]. In addition, it contains about 35–45 wt.% of iron, which indicates that the grade of iron in copper slag is much higher than that of ordinary iron ore [6]. Therefore, the recovery ratio of iron in copper slag is an important index for comprehensive recovery and utilization of copper slag resources. More than 80% of iron in copper slag exists in the form of ferric silicate (Fe2SiO4), and only a few Fe exists in magnetite phase. However, the physical and chemical properties of ferric silicate are extremely stable. It is difficult to recover iron efficiently by conventional methods [7]. In order to improve the iron recovery ratio and the iron grade of the concentrate, many scholars have carried out experimental and theoretical studies on the recovery of iron from copper slag. Zeng and Xiao [8] proposed a physical beneficiation method combining direct flotation with three times magnetic separation to recover copper and iron from copper slag. Under this condition, iron-bearing concentrate with an iron content of 52.21 wt.% and silicon dioxide content of 13.2 wt.% as well as iron recovery ratio of 38.9% was eventually obtained. Iron-bearing concentrate with low grade and recovery can only be obtained by direct magnetic separation, because most of iron in copper slag exists in the form of fayalite [9]. In the study of Cao et al. [10] and Kim et al. [11], the direct reduction iron powders with TFe of 90.35 and 65.00 wt.% as well as the recovery ratio of Fe of 89.7% and 85.0%, respectively, were obtained by smelting reduction and subsequent grinding/magnetic separation process. Recovering iron by high temperature smelting reduction needs a lot of power to make copper smelting and reduction, and the waste heat cannot be used effectively when the reduction slag is slowly cooled, resulting in a large amount of energy waste. Therefore, Jiao et al. [12] proposed an innovative approach to recover iron from copper tailings via low-temperature direct reduction and magnetic separation, in which copper slag was reduced by pulverized coal and then recovered by magnetic separation, and the reduced iron powder with iron grade above 90% and recovery ratio of 95% was finally obtained. Generally, reduction and beneficiation of iron from copper slag result in high iron grade and recovery of reduced iron powder, but coal or carbon powder as reductants will produce carbon dioxide and other greenhouse gases during reduction and recovery process, which does not meet the requirements of energy saving and emission reduction. In recent years, oxidized modification and magnetic separation of copper slag were used as an advanced technology to recover iron from copper slag by many researchers such as Cao et al. [6], Ma et al. [13], Fan et al. [14,15,16] and Guo et al. [17, 18]. In addition, the mechanism of oxidized modification of copper slag was studied by analyzing the phase and structure transformation during oxidized modification. The oxidized modification of copper slag is usually carried out in the presence of oxygen or other oxidizing gas. As we all know, the internal porosity of molten liquid is much lower than that of solid. Although the chemical reaction activity between molten liquid surface and oxidizing gas is greater than that of solid, the internal diffusion efficiency of gas is far lower than that of solid. Therefore, it is not certain that the overall oxidation effect of molten copper slag is better than that of copper slag solid. And when copper slag has been oxidized, a large amount of gas has been consumed, and the uncontrollability of the gas will easily lead to the waste of gas. In addition, the ventilation rate is an important parameter affecting the recovery ratio of iron, but the different operating personnel and equipment conditions may cause insufficient or excessive oxidation of copper slag, which is not conducive to the subsequent magnetic separation.
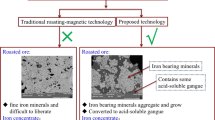
Based on the above analysis, a new method of recovery of iron from copper slag via modified roasting and magnetic separation is proposed, where the whole system is in a weak oxidizing atmosphere due to stable equilibrium of CO2–CO [19], and ferrous oxide (FeO) in fayalite is separated by adding flux (CaO) [20], and then the free FeO is oxidized and roasted to magnetite at lower temperature. After grinding, crushing and magnetic separation of the modified slag, the iron-bearing concentrate meeting the requirement of ironmaking is obtained and the tailing can be used as raw materials for building materials. The new method has the following advantages: CO2 and CO were used as gas oxidants; no additional greenhouse gases were emitted, and the modification conditions will not cause excessive energy consumption. Recovery of iron from copper slag can be realized while the secondary resources are rationally utilized.
