Abstract
FeO-containing slag originated from the basic oxygen furnace to the ladle is a major reoxidation source during the following secondary refining. Ladle slag reduction treatment (slag treatment) is one of the common countermeasures adopted to eliminate the steel contamination by FeO reoxidation. The oxygen transfer phenomenon between molten steel and slag was studied during the industrial production of interstitial-free (IF) steel, the measured and calculated oxygen activities in steel were compared, and the Fe–O equilibrium at the slag–molten steel interface was investigated by thermodynamic analysis. With slag treatment, the oxygen potential is higher in the molten steel than in the pre-deoxidation slag; this causes oxygen transfer from the molten steel to the slag, decreasing the efficiency of slag treatment. Based on this, a two-step slag deoxidation process was optimized. The second step further reduced the FeO content. On the other hand, the CaO/Al2O3 (C/A) ratio in the refining slag must be controlled, because it affects the FeO activity and inclusion absorption capacity of the slag. The results suggest that the C/A ratio of 1.2–1.5 and the FeO content of < 6% are beneficial to refine IF steel.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Interstitial-free (IF) steels are widely used in the industry of automobiles and household electrical appliances. As the demand for high-quality steels continues to increase, the control of surface quality and the uniformity of mechanical property for IF steels has become increasingly important in recent times. Al2O3 inclusion is one of the common inclusions observed in molten steel and the final product for the production of IF steel. The Al2O3 inclusion is generally considered harmful because several studies have shown that the surface deterioration of steel is often related to Al2O3 inclusions [1,2,3,4,5]. In addition, Al2O3 can easily deposit on the inner wall of submerged entry nozzle and disturb the casting process [6, 7]. Therefore, the control of the generation of Al2O3 inclusions is currently attracting worldwide research attention [8, 9].
Al2O3 usually forms in molten steel during deoxidation in the RH process. Nevertheless, when using a basic oxygen furnace (BOF), some BOF slags are unavoidably poured into ladle along with molten steel during tapping even when lots of countermeasures are conducted to prevent slag carry-over. FeO is one of the main components of BOF slag; therefore, the FeO content in the refining slag is high. Generally, after deoxidation of molten steel, oxygen is transferred from the FeO-containing slag to the molten steel because the molten steel has a lower oxygen potential than the slag, which leads to the steel reoxidation and the new generation of inclusions in steel [10]. Lee et al. [11] found that the number of defects on cold-rolled sheets diminishes with a diminishing FeO + MnO content of ladle slag at the end of the secondary refining. Wang et al. [12] built a prediction model of the content of total oxygen (TO) in molten steel based on the equilibrium between reoxidation rate and inclusion removal rate, and their research showed that reoxidation rate of oxidizing slag to the molten steel was dominated by the mass transfer of reducible oxides (FeO + MnO) in the slag. Lee et al. [13] found that the reoxidation rate increased linearly with increasing FeO content if the FeO content in slag was higher than 1.5 wt.%, and it decreased with the increasing (CaO)/(Al2O3) ratio of the slag for a given FeO content. Qin et al. [14] found that the cleanliness of molten steel refined through slag with a lower content of FeO (7−9 mass%) is much better than that refined by slag with a higher content of FeO (21 mass%). From the above analysis, it can be concluded that the FeO content in slag should be reduced to improve the cleanliness of the steel.
Slag treatment is a process after tapping of BOF to lower the FeO content in slag. In this process, a slag conditioner (i.e., slag reduction or deoxidation treatment), which is a mixture of aluminum and burnt lime or limestone, is added into the slag to lower FeO and MnO contents of the ladle slag [9]. During RH refining, the slag can solidify easily after decarburization (de-C) of molten steel, which makes it difficult to conduct deoxidation of slag and molten steel simultaneously. Therefore, slag treatment after tapping is generally adopted, such as the steelmaking process of Baosteel and POSCO [13, 15]. However, this process has the disadvantage that the deoxidized slag can be oxidized by un-killed steel during decarburization process and subsequently become a potential source of reoxidation for molten steel after molten steel deoxidation. In addition, the mechanism of the transfer of oxygen between steel and slag remained unclear during this process.
