Abstract
The effect of Al2O3/SiO2 and CaO/Al2O3 ratios on the molten structure of CaO–SiO2–Al2O3-based mold flux system was analyzed by Fourier transform infrared spectroscopy, Raman spectroscopy, and X-ray photoelectron spectroscopy. Also, the variation in the wettability between the mold flux system and an interstitial free (IF) steel substrate was investigated using the sessile drop method. The results indicate that the contact angle and interfacial tension between the molten slag and solid steel increase slightly with the increase in the Al2O3/SiO2 ratio, while they decrease with the increase in the CaO/Al2O3 ratio. The network structure for the designed mold flux system changes gradually from silicate to aluminosilicate and aluminate with the increase in the Al2O3/SiO2 ratio, and the network is simplified with the increase in the CaO/Al2O3 ratio. Besides, combining the results of sessile drop method and melt structure analyses, it suggests that the variation in interfacial properties of mold flux/IF steel substrate is mainly caused by the change in melt structure, especially the variation in free oxygen ions (O2−) and non-bridged oxygen (O−) at the interface.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In recent years, researches regarding the advanced high strength steels (AHSS) have been conducted intensively [1, 2]. However, the high content of Al in advanced high strength steel tends to react with the reducing oxides (e.g., SiO2) in the mold flux during the continuous casting process, which results in the pickup of Al2O3 in the spent mold flux and deteriorates the process of continuous casting. In order to avoid the problems caused by slag/steel reactions, a new non-reactive mold flux system based on CaO–Al2O3 system has been developed. The change of mold flux system from CaO–SiO2 to CaO–Al2O3 leads to variation in melt structure of mold flux, which further affects the interfacial properties between the molten mold flux and steel.
Slag/metal interfacial tensions have a marked impact on many metallurgical processes [3]. Interfacial tension determines the wettability among refractory/slag/metal in the iron and steelmaking processes [4, 5] and affects the final quality of welding in the welding process [6]. Summarizing the previous researches, there are many factors that affect the interfacial tension, such as oxygen in the molten iron or steel [7, 8], alloying elements [9, 10], and structure of the slag melt [11]. However, many previous studies focused on the effect of oxygen or alloying elements in the molten iron or steel on the interfacial tension, but few of them concentrated on the influence of the structure of slag melt on the interfacial tension. Thus, a comprehensive understanding of the effect of melt structure on the interfacial behavior during metallurgical processes is very crucial.
Interfacial properties also play very important roles in the continuous casting process [12]. It can affect the emulsification [13, 14], inclusion absorption [15, 16], and corrosion of mold nozzle [17]. To evaluate the effect of slag structure on interfacial tension, most recent studies were conducted using the interaction of solid metal with mold flux in the experiments. For example, Wang et al. [18] investigated the effect of MnO content on the interfacial property between the solid ultra-low carbon steel substrate and CaO–SiO2-based mold flux system. Jung et al. [19, 20] investigated the effect of basic oxides (MnO, FeO) and inclusion oxides (Al2O3, MgO) on the interfacial tension between the interstitial free (IF) steel substrate and CaO–SiO2-based mold flux system. However, these works are mainly based on the traditional CaO–SiO2-based mold flux system, and the research concerning the relationship between CaO–Al2O3-based mold flux structure and slag/steel interfacial property has rarely been done.
Therefore, in this study, the wettability of the CaO–SiO2–Al2O3-based mold flux system on the ultra-low carbon IF steel substrate was studied by using the sessile drop method at 1773 K. Also, the relationship between the interfacial tension and the melt structure of mold flux was investigated using Fourier transform infrared spectroscopy (FTIR), Raman spectroscopy, and X-ray photoelectron spectroscopy (XPS). The results obtained in this paper would provide the guidance for the designing and optimizing of CaO–Al2O3-based mold flux.