2 Experimental
2.1 Materials
2.1.1 Copper slag
The copper slag used in the experiments is by-product generated during refining of copper in a non-ferrous metals group located in the east China. It is gray after drying, and 95 wt.% of the copper slag has a particle size less than 0.06 mm. The chemical composition of copper slag was analyzed by inductively coupled plasma atomic emission spectroscopy (ICP-AES) and X-ray fluorescence, and the result is given in Table 1, which shows that the main ingredients of the copper slag are 40.44 wt.% Fe and 28.41 wt.% SiO2, and the Cu content also reaches as high as 0.27 wt.%. In addition, the contents of harmful elements such as sulfur and phosphorus are relatively low, which can be removed by other treatments.
The X-ray diffraction (XRD) pattern of raw copper slag used in the experiments is shown in Fig. 1, which indicates that copper slag mainly consists of fayalite (Fe2SiO4) and magnetite (Fe3O4). In addition, a broad band indicating the presence of glass phase materials was observed between 10° and 40° in XRD patterns. It can be referred that there are still some glass phases in the copper slag, which is similar as the study of Hu et al. [21]. Microstructure and elemental scanning figures of raw copper slag were detected by scanning electron microscopy with energy-dispersive X-ray spectrometry (SEM–EDS), and the results are shown in Fig. 2, which confirm that Fe, Si, Ca, O and Cu distribute uniformly in slag.
2.1.2 Flux and gas
Calcium oxide (CaO) powder with a purity of 99.9% (AR), supplied by Xilong Chemical Co., Ltd. (Shantou, China), was employed as the modifier. The purity of the gas (CO2, CO) used in the experiment is 99.99%, which is provided by Jianli Gas Co., Ltd. (Ganzhou, China).
2.1.3 Experimental procedure
The whole treatment process is shown in Fig. 3. First, raw copper slag was uniformly mixed with the required amount of calcium oxide in the ball mill for 2 h. Then, approximately 25 g of dry mixed material was charged into a corundum crucible with an inner diameter of 44 mm and height of 58 mm. After that, the corundum crucible was placed in a MoSi2 electric furnace (GWL-LKQGA) and roasted between 1173 and 1373 K for a predetermined time (1–4 h) under CO2–CO atmosphere. Simultaneously, the mixed gas of CO2–CO with a certain ratio of CO2/CO was always blown into the reaction system by adjusting the flowrate of CO2 and CO during keeping-temperature processes and with the constant flowrate of CO2 of 250 mL/min during heating-up and cooling stage. Once the temperature of furnace was cooled to ambient temperature, the samples were taken out and crushed by a hammer and a pulverizer, and the standard sieves with different pore sizes were used to screen the broken copper slag. Various standard sieves, such as 125, 74, 38.5 and 25 μm, were used to classify the modified copper slag of different sizes. Subsequently, a 15 g batch of dry modified slags was sorted out to iron-bearing concentrate and tailing by wet magnetic separation for 10 min in a magnetic tube (CXG-08SD(A)) at a magnetic field strength in the range of 120–320 mT. Ultimately, the produced iron-bearing concentrate can be used for steel-making and the tailing can be used for building materials.
2.2 Equipment and analytical method
The heating device used in the experiment is a vertical furnace (GWL-LKQGA, Luoyang Guoju Experimental Electric Furnace Co., Ltd., Luoyang, China), and the flowrate and the gas composition of reaction gas mixture were controlled by gas mass flow controllers (ALICAT). Their schematic diagram is shown in Fig. 4. The modified slag was crushed and ground to a specific size via a crusher (GJ-2A, Hebi Tianguan Instrument Co., Ltd., Hebi, China) and a ball mill (YXQM-1L, Changsha Miqi Instrument and Equipment Co., Ltd., Changsha, China). The magnetic separation was performed on a wet feebleness magnetic separation machine (CXG-08SD(A), Shicheng Yongsheng Mineral Processing Equipment Co., Ltd., Ganzhou, China) with a maximum flux of 600 mT.