In this study, the Fe–O equilibrium at the slag–steel interface was investigated by both industrial experiment and thermodynamic analysis. Based on the results, the mechanism of oxygen transfer between molten steel and slag was proposed. In addition, based on the proposed mechanism, a new process for the production of IF steel is proposed.
2 Experimental method
In this study, experiments were conducted during the production of IF steel and the typical composition of the IF steel is shown in Table 1. The general production process of IF steel follows the route of KR (knotted reactor) desulfurization → BOF → RH → CC (continuous casting). After KR desulfurization, the hot metal was sent to a converter for steelmaking. Then, the steel was tapped. Slag deoxidant which was a mixture of aluminum and burnt lime was added into the slag after BOF tapping to reduce the FeO content in the slag. From the end of RH treatment to CC, the steel was not stirred, which is named as holding process, to promote the removal of inclusions for approximately 10 min.
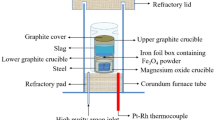
The schematic of the sampling is shown in Fig. 1. Steel sample was obtained by barrel type samplers (inner diameter of 45 mm and height of 100 mm) at the beginning of RH, the end of decarburization, as well as 0, 5, and 10 min after RH treatment. Slag sample was obtained by immersing a steel rod into the bulk of slag. Slag samples were collected just after tapping, the beginning of RH, the end of decarburization, the end of RH treatment and the end of holding process. In this study, two heats of experiments on a scale of 210 t were conducted.
The Al content in molten steel was analyzed by inductively coupled plasma–atomic emission spectroscopy (ICP-AES). The dissolved oxygen content in molten steel was measured using a solid electrode sensor with Cr/Cr2O3 as reference electrode. The other alloying elements in molten steel were measured by spark discharge emission spectrometry method. The composition of slag was analyzed using an X-ray fluorescence spectrometer. The details of these methods were summarized by Zhang et al. [9].
3 Experimental results
The compositions of Al and dissolved oxygen in molten steel during the production of IF steel are shown in Fig. 2. The contents of other elements in steel are shown in Table 1. According to Fig. 2, it is clear that before steel deoxidation during RH treatment, the dissolved oxygen content in steel was high (0.04–0.05 mass%) as the Al content in steel was almost zero. The high concentration of dissolved oxygen was used for decarburization. After steel deoxidation by Al during RH treatment, the Al content in steel increased to about 0.03–0.04 mass% and the content of dissolved oxygen decreased simultaneously to about 3 × 10−6. The concentration of other elements in steel did not show much change as the surface of the top slag was solidified. During IF production, Ti was added into the steel for the stabilization of remained elements of C and N; therefore, the content of Ti increased immediately after the addition of Ti alloy during RH treatment.
The content of FeO + MnO (reducible oxide) in slag during the production of IF steel is shown in Fig. 3. The contents of other components in slag are shown in Table 2. From Fig. 3, it is clear that the initial content of reducible oxide was high (more than 20 mass%) before slag treatment. By the slag treatment during tapping, the content of reducible oxide decreased greatly to lower than 10 mass%. However, after slag treatment, the concentration of reducible oxide increased to about 12 mass% and then began to decrease after steel deoxidation. The concentrations of other oxides in slag did not show much change after slag treatment.
The typical morphology of the observed inclusion is shown in Fig. 4. As seen, single Al2O3 (including irregular, spherical and rod-like), Al2O3 cluster, and Al2O3–TixO inclusions (including single and cluster) were observed, and some Al2O3 inclusions are larger than 50 µm. During the holding period at the end of the ladle process, the concentration of reducible oxide in slag was high (12 mass%); therefore, no MgO·Al2O3 spinel inclusion was observed in steel, and only Al2O3 and Al2O3–TixO inclusions were detected. Inclusion of large size is harmful to the surface quality of IF steel regardless of the inclusion type [2]. Therefore, in this study, the inclusion is classified by the size of inclusion. The size distribution of the inclusion in heats 1 and 2 is shown in Fig. 5, respectively. It is clear that most inclusions were in small size (< 10 μm) during the holding period at the end of the ladle process. The number density of inclusions increased as the treatment time extended, which indicated that the rate of reoxidation by reducible oxide in slag is higher than that of inclusion removal by floatation.