2 Experimental
2.1 Sample preparation
The mold flux samples used in the experiments were made of reagent grade chemicals of SiO2, CaCO3, Al2O3, Na2CO3, MgO, and MnO, and their major chemical compositions are shown in Table 1. For Samples 1–4, Sample 1 was designed as a benchmark, and the content of SiO2 in Samples 2, 3, and 4 was reduced with the substitution of Al2O3 content. For Samples 4–7, Sample 5 was set as a benchmark, and the content of CaO in other samples was varied with the corresponding change in Al2O3. In order to uniform the chemical composition of mold flux, the raw materials were firstly mechanically mixed and then pre-melted in an induction furnace at 1773 K for 10 min under an Ar atmosphere protection. After that, the molten slag was quenched on a water-cooled copper plate. When the slag was cooled and solidified, it was polished into the length of about 5-mm cubic blocks for the wettability measurements. The post-experimental compositions of the pre-melted samples were then analyzed using X-ray fluorescence (XRF) spectroscopy (S4 Explorer; Bruker AXS GmbH, Karlsruhe, Germany) and are provided in Table 1. It can be seen from the results of XRF that the variation in major components was very small, which indicates that the volatilization loss of the mold flux could be ignored. The substrates, which were made of IF steel, were cut into size of 30 mm (length) × 30 mm (width) × 5 mm (height) and then polished by SiC sandpaper with the grit size down to 2000 to ensure the surface roughness, and the major chemical composition of IF steel is listed in Table 2.
2.2 Wettability test procedure
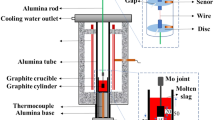
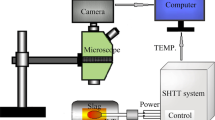
The wetting experiments were carried out by a sessile drop apparatus. The details of the experimental apparatus have been provided elsewhere [21,22,23], and its schematic is presented in Fig. 1.
During the experiments, the cubic slag sample was first placed on the polished IF steel substrate, and then both the cubic slag sample and IF steel substrate were pushed together into the center of the horizontal Al2O3 tube. Later, the entire system was sealed off and evacuated by a vacuum pump to − 0.1 MPa. Next, the gas mixture with 200 mL/min high-purity Ar (99.999%) and 19.8 mL/min H2 (99.999%) was introduced into the system to prevent the oxidation of the surface of IF steel substrate during the experiment. The gas mixture was firstly heated up to 673 K and then deoxidized by slowly passing through the gas purification furnace, where the gas was passed through getters such as Mg, Cu, Ti, and anhydrous calcium chloride to remove potential residual oxygen and water vapor. Then, the preheated gas mixture was continuously flushed into the reaction tube. After that, the sample was heated with a rate of 5 K/min to reach the target temperature of 1773 K and held for 30 min to ensure shape stability. A digital charge-coupled device (CCD) camera with high resolution was used to record the evolution of contact angle between the molten slag sample and substrate during the experiment. The contact angle was measured by the ImageJ software. Each measurement was taken at least three times to ensure the accuracy and reliability, and standard deviation was used within the results of the present study. One typical measurement of contact angle in the present study is illustrated in Fig. 2.
2.3 Structural analysis
FTIR, Raman spectroscopy, and XPS were employed to identify the structure of the molten slag. All the amorphous samples used for the spectroscopic analysis were obtained by quenching in liquid nitrogen from wetting tests at 1773 K and identified by X-ray diffraction (XRD) test using a Rigaku-TTR III diffractometer (Rigaku Corporation, Japan), as shown in Fig. 3. In order to ensure the accuracy of the test, at least three samples of each composition were analyzed and tested.
FTIR spectra were measured under ambient condition using the FTIR spectrometer (Spectra 100; PerkinElmer Corporation, USA) equipped with a KBr detector. The quenched samples were ground into powders with diameter less than 100 μm. And then, 2.0 mg of each powder sample mixed with 200 mg of KBr was pressed into thin section disk for FTIR measurement. The spectra were recorded in the wavenumber range of 1200–400 cm−1 with a resolution of 2 cm−1 [24].