The iron content of products was evaluated by the titration method according to Chinese standard GB/T 6730.73-2016. XRD patterns of modified slag were recorded using a diffractometer (EMPYREAN, PANalytical B.V., 40 kV, 40 mA, Cu-Kα, Almelo, Netherlands). Microstructures of reduced briquettes were identified using an MLA650F scanning electron microscope (FEI Company, Hillsboro, USA) equipped with an energy-dispersive spectrometer (EDAX, Bruker Analysis Instrument, Inc., Billerica, USA). SEM images were recorded in backscatter electron modes operating in low vacuum mode at 66.67 Pa and 20 keV. The particle size of the copper slag was obtained with a laser particle size analyzer (Mastersizer 3000, UK Malvern Instruments Co., Ltd., Malvern, UK) with a refractive index of 1.330.
2.3 Evaluation indexes
The purpose of this experiment is to effectively recycle iron from copper slag. Therefore, after modified roasting and magnetic separation, the quality of magnetic product iron-bearing concentrate and tailing and the corresponding iron grade are calculated, and the iron yield and the final recovery ratio of iron are obtained. The formula is as follows [16]:
where P1 and P2 are the yield of concentrate and tailing, respectively, %; m1 and m2 are the mass of concentrate and tailing, respectively, g; m0 is the feed mass of modified slag subjected to magnetic separation, g; R is the final recovery ratio of iron, %; and C1 and C2 are the iron grade of concentrate and tailing, respectively, %.
3 Results and discussion
3.1 Thermodynamic analysis
The modified roasting experiment of copper slag is to oxidize Fe2SiO4 from copper slag to magnetite under weak oxidation condition by adjusting the flowrate of CO2 and CO because lower oxygen partial pressure was beneficial to the transition of magnetite [15]. High roasting temperature is beneficial to the separation of SiO2 and FeO in copper slag, but the free magnetite will combine with SiO2 to form fayalite again at too high temperature [22]. Previous studies have found that the addition of calcium oxide can bind SiO2 in the slag to form CaSiO3, and the binding energy of CaSiO3 is lower than that of Fe2SiO4; thus, CaO is favorable for dissociating FeO to promote the formation of magnetite [20, 23]. However, when insufficient calcium oxide exists in the reaction system, the newly formed ferrous oxide will recombine with CaSiO3 to form kirschsteinite (CaFeSiO4), which is not conducive to magnetic separation. The main reactions involved are as follows:
The standard Gibbs free energy (ΔGθ) of Eqs. (4)–(8) and the phase-stability diagram of Fe–Ca–O–Si at 1273 K were calculated under one atmospheric pressure by FactSage™7.0, and the results are shown in Fig. 5. It can be seen from Fig. 5a that the ΔGθ values for Eqs. (4)–(9) are negative over the temperature range of 200–1600 K, indicating that those reactions are thermodynamically possible, and the ΔGθ value of Eq. (8) is smaller than others, which indicates that the equilibrium reaction between CO and CO2 was first completed in the oxidizing roasting of copper slag, providing a stable partial pressure of oxygen for the whole system. Furthermore, the ΔGθ value of Eq. (9) is smaller than those of Eqs. (4)–(7) in the temperature range of 1000–1600 K, which reveals that fayalite in copper slag is more easily converted into magnetite and calcium silicate in the presence of calcium oxide and oxygen. In addition, as shown in Fig. 5b, magnetite and calcium silicate can exist in the same region as long as the proper CO and CO2 partial pressures in the system are controlled. Equation (10) illustrates that the kirschsteinite will be formed in copper slag when calcium oxide and oxygen partial pressure are unstable. Therefore, the determination of these two important parameters will be investigated in the following research.