4 Discussion
For Al-killed steel, the content of dissolved oxygen is controlled by reaction (1). The equilibrium constant (K1) of reaction (1) is shown in Eq. (2), which is a constant related to temperature.
where \(a_{({{\text{Al}}_2}{{\text{O}}_3})}\) is the activity of Al2O3; a[Al] and a[O] are the activities of aluminum and oxygen, respectively; f[O] and f[Al] are the activity coefficients of oxygen and aluminum in the molten steel, respectively; w[j] is the concentration of element j in the molten steel; and ejO and ejAl are the interaction coefficients of oxygen and aluminum in the molten steel, respectively.
Many studies have been conducted to investigate the value of K1 and the frequently used data collected at 1873 K are presented in Table 3 [16,17,18,19]. The published values of K1 did not show much difference; therefore, lg(K1) = 13.6 was used in the present study.
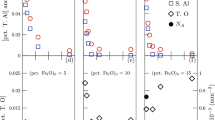
After steel deoxidation by Al, dissolved Al and dissolved O react with each other as shown in Eq. (1). When reaction (1) reaches equilibrium, the contents of Al and O in the molten steel can be calculated by Eqs. (2–6). The interaction coefficients used for thermodynamic calculation are listed in Table 4 [16]. For the calculation, the activity of Al2O3 is very important. A lot of researchers took the value of the Al2O3 activity in top slag into consideration because they assumed that the equilibrium between slag and molten steel was achieved at the slag–steel interface. However, in this study, the top slag contained a high concentration of reducible oxide, which had the priority to react with Al in molten steel and the treatment time in this study was too short (10 min after steel deoxidation) to reach slag-steel equilibrium. Recently, Liu et al. [20] found that the reaction rate between steel and inclusion was quick and the steel–inclusion equilibrium could be reached within seconds. Therefore, in this study, the Al2O3 activity in Al2O3 inclusions, which is assumed to be unity, and the Al2O3 activity in slag were both taken into consideration. The activity of Al2O3 in slag was calculated by FactSage. The temperature for calculation was 1873 K, and the slag composition for calculation was the values listed in Table 5. The calculated results together with more than 100 sets of measured data are shown in Fig. 6.
In Fig. 6, the experimental results agreed well with the calculation assuming that the activity of Al2O3 is unity, which indicated that dissolved oxygen and aluminum in molten steel were in equilibrium with Al2O3 inclusions rather than Al2O3 in the top slag under the condition of this study.
After the determination of the Al–O equilibrium in molten steel, the transfer of oxygen between slag and steel during IF steel production is discussed in the following part. As shown in Fig. 3, by the slag treatment, the content of reducible oxide decreased greatly. However, after slag treatment, the content of reducible oxide increased gradually.
The equilibrium relationship between FeO in slag and molten steel can be expressed as Eq. (7). In fact, the activity of Fe in Eq. (7) can be considered as unity; thus, according to Eq. (8), the dissolved oxygen activity is only determined by the FeO activity at a certain temperature T [Eq. (9)]. The relationship between the dissolved oxygen content in the molten steel and the FeO content in the slag can be described as Eq. (10). In this calculation, f[O] and the activity coefficient of FeO in slag γFeO can be calculated by Eqs. (2) and (11) [10], respectively.
where a(FeO) is the activity of FeO; a[Fe] is the activity of Fe; and NFeO is the molecular weight of FeO in slag, g/mol.