The Raman spectra were measured at room temperature using a micro-Raman spectrometer coupled with a CCD detector (LabRAM HR Evolution, HORIBA Corporation, France). The Raman spectra were tested between 400 and 1200 cm−1 at the spectral resolution ranging from 2 to 3 cm−1 through a 532 nm excitation source laser. Then, the baseline of the Raman spectra was subtracted by using the LabSpec software, while the Raman spectra were deconvoluted with Gaussian functions using the Origin MicroCal software.
In order to investigate the effect of different oxygen ion species at the slag/steel interface on the wetting behavior, the O1s XPS spectra of the amorphous samples, taken from the slag/steel interface, were obtained using an X-ray photoelectron spectrometer (PHI-5300/ESCA, PerkinElmer Corporation, USA). The X-ray photoelectron spectrometer was equipped with an Al Kα X-ray source (1253.6 eV) and operated at 3.0 kV and 25 mA. And, the binding energy was calibrated with C1s (284.5 eV). The surface of slag sample was etched by an argon ion beam for 30 min to eliminate the surface contamination before XPS measurement. The Shirley’s method was employed to subtract the background, while the Origin MicroCal software was used for the deconvolution through Gaussian functions.
3 Results and discussion
3.1 Effect of Al2O3/SiO2 and CaO/Al2O3 ratios on contact angle
The variation in contact angle between the molten slag and IF steel at 1773 K with different Al2O3/SiO2 and CaO/Al2O3 ratios was investigated via the sessile drop experiments and is shown in Fig. 4. It can be found that the contact angle increased slightly with the increase in Al2O3/SiO2 ratio. The reason is that Al2O3 is a kind of amphoteric oxide, and it mainly plays the role of network former in slag containing sufficient basic oxides. The continuous substitution of SiO2 with Al2O3 might lower the amounts of O2− ions (free oxygen ions) that exist at the interface, as Al2O3 needs more free O2− to form the [AlO4]5−-tetrahedral than SiO2 to form [SiO4]4− under the condition of the same mass, and then it results in poor wettability between the molten mold flux and steel substrate. Besides, Fig. 4 also shows that the contact angle decreased with an increase in CaO/Al2O3 ratio. It may be because there are more O2− ions released with the excess addition of CaO when CaO/Al2O3 ratio increases. Thus, the amount of O2− at the interface increases, which improves the wettability and reduces the contact angle between mold flux and the substrate.
3.2 Effect of Al2O3/SiO2 and CaO/Al2O3 ratio on interfacial tension
The interfacial tension of mold flux/steel was calculated using the Young’s equation shown below [25]:
According to Brooks et al. [26], the surface tension of Fe can be calculated by the following equation in the temperature range of 1740–1920 K.
where T is temperature, K.
Considering that the substrate is made of ultra-low carbon IF steel, the effect of the alloy components on the surface tension of substrate was ignored here for the simplification of study [19, 20]. Thus, the surface tension of the substrate can be obtained from Eq. (2).
Besides, the surface tensions of mold fluxes can be calculated using Boni’s empirical equation [27, 28], which is shown in the following equation.
where γi is the surface tension factor of slag component i, (mN/m); and Ni is molar fraction of component i. The surface tension factors used in the present work are obtained from some references as provided in Table 3 [29,30,31,32,33].
Based on Eqs. (1)–(3), the interfacial tensions between mold flux and IF steel are calculated and illustrated in Fig. 5a, b, respectively, where it can be observed that the interfacial tensions increase slightly with the increase in Al2O3/SiO2 ratio, whereas they decrease with the increase in CaO/Al2O3 ratio.
The increasing trend of interfacial tension with the increase in Al2O3/SiO2 ratio obtained in this experiment is consistent with the results of Sharan and Cramb [34] and Sun et al. [35] obtained in the CaO–SiO2–Al2O3 system, as illustrated in Fig. 5a. It is worth noticing that the values of the two studies mentioned above increased more remarkably compared with the present study. The reason is that the substrate metal employed in their studies tended to interact with the molten slag and the surface active oxygen in the molten metal would exist at the slag/metal interface, which resulted in a significant change in interfacial tension.