3.2 Roasting modification of copper slag
3.2.1 Effect of roasting temperature
The effect of modification temperature on grade and recovery of iron was investigated, and the results are shown in Fig. 6 with constant roasting time, gas flowrate of CO2 and CO, and dosage of CaO of 2 h, 180 mL/min, 20 mL/min, and 25 wt.%, respectively. It can be seen that the iron recovery ratio and grade of concentrate significantly increase with increasing modification temperature from 1173 to 1323 K. Namely, the iron recovery ratio and grade of concentrate are increased from 56.43% and 42.16% to 74.16% and 54.89% with increasing modification temperature from 1173 to 1323 K, respectively. However, with further increasing the modification temperature to 1375 K, both the iron recovery ratio and grade of concentrate show a downward trend from 74.16% and 54.89% to 67.67% and 53.82%, respectively. Meanwhile, it can be clearly seen that the change of iron grade of tailing is different from the other two situations, which fluctuated with increasing modification temperature and the maximum 28.12% and minimum 24.57% appear in 1173 and 1323 K, respectively. Higher temperature is favorable for dissociation of fayalite in copper slag and oxidation of free FeO, and the binding reaction of CaO with free SiO2 is also an endothermic reaction. Therefore, the mass of magnetite and calcium silicate gradually increases with increasing modification temperature, which contribute to beneficiation and recovery of iron from copper slag by following magnetic separation. However, as the temperature continues to rise to be higher than 1323 K, CaSiO3 will recombine with the excess FeO to form CaFeSiO4 because of high dissociation rate of fayalite, which hinders the recovery of iron from copper slag.
Figure 7 shows the XRD patterns of the copper slag at different modification temperatures from 1173 to 1373 K. As we all know, the intensity and quantity of peaks in XRD patterns are important parameters to exhibit the mass content of samples [24]. The original copper slag (Fig. 7, copper slag) contains intensive fayalite and magnetite peaks due to the matte smelting process or cooling. The higher modification temperature provided an excellent oxidation conditions to slag to produce considerable magnetite. As can be seen from the modified slag (Fig. 7, 1173 K), the fayalite peaks were reduced to become smaller and the magnetite peaks were increased to become stronger, which indicated that the fayalite in copper slag was gradually oxidized to magnetite. In addition, there are less intensive kirschsteinite peaks and hardly any other peaks were observed in modified copper slags with modification temperature at 1223 and 1273 K, which indicated that the purity of magnetite in modified copper slags is very high as we expected. However, the peaks of kirschsteinite have increased a lot and the peaks of fayalite reproduced in Fig. 7 at 1373 K, which explained the tendencies of iron grade and recovery ratio in Fig. 6. In consideration of the efficiency and energy saving of the experiment, 1323 K was selected as the optimum modification temperature.
3.2.2 Effect of roasting time
The effect of roasting time on the oxidized modification followed by magnetic separation of copper slag was investigated. The experiments were performed at a modification temperature of 1323 K, CaO content of 25 wt.%, and the gas flowrate of CO2 and CO of 180 and 20 mL/min, respectively. The experimental results are shown in Fig. 8.
As shown in Fig. 8, when the roasting time ranges from 1 to 2 h, increasing the roasting time benefits iron recovery of iron-bearing concentrates, the total iron grade of concentrate increases from 47.22% to 55.07% and iron recovery ratio significantly increases from 63.81% to 72.62%, because prolonging time is beneficial to the dissociation of fayalite and free FeO can be oxidized as much as possible to magnetite. However, when roasting time is extended to 4 h, the total iron grade of concentrate dramatically decreases from 55.07% to 45.44% and iron recovery ratio significantly decreases from 72.62% to 67.23%, and then, both reach a platform. The total iron grade of magnetite tailing changes slightly between 29.26% and 31.12% when the roasting time ranges from 1 to 4 h. This means that too long roasting time is not conducive to the most effective recovery of iron from copper slag, because FeO has been greatly oxidized in 1–2 h of reaction time and magnetite is constantly precipitated. These magnetites adhere to the surface of unoxidized copper slag, hindering the separation of copper slag and the contact of gas oxidants. Furthermore, calcium oxide will combine with iron olivine to form calcium iron olivine due to the poor reaction kinetics, as a result of the attachment of magnetite. Therefore, from a practical point of view, the roasting time is fixed at 2 h in the subsequent experimental series.