The calculated equilibrium content of dissolve oxygen in molten steel in equilibrium with the FeO-containing slag together with the experimental results in the experiment of heat 2 is shown in Fig. 7. It can be seen that the calculated oxygen content in molten steel was more than 200 × 10−6 before steel deoxidation (tapping, beginning of RH, and end of de-C), which is much lower than the measured oxygen content in molten steel. This indicated that the oxygen should transfer from the molten steel to the slag at these stages, which led to an increase in the reducible oxide content in top slag and a decrease in oxygen content in steel, as shown in Figs. 2 and 3. At the end of decarburization, the FeO content in slag increased to 11.24 mass% from 6.34 mass% after slag treatment during tapping. After steel deoxidation, the content of dissolved oxygen in the molten steel decreased immediately to (3–5) × 10−6, while the FeO content in slag was still high. Therefore, the oxygen at the molten steel–slag interface was transferred from the slag to the molten steel, which led to the reoxidation of steel by slag. From Fig. 3, it is clear that the content of reducible oxide decreased slightly after steel deoxidation, which confirmed that oxygen transferred from the slag to the molten steel after molten steel deoxidation.
The reoxidation is harmful to steel quality. As shown in Fig. 2, the average Al loss is approximately 21 × 10−6 for heat 1 and 14 × 10−6 for heat 2 from the end of RH treatment to the beginning of CC. In addition, the reoxidation led to the new generation of inclusions in steel, as shown in Fig. 5.
Based on the above analysis, the reoxidation of molten steel by slag should be suppressed to improve the cleanliness of molten steel. The best method is to conduct deoxidation of slag and molten steel simultaneously. However, during RH refining, the difficulty for the slag treatment is the solidification of the surface of slag. From Fig. 3, it is clear that the content of reducible oxide increased gradually before molten steel deoxidation, which indicated that the molten steel and slag did not reach equilibrium during the period from the slag treatment to the molten steel deoxidation. Therefore, in order to suppress the reoxidation of molten steel by slag after molten steel deoxidation, it is better to strengthen the slag treatment after tapping. By the application of ladle lid to melt the solidified slag, it is better to conduct slag treatment again after molten steel deoxidation if possible. The proposed new process is shown in Fig. 8.
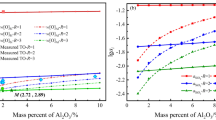
During holding process, inclusion removal by floatation and inclusion generation by reoxidation happened simultaneously. If the rate of inclusion formation by reoxidation was lower than that of inclusion removal, holding process can be applied to improve the cleanliness of the molten steel. The rate of inclusion formation by reoxidation increased with the activity of reducible oxide in slag [21]. Therefore, it is necessary to decrease both the content of reducible oxide and the activity coefficient of reducible oxide in slag as low as possible. The countermeasures for the decrease in FeO content in slag was discussed in the previous part by the slag treatment twice. For the countermeasures to decrease the activity coefficient of reducible oxide in slag, researchers found that the C/A ratio significantly affects the activity of FeO [22, 23]. The activity coefficient lines of CaO–Al2O3–FeO slag calculated by FactSage software are shown in Fig. 9. The area outlined by solid lines is the liquid region of the slag at 1873 K. In the liquid region, with increasing the C/A ratio in the slag, the activity of FeO in the slag decreased.
Meanwhile, the removal rate of inclusion should be enhanced to increase the cleanliness of molten steel. Valdez et al. [24] investigated the capacity of slag of Al2O3–CaO–SiO2 system to absorb inclusions. The capacity of inclusion absorption was clearly increased with the C/A ratio in slag, thus explaining the improvement of the cleanliness of the molten steel. These indicate that the C/A ratio in the refining slag should be controlled as well. The optimal composition of slag for the production of IF steel is marked in Fig. 9. In this study, the C/A ratio of 1.2–1.5 and the FeO content of less than 6% are recommended to refine IF steel with high cleanliness.
5 Conclusions
-
1.
The Al and O in molten steel are in equilibrium with the Al2O3 in inclusion rather than that in top slag.
-
2.
After slag treatment, the activity of oxygen in molten steel is larger than that in slag; thus, the oxygen is transferred from the molten steel to the slag. After steel deoxidation, the activity of oxygen in molten steel became extremely low (3 × 10−6) and the oxygen transferred from the slag to the molten steel.
-
3.
Besides FeO, the C/A ratio is an important factor affecting the activity of FeO and the inclusion absorption capacity of the slag. In this study, the C/A ratio of 1.2–1.5 and the FeO content of < 6% are recommended to refine IF steel.