Figure 5b illustrates that the interfacial tension decreases with the addition of CaO/Al2O3 ratio. This variation trend of the interfacial tension correlates well with the work carried out by Sharan and Cramb [34] and Sun et al. [35]. The increase in CaO/Al2O3 ratio may lead to the increase in O2− ions released from higher CaO content in mold flux; then, the increase in O2− breaks the aluminate and silicate structure and simplifies the network structure. Besides, the increase in the total number of O2− at the slag/metal interface may interact with the IF steel plate, which further leads to a lower interfacial tension. The details about the variation in melt structure with different Al2O3/SiO2 and CaO/Al2O3 ratios are discussed in Sect. 3.3. Although the variation trends of effect of Al2O3/SiO2 and CaO/Al2O3 ratios on the interfacial tension obtained in this study are consistent with the results of Sharan and Cramb [34] and Sun et al. [35], the specific values in Fig. 5a, b are still different, which is mainly due to the different compositions of slags and metals used in those experiments.
3.3 FTIR structural analysis
The FTIR results of various samples with the variation in Al2O3/SiO2 and CaO/Al2O3 ratio are illustrated in Fig. 6a, b. Generally, the convoluted [SiO4]4−-tetrahedral symmetric stretching (or Si–O symmetric stretching) bands exist between the wavenumbers of 1200–800 cm−1. According to the previous works [36, 37], the wavenumbers in the range of 880–850 cm−1, 920–900 cm−1, and 980–950 cm−1 correspond to Q0, Q1, and Q2 units, respectively. For typical Qn, Q is the [SiO4]4−-tetrahedral species unit and n is the number of bridging oxygen atoms per silicon atom. Qn usually represents the degree of polymerization of slag, and a larger n value indicates a higher degree of polymerization of slag. The [AlO4]5−-tetrahedral stretching vibrations exist in the range approximately from 800 to 600 cm−1, which relates to the symmetric and asymmetric stretching vibrations of the Al-O bonding [38].
It can be seen from Fig. 6a that the convoluted [SiO4]4−-tetrahedral symmetric stretching bands between the wavenumbers of 1200–800 cm−1 become less dominant; particularly, the wavenumbers that represent the [SiO4]4−-tetrahedral symmetric stretching gradually disappear with the substitution of SiO2 with Al2O3, while the [AlO4]5−-tetrahedral stretching bands become more pronounced, which suggests that the dominant slag structure transformed gradually from silicate structure to the aluminate structure. Figure 6b shows that the wavenumbers peaks of Q2 and Q1 and the [AlO4]5−-symmetric stretching transmittance all become less pronounced with the increase in the CaO/Al2O3 ratio, which illustrates that the complex network structure was depolymerized gradually as a result of the increase in O2− content released from higher CaO content.
3.4 Raman structural analysis
The Raman spectra of samples of the above samples are illustrated in Fig. 7. Based on the results of previous studies [39,40,41], the characteristic peaks related to the Al–O–Al linkage (Al–O0) are near 550 cm−1, while the characteristic peaks in the range of 700–1000 cm−1 are envelopes formed by the overlap of characteristic peaks of [AlO4]5−-tetrahedral and [SiO4]4−-tetrahedral stretching vibration. The typical deconvoluted peaks of the Raman spectra fitted by Gaussian function are shown in Fig. 8, and the characteristic peaks in the Raman spectra of the various structural units were obtained by typically fitting until an R2 (R2 stands for the correlation coefficient of the deconvolution of the Raman spectra curve fitted by Gaussian function) value above 99.8% was achieved. The characteristic peaks in the range of 770–800, 830–850, 850–880, 890–920, and 950–1000 cm−1 correspond to Al–O− bands, Al–O–Si linkage, Q0, Q1, and Q2 units, respectively [39,40,41]. The specific details of the Raman shift after the Gaussian deconvolution are shown in Table 4.