3.2.3 Effect of gas flowrate of CO and CO2
The effect of the gas flowrate of CO and CO2 on the oxidized modification followed by magnetic separation of copper slag was investigated. The total gas flowrate remains constant at 200 mL/min, and the CO2 and CO gas flowrates are 170–200 and 0–30 mL/min, respectively, and other parameters are constant. The experimental results are shown in Fig. 9.
With an increase in the gas flowrate of CO2 from 170 to 200 mL/min and a decrease in the gas flowrate of CO from 30 to 0 mL/min, the iron grade of concentrate has a tendency of first rise before declining, while the iron grade of tailing has a tendency of first decline before rising, and iron recovery has continued to increase but slowly when the gas flowrate of CO2 is greater than 190 mL/min. When the gas flowrates of CO2 and CO are 190 and 10 mL/min, respectively, an iron grade of 55.56% and 26.24% of concentrate and tailing can be obtained at the iron recovery ratio of 65.29%. No direct measurements have been made for the interracial rate of oxidation of iron oxide by CO2, but it is now well accepted that the linear stage of oxidation of solid iron by CO2 at elevated temperatures is controlled by the rate of the interfacial reaction between CO2 and solid iron oxide [25, 26]. With the increase in CO2 gas flowrate, the partial pressure of CO2 increases gradually in the whole reaction system, which effectively improved the reaction rate of CO2 and free solid ferric oxide in copper slag. Based on the database of FactSage, iron oxides exist in magnetite in the Fe–C–O phase diagram with temperature of 1273 K [19]. Therefore, it is beneficial to the separation of iron from copper slag by magnetic separation.
According to the usual understanding, CO2 dissociation on the surface of metal and oxides containing iron oxide can be expressed as follows [27]:
With the continuous increase in CO2 flowrate, the oxygen partial pressure in the system will increase. Excessive partial pressure of oxygen may oxidize magnetite to iron oxide (Fe2O3), resulting in loss of recovered iron concentrate, which coincides with the change trend of iron grade in Fig. 9. Therefore, the flowrates of CO2 and CO were 180 and 20 mL/min, respectively, in a series of experiments.
3.2.4 Effect of dosage of flux (CaO)
The experiments were performed under the conditions of modification temperature of 1323 K, gas flowrates of CO2 and CO of 180 and 20 mL/min, respectively, CaO content from 0 to 25 wt.%, and roasting for 2 h. The histograms in Fig. 10 show the effect of dosage of CaO on the oxidized modification and magnetic separation.
As shown in Fig. 10, the iron grade of concentrate and tailing and iron recovery ratio of raw copper slag by direct magnetic separation are 48.97%, 39.64% and 33.93%, respectively. When the dosage of calcium oxide was added from 0 to 25 wt.%, the iron recovery ratio of modified slag increased significantly from 47.16% to 74.16%, and the iron grade of concentrate increased slowly from 51.16% to 54.89%, while the iron grade of tailing reduced dramatically from 33.48% to 24.27%, indicating that CaO is well-suited to cause the magnetite to reshape and coalesce. Similar to Fe2+ in fayalite, Ca2+ is the network modifier cation that destroys the 3D interconnected network of the fayalite; thus, the presence of calcium oxide together with Fe2+ decreases the viscosity of the silicate [28] and calcium oxide accelerates the oxidation reaction of Fe2SiO4 at lower temperature [29].