References
X.X. Deng, C.X. Ji, W.L. Dong, L.P. Li, X. Yin, Y.D. Yang, A. McLean, Ironmak. Steelmak. 45 (2018) 592-602.
X.X. Deng, C.X. Ji, Y. Cui, Z.H. Tian, X. Yin, X.J. Shao, Y.D. Yang, A. McLean, Ironmak. Steelmak. 44 (2017) 739-749.
C. Bonilla, in: 78th Steelmaking Conference Proceedings, Iron and Steel Society of AIME, Warrendale, PA, USA, 1995, pp. 743–752.
G. Domizzi, G. Anteri, J. Ovejero-García, Corros. Sci. 43 (2001) 325–339.
H. Yasunaka, R. Yamanaka, T. Inoue, T. Saito, Tetsu-to-Hagane 81 (1995) 529–534.
K.G. Rackers, B. Thomas, in: 78th Steelmaking Conference Proceedings, Iron and Steel Society of AIME, Warrendale, PA, USA, 1995, pp. 723–734.
S. Basu, S.K. Choudhary, N.U. Girase, ISIJ Int. 44 (2004) 1653–1660.
Y. Sahai, Metall. Mater. Trans. B 47 (2016) 2095–2106.
L. Zhang, B. Thomas, X. Wang, in: 85th Steelmaking Conference Proceedings, Iron and Steel Society of AIME, Warrendale, PA, USA, 2002, pp. 431–452.
H. Ohta, H. Suito, Metall. Mater. Trans. B 29 (1998) 119–129.
K.K. Lee, J.M. Park, J.Y. Chung, S.H. Choi, S.B. Ahn, Rev. Met. Paris 93 (1996) 503-509.
M. Wang, Y.P. Bao, H. Cui, W.S. Wu, H.J. Wu, J. Univ. Sci. Technol. Beijing 32 (2010) 432-437.
K.Y. Lee, J.M. Park, C.W. Park, in: VII International Conference on Molten Slags Fluxes and Salts, The South African Institute of Mining and Metallurgy, Stockholm, Sweden, 2004, pp. 601–606.
Y. Qin, X. Wang, F. Huang, B. Chen, C. Ji, Metall. Res. Technol. 112 (2015) 405.
L. Zhang, J. Zhi, F. Mei, L. Zhu, X. Jiang, J. Shen, J. Cui, K. Cai, B.G. Thomas, Ironmak. Steelmak. 33 (2006) 129-139.
Japan Society for the Promotion of Science (JSPS), Steelmaking data sourcebook, Revised ed., Gordon and Breach Science Publishers, New York, USA, 1988.
H. Itoh, M. Hino, S. Ban-Ya, Metall. Mater. Trans. B 28 (1997) 953–956.
J.D. Seo, S.H. Kim, K.R. Lee, Steel Res. Int. 69 (1998) 49–53.
R.J. Fruehan, Metall. Trans. 1 (1970) 2083–2088.
C. Liu, M. Yagi, X. Gao, S.J. Kim, F. Huang, S. Ueda, S.Y. Kitamura, Metall. Mater. Trans. B 49 (2018) 113-122.
Y. Qin, X. Wang, L. Li, F. Huang, Steel Res. Int. 86 (2015) 1037–1045.
V. Espejo, M. Iwase, Metall. Mater. Trans. B 26 (1995) 257-264.
Y. Ji, C. Liu, Y. Lu, H. Yu, F. Huang, X. Wang, Metall. Mater. Trans. B 49 (2018) 3127-3136.
M. Valdez, G.S. Shannon, S. Sridhar, ISIJ Int. 46 (2006) 450–457.
Acknowledgements
The authors are grateful for financial support from the National Natural Science Foundation of China under Grant No. 51404020 and the National Key R&D Program of China under Grant Nos. 2017YFB0304000 and 2017YFB0304001.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ji, Yq., Liu, Cy., Yu, Hx. et al. Oxygen transfer phenomenon between slag and molten steel for production of IF steel. J. Iron Steel Res. Int. 27, 402–408 (2020). https://doi.org/10.1007/s42243-019-00285-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42243-019-00285-z