The integrated area of each characteristic peak provides a semiquantitative method to evaluate the amount of the characteristic structural units in the molten mold flux. The proportion of the integrated area of each characteristic peak over the sum of the integrated area of all characteristic peaks represents the fraction of each structural unit [41]. Figure 9 shows that the area percent of each deconvoluted characteristic peak is plotted as a function of various Al2O3/SiO2 ratios. It suggests that the fractions of Q0 to Q2 in the [SiO4]4−-tetrahedral structure units decrease significantly with the reduction in the amount of SiO2. In contrast, the fractions of Al-O0 and Al-O− in the [AlO4]5−-tetrahedral structure and Si–O–Al units increase obviously with the addition of Al2O3 content. The results indicate that the original dominant silicate structure has been transferred into a more complex structure with the major Si–O–Al structural and [AlO4]5−-tetrahedral units, with the addition of Al2O3 in the molten slag. Thus, in the present slag system, the network structure in molten mold flux becomes more complex with the increase in Al2O3/SiO2 ratio, and the silicate or aluminate structural units connect with each other to form a complex aluminosilicate network structure.
The area percentage of each deconvoluted characteristic peak is also plotted in Fig. 10 as a function of various CaO/Al2O3 ratios, where it could be observed that the fractions of Q1 and Q2 structural units decrease, while the fraction of Q0 units increases with the increase in CaO/Al2O3 ratios, and the fractions of the Al–O0 and Si–O–Al structural units reduce with a higher CaO/Al2O3 ratio. The results indicate that the melt structure has become simplified, as a large number of free O2− ions supplied by CaO tend to break Si–O–Al bonds to depolymerize the complex aluminosilicate structure.
3.5 XPS analysis
A typical example of the deconvoluted peaks of the O1s binding energy from the XPS spectra is depicted in Fig. 11. Peak fitting is conducted with a full width at half maximum (FWHM) of less than 2.0, and three kinds of oxygen ions could be deconvoluted from the XPS spectra, which contain the bridged oxygen (O0), non-bridged oxygen (O−), and free oxygen (O2−) ions. According to the previous studies [19, 20, 41], the binding energies around 532, 531, and 530 eV correspond to O0, O−, and O2− ions, respectively. The characteristic peaks of these three oxygen species in the XPS spectra are obtained by a typical fitting, till R2 value above 99.8% is achieved. The specific information of the binding energy of the O0, O−, and O2− after the Gaussian deconvolution is shown in Table 5.
The relative percent of O2−, O−, and O0 can be obtained from the integrated area of the deconvoluted peaks for each oxygen species, and the results are represented in Fig. 12. Figure 12a shows that the total percent of O− and O2− decreases, while that of O0 gets increased with the increase in Al2O3/SiO2 ratio. In Fig. 12b, for a higher CaO/Al2O3 ratio, the percent of O− and O2− increases with the reduction in that of O0.
According to Toop and Samis [42], one free oxygen O2− can react with the O0 to obtain two O− ions in equilibrium at high temperature, as expressed in Eq. (4).
It has been suggested that only free oxygen ions can have an impact on the interfacial tension between the molten slag and the substrate [19, 20]. However, in a recent study, it was found that the O− can also affect the interfacial tension [11]. Thus, in Fig. 12a, the sum of O2− and O− slightly decreases with the addition of Al2O3/SiO2 ratio, which results in a more complex aluminosilicate network structure, leading to a decrease in the localized concentration of oxygen anions at the interface between the molten slag and the IF steel substrate. Figure 12b represents that O2− break the O0 in the molten slag, which leads to a higher amount of O2− and O− with the improvement of CaO/Al2O3 ratio and then results in an increase in the local oxygen anions concentration at the slag/metal interface.
According to the Gibbs adsorption isotherm, the relationship between the oxygen activity and the interfacial tension at the interface can be expressed as the following equation [19]:
where Γ is the saturation coverage; R is the ideal gas constant, 8.314 J/(mol K); and \(a_{\text{O}}\) and \(X_{\text{O}}\) are the activity and mole fraction of oxygen at the interface, respectively.