The XRD patterns of modified slag with different dosages of CaO are shown in Fig. 11. Compared with the XRD pattern of original copper slag, shape of most peaks in XRD pattern of modified slag without CaO has remained little change, and the fayalite peaks were still strong, indicating that oxidizing roasting of copper slag without CaO is not conducive to iron recovery. Owing to the presence of CaO, more and more calcium silicate and magnetite have been produced, which can be seen from the changes of peaks in the XRD diagram. However, there are many high intensity kirschsteinite peaks found in the modified slag with 15 wt.% CaO, which leads to a reduction in iron recovery. Therefore, the optimum dosage of CaO is 25 wt.% in experiments.
3.3 Magnetic separation of modified copper slag
It was found that the modified slag contained not only Fe element, but Mg, Cu, Si and Ca elements, implying that iron grade was at a relatively low level (about 45%). It was necessary to consider the influence factors on magnetic separation in more detail.
3.3.1 Effect of magnetic flux density
The magnetic flux density is an important factor on the magnetic separation. Lower magnetic flux density is used to separate ferromagnetic materials, such as magnetite and metallic iron from the surrounding gangue and so on. In magnetic separation of nonmagnetic minerals, a higher magnetic flux density is necessary. The effect of magnetic flux density on the magnetic separation of modified slag was investigated, and the experimental results are shown in Fig. 12.
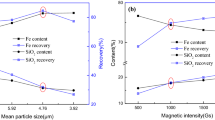
Figure 12 shows that the iron grade of concentrate decreases, and the iron grade of tailing and iron recovery increase with increasing field intensity, which possibly keeps intermixed or weak magnetism particles from becoming trapped in the concentrate. Although a high magnetic field intensity of 320 mT can recover 88.05% of iron in copper slag, the iron grade of concentrate is very low (only 38.8%), and the grade of iron of tailing is too much (26.32%). A low field intensity of 120 mT may lead to an increase in the exclusion of magnetite particles by the separator, which leads to a reduction in the recovery of iron. Therefore, 170 mT is selected as the most suitable magnetic flux density in the current experiments.
3.3.2 Effect of particle size
It is well known that particle size is the other important factor in magnetic separation, and too small or too large size results in loss of magnetite. The histograms in Fig. 13 show the effect of particle size of modified slag on the magnetic separation.
It can be seen from Fig. 13 that with decreasing particle size, the iron recovery and iron grade of concentrate firstly increased to a maximal value at 25.0–38.5 μm and then decreased, which might mainly be attributed to the liberation of magnetite from calcium silicate and kirschsteinite. Meanwhile, with the increase in fineness, the loss of fine magnetite particles can be easily observed, and the iron recovery will be reduced. As the particle size was smaller than 25 μm, it was difficult to separate magnetite particles because of magnetic agglomeration, which prevented the removal of nonmagnetic silicate particles. At the optimum particle size of 25.0–38.5 μm, the recovery ratio of iron is 80.14%, and the iron grades of concentrate and tailing are 54.79% and 22.12%, respectively.
3.4 Product examination
3.4.1 Mineralogical analysis of modified slag
Through the analysis of the above experiments, a satisfactory modified product was obtained under the conditions of mixing the raw copper slag with 25 wt.% CaO for 2 h and oxidizing roasting mixed material at 1323 K for 2 h with the gas flowrates of CO2 and CO of 180 and 20 mL/min, then grinding the modified slag to 25.0-38.5 μm, and magnetic separation at 170 mT. The XRD analysis was carried out to reveal the mineral compositions of satisfactory modified slag, and the result is shown in Fig. 14.
It can be seen from Fig. 14 that the major minerals constituents are magnetite, wollastonite (CaSiO3) and kirschsteinite. In addition, a broad band indicating the presence of glass phase materials was observed between 10° and 40° in XRD patterns, which could be inferred that there was glass phase formed in the modified slag during oxidizing roasting. Furthermore, the intensity and quantity of magnetite peaks are more than those of others, and there are only a few weak kirschsteinite peaks. These results indicate that most of the fayalite in copper slag has been converted into magnetite and wollastonite by oxidizing roasting, as expected. Moreover, the modified slag can be separated by simple magnetic separation to obtain concentrate which meets the requirements of ironmaking and tailing which can be used for building materials such as cement.