In this study, it is assumed that the interfacial tension is affected by both O2− and O−. Figure 13 represents the effects of the sum of X(O2−) + X(O−) on the interfacial tension between mold flux and the IF steel at 1773 K. Results from the published literature are also included in Fig. 13, and all data have been fitted with a linear regression analysis [11, 20]. It suggests that the interfacial tension decreases linearly with the sum of X(O2−) + X(O−) at the interface, indicating that the saturation coverage is independent of slag concentration [19]. It is worth noticing that the slope of each fitting line is different, which is because the network structure of various slag systems is different, resulting in the different oxygen ions content in molten slag; or it may be related to the characteristics of cations in various slag systems, such as the radius of cations, which affects the saturation coverage of O2− and O− at the surface [20] and then decreases the interfacial tension. The XPS results are consistent well with the results of the interfacial tension.
4 Conclusions
-
1.
The sessile drop method results indicate that the contact angle and interfacial tension for the mold flux with IF substrate increase slightly with the addition of the Al2O3/SiO2 ratio and decrease with the increase in CaO/Al2O3 ratio.
-
2.
The FTIR results show that the original dominant silicate structure transforms gradually to aluminosilicate and aluminate structure with the substitution of SiO2 with Al2O3. However, with the increase in CaO/Al2O3 ratio, the complex network structure is depolymerized gradually as a result of the increase in O2− content released from higher CaO content.
-
3.
The Raman results suggest that the aluminate structure increases accompanied with the decrease in the silicate structures, with the addition of Al2O3/SiO2, while the amounts of the symmetric Al–O− stretching vibrations increase; in the meantime, the symmetric Al-O0 and Si–O–Al stretching vibrations decrease, with the addition of CaO/Al2O3, which results in a more simplified melt network.
-
4.
The XPS results indicate that the total percent of O− and O2− slightly decrease, whereas that of O0 slightly increases, with the addition of Al2O3/SiO2 ratio. And, the increase in the percent of O− and O2− is accompanied with the decrease in that of O0, for a higher CaO/Al2O3 ratio.
-
5.
Combining the results of sessile drop method with melt structure analysis, it suggests that the interfacial tension decreases linearly with the increase in sum of the amounts of O2− and O− at the interface, which suggests that the variation in interfacial properties for mold flux/IF steel substrate is mainly caused by the change in melt structure, especially the variation in amounts of O2− and O− at the interface.
References
W. Wang, K. Blazek, A. Cramb, Metall. Mater. Trans. B 39 (2008) 66–74.
L. Zhou, W. Wang, K. Zhou, Metals 6 (2016) 139.
A. Jakobsson, D. Sichen, S. Seetharaman, N.N. Viswanathan, Metall. Mater. Trans. B 31 (2000) 973–980.
J. Safarian, M. Tangstad, Metall. Mater. Trans. B 40 (2009) 920–928.
H. Sun, W. Easman, Energy Fuels 21 (2007) 413–418.
I. Egry, G. Lohöfer, I. Seyhan, S. Schneider, B. Feuerbacher, Int. J. Thermophys. 20 (1999) 1005–1015.
K. Ogino, S. Hara, T. Miwa, S. Kimoto, Trans. ISIJ 24 (1984) 522–531.
H. Sun, K. Nakashima, K. Mori, ISIJ Int. 37 (1997) 323–331.
C. Dumay, A.W. Cramb, Metall. Mater. Trans. B 26 (1995) 173–176.
A. Sharan, A.W. Cramb, Metall. Mater. Trans. B 28 (1997) 465–472.
J.B. Kim, J.K. Choi, I.W. Han, I. Sohn, J. Non-Cryst. Solids 432 (2016) 218–226.
Y. Chung, A.W. Cramb, Metall. Mater. Trans. B 31 (2000) 957–971.
M.A. Rhamdhani, G.A. Brooks, K.S. Coley, Metall. Mater. Trans. B 37 (2006) 1087–1091.