3.4.2 Microstructural analysis of modified slag
The SEM image and EDS analysis of modified slag obtained under optimum conditions are shown in Fig. 15. The bright phase (point 1), the dark gray phase (point 2) and the light gray phase (point 3) are observed by EDS and found to be magnetite, kirschsteinite and calcium silicate (CaSiO3), respectively. This is consistent with the analysis result of X-ray diffraction pattern. Magnetite in the SEM image occupies a larger area than others, and its maximum size is more than 40 μm, indicating that the iron-bearing minerals in the copper slag were oxidized, the generated magnetite has grown into large particles, and the silicate in copper slag has been combined with calcium oxide to form calcium silicate. In addition, the Fe content of magnetite (point 1) is 73.12 wt.%, which indicates that the purity of magnetite is very high and there is hardly any other impurity element. Although kirschsteinite (point 2) and CaSiO3 also contain 33.74 and 9.54 wt.% iron, respectively, their proportion in modified copper slag is small, especially kirschsteinite can hardly be seen in SEM image of modified copper slag, which reveals that the fayalite in copper slag has been oxidized to magnetite and nonmagnetic calcium silicate; with the help of a mixture of calcium oxide and CO and CO2 mixed gas, the desired separation of iron and other impurities has already realized.
3.4.3 Chemical analysis of magnetic separation production
For iron and steel metallurgy, the Cu content in Fe-bearing concentrate is very important; if the Cu content is over 0.3 wt.%, the concentration is not suitable for ironmaking blast furnace. Therefore, the impurity content of Fe-bearing concentrate and tailing was analyzed by ICP-AES and X-ray fluorescence. Table 2 shows the impurity contents of the concentrate and the tailing. In addition, Table 3 shows chemical composition of Fe-bearing concentrate. From the above analysis, it is obvious that the effective recovery of iron in copper slag was realized, and the concentrate and tailings used for ironmaking and building materials are obtained synchronously.
4 Conclusions
-
1.
The copper slag was assayed and found to be composed of 40.44 wt.% iron, and the main ferrous minerals in copper slag were magnetite and fayalite. It is difficult to recover iron from copper slag by traditional separation method.
-
2.
Modified roasting in CO–CO2 mixed gas followed by magnetic separation was used to recover iron from copper slag. By analyzing the influence of various factors on the experimental results, the obtained optimum conditions for separation by oxidizing roasting and magnetic separation are as follows: calcium oxide content of 25 wt.%, mixed gas of CO2 and CO with flowrate of 180 and 20 mL/min, oxidizing roasting at 1323 K for 2 h, grinding the modified slag to 25.0–38.5 μm, and magnetic separation at 170 mT. Under these conditions, the iron-bearing concentrate with an iron grade of 54.79% and iron recovery ratio of 80.14% were effectively obtained.
-
3.
The mineralogical and microstructural characteristics of modified slag revealed that the iron-bearing minerals in the copper slag were oxidized, and the generated magnetite has grown into large particles and the silicate in copper slag has been combined with calcium oxide to form calcium silicate. After magnetic separation, the effective recovery of iron in copper slag was realized, and the concentrate and tailings used for ironmaking and building materials are obtained synchronously.
References
C.J. Shi, C. Meyer, A. Behnood, Resour. Conserv. Recycl. 52 (2008) 1115–1120.
B. Gorai, R.K. Jana, Resour. Conserv. Recycl. 39 (2003) 299–313.
H.T. Shen, E. Forssberg, Waste Manag. 23 (2003) 933–949.
P.K. Gbor, V. Mokri, C.Q. Jia, J. Environ. Sci. Health Part A 35 (2000) 147–167.
M. Najimi, A.R. Pourkhorshidi, Mag. Concr. Res. 63 (2011) 605–615.
H.Y. Cao, N.X. Fu, C.G. Wang, L. Zhang, F.S. Xia, Z.T. Sui, N.X. Feng, Multipurp. Util. Min. Resour. (2009) No. 2, 8–10.