Z. Liu, B. Li, L. Zhang, G. Xu, ISIJ Int. 54 (2014) 2324–2333.
P. Rocabois, J. Lehmann, C. Gatellier, J.P. Teres, Ironmak. Steelmak. 30 (2003) 95–100.
J. Wikström, K. Nakajima, H. Shibata, A. Tilliander, P. Jönsson, Mater. Sci. Eng. A 495 (2008) 316–319.
C.G. Aneziris, E.M. Pfaff, H.R. Maier, J. Eur. Ceram. Soc. 20 (2000) 159–168.
W. Wang, J. Li, L. Zhou, J. Yang, Met. Mater. Int. 22 (2016) 700–706.
E.J. Jung, W. Kim, I. Sohn, D.J. Min, J. Mater. Sci. 45 (2010) 2023–2029.
E.J. Jung, D.J. Min, Steel Res. Int. 83 (2012) 705–711.
L. Zhou, J. Li, W. Wang, I. Sohn, Metall. Mater. Trans. B 48 (2017) 1943–1950.
E. Gao, G. Zou, W. Wang, F. Ma, X. Luo, Metall. Mater. Trans. B 48 (2017) 1014–1023.
X. Luo, W. Wang, F. Ma, ISIJ Int. 56 (2016) 1333–1341.
H. Kim, W.H. Kim, I. Sohn, D.J. Min, Steel Res. Int. 81 (2010) 261–264.
T. Young, G. Peacock, Miscellaneous Works of the Late Thomas Young V1: Including His Scientific Memoirs, Kessinger Publishing LLC, Montana, 2007.
R.F. Brooks, I. Egry, S. Seetharaman, D. Grant, High Temp.-High Press. 33 (2001) 631–637.
R.E. Boni, G. Derge, JOM 8 (1956) 53–59.
K. Nakashima, K. Mori, ISIJ Int. 32 (1992) 11–18.
M. Nakamoto, A. Kiyose, T. Tanaka, L. Holappa, M. Hämäläinen, ISIJ Int. 47 (2007) 38–43.
M. Nakamoto, T. Tanaka, L. Holappa, M. Hämäläinen, ISIJ Int. 47 (2007) 211–216.
N. Ikemiya, J. Umemoto, S. Hara, K. Ogino, ISIJ Int. 33 (1993) 156–165.
K. Ogino, H. Taimatsu, F. Nakatani, J. Jpn. Inst. Metals 46 (1982) 957–962.
D. Chatain, F. Chabert, V. Ghetta, J. Fouletier, J. Am. Ceram. Soc. 77 (1994) 197–201.
A. Sharan, A.W. Cramb, Metall. Mater. Trans. B 26 (1995) 87–94.
H. Sun, K. Nakashima, K. Mori, ISIJ Int. 46 (2006) 407–412.
D.W. Matson, S.K. Sharma, J.A. Philpotts, J. Non-Cryst. Solids 58 (1983) 323–352.
B.O. Mysen, Contrib. Mineral. Petrol. 133 (1998) 38–50.
G.H. Kim, I. Sohn, ISIJ Int. 52 (2012) 68–73.
T.S. Kim, J.H. Park, ISIJ Int. 54 (2014) 2031–2038.
Y. Tsunawaki, N. Iwamoto, T. Hattori, A. Mitsuish, J. Non-Cryst. Solids 44 (1981) 369–378.
G.H. Kim, I. Sohn, J. Non-Cryst. Solids 358 (2012) 1530–1537.
G.W. Toop, C.S. Samis, Trans. Metall. Soc. AIME 224 (1962) 878–887.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (U1760202, 51661130154, 51874363) and Newton Advanced Fellowship (NA150320).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, Wl., Gao, Ez., Zhou, Lj. et al. Effect of Al2O3/SiO2 and CaO/Al2O3 ratios on wettability and structure of CaO–SiO2–Al2O3-based mold flux system. J. Iron Steel Res. Int. 26, 355–364 (2019). https://doi.org/10.1007/s42243-018-0207-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42243-018-0207-z