U. Brinkmann, W. Laqua, Phys. Chem. Miner. 12 (1985) 283–290.
J.L. Zeng, K.M. Xiao, Nonferrous Met. Sci. Eng. 2 (2011) No. 6, 71–73.
T.J. Veasey, J. Process Mech. Eng. 211 (1997) 61–64.
Z. Cao, T. Sun, X. Xue, Z. Liu, Minerals 6 (2016) 119.
B.S. Kim, S.K. Jo, D. Shin, J.C. Lee, S.B. Jeong, Int. J. Miner. Process. 124 (2013) 124–127.
R.M. Jiao, X. Peng, C.Y. Wang, B.Z. Ma, Y.Q. Chen, Int. J. Miner. Metall. Mater. 24 (2017) 974–982.
Y.B. Ma, X.Y. Du, Y.Y. Shen, G.Z. Li, M. Li, Metals 7 (2017) 321.
Y. Fan, E. Shibata, A. Iizuka, T. Nakamura, Mater. Trans. 55 (2014) 958–963.
Y. Fan, E. Shibata, A. Iizuka, T. Nakamura, Metall. Mater. Trans. B 46 (2015) 2158–2164.
Y. Fan, E. Shibata, A. Iizuka, T. Nakamura, Metall. Mater. Trans. B 47 (2016) 2754–2760.
Z.Q. Guo, D.Q. Zhu, P. Jian, T.J. Wu, F. Zhang, Metals 6 (2016) 86.
Z.Q. Guo, D.Q. Zhu, P. Jian, F. Zhang, JOM 68 (2016) 2341–2348.
X.J. Hu, T. Zhang, H.Y. Yan, H. Matsuura, F. Tsukihashi, K.C. Chou, ISIJ Int. 52 (2012) 1529–1534.
J.H. Heo, B.S. Kim, J.H. Park, Metall. Mater. Trans. B 44 (2013) 1352–1363.
J.H. Hu, H. Wang, L.M. Zhao, L. Li, H.L. Liu, J. Saf. Environ. 11 (2011) No. 2, 90–93.
D. Durinck, F. Engström, S. Arnout, J. Heulens, P.T. Jones, B. Björkman, B. Blanpain, P. Wollants, Resour. Conserv. Recycl. 52 (2008) 1121–1131.
Z.Q. Guo, D.Q. Zhu, J. Pan, F. Zhang, J. Clean. Prod. 187 (2018) 910–922.
J.R. Garcia, M. Suarez, C.G. Guarido, J. Rodriguez, Anal. Chem. 56 (1984) 193–196.
E.T. Turkdogan, J.V. Vinters, Metall. Trans. 3 (1972) 1561–1574.
Y. Sasaki, S. Hara, D.R. Gaskell, G.R. Belton, Metall. Trans. B 15 (1984) 563–571.
Y. Li, J.A. Lucas, G.M. Evans, I.P. Ratchey, G.R. Belton, Metall. Mater. Trans. B 31 (2000) 1049–1057.
H.R. Fernandes, A. Gaddam, D.U. Tulyaganov, J.M.F. Ferreira, J. Non-Cryst. Solids 406 (2014) 54–61.
Z.H. Yang, Z.H. Ma, Steel Res. Int. 88 (2017) 1600145.
Acknowledgements
The authors wish to express thanks to National Natural Science Foundation of China (Grant No. 51774154) and the Jiangxi Natural Science Foundation (Grant No. 20151BAB206029) for the financial support for this research. And we also thank the Testing Center of Jiangxi University of Science and Technology for testing of samples.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jiang, Pg., Liu, Js., Xiao, Yy. et al. Recovery of iron from copper slag via modified roasting in CO–CO2 mixed gas and magnetic separation. J. Iron Steel Res. Int. 27, 796–806 (2020). https://doi.org/10.1007/s42243-020-00413-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42243-020-00413-0