Abstract
Chemical compounds, which are used on beans to manage common bacterial blight (CBB) disease, cause serious damage to natural ecosystems and often fail to control CBB. A greenhouse experiment was conducted to assess the effectiveness of foliar sprays of Bacillus subtilis str. QST 713 against CBB in a susceptible bean cultivar in comparison with copper hydroxide (CH) and its mixes. A concentration of 108 CFU mL− 1 for Xanthomonas axonopodis pv. phaseoli (Xap) was used to inoculate 21-day-old plants after 3 days of first-time treatment applications. The 2nd and 3rd time treatment applications were applied every 7 days after bacterial inoculation. The disease severity, disease infection rate, AUDPC and disease incidence were significantly reduced over the control treatment. The lowest disease severity, incidence and AUDPC were observed from the mix applications with str. QST713 and CH followed by str. QST 713 and CH alone compared to control treatments. The plants treated with mixes and str. QST 713 showed a reduction of the infection rate by 51.21 and 40.36%, and the disease incidence by 53 and 52.25%, respectively. In addition, the lowest bacterial population per gram of fresh weight of leaves was recorded in plants that had been treated with the mix treatments followed by str. QST713 and CH. Results suggest that integrated management with biocontrol agents is the best strategy for effective control of CBB in sustainable and organic agriculture.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Xanthomonas axonopodis pv. phaseoli (Xap) is a destructive pathogen which causes the common bacterial blight (CBB) disease in all bean producer countries (Dursun et al. 2002; Popovic et al. 2012; Donmez et al. 2013). Depending on the intensity of the disease, degree of cultivar susceptibility and environmental conditions, the seed quality is affected by 45% and the yield loss may be reach up to 100% (Opio et al. 1996; Allen and Lenne 1998; Belachew et al. 2015; Belete and Bastas 2017). Researchers are still trying to develop different strategies from conventional methods to molecular techniques for managing of the CBB. In addition to the use of pathogen-free seed and hygienic cultivation (Schuster and Coyne 1981), new approaches like plant resistance activators and plant extracts, have recently been investigated as alternative tools to reduce CBB disease (Balestra et al. 1999, 2009; Quattrucci et al. 2013; Fortunati et al. 2019).
Pesticides that are incessantly used on beans to control the disease cause serious damage to agricultural land and ecosystems, despite often failing to control CBB (Corrêa et al. 2017). In addition, the use of synthetic chemicals is increasingly restricted due to the development of antibiotic resistant microorganisms and deposits of toxic residue which have human health and environmental consequences (Rahman et al. 2008). As a result, innovative eco-friendly alternative disease management strategies are needed. In other words, the use of biological fungicides and/or bactericides offer alternatives to chemical control of plant diseases (Lugtenberg and Kamilova 2009). In this connection the development of biological control method using bacterial bio-control agents show good results for controlling CBB disease (Corrêa et al. 2017).
The different strains of Bacillus subtilis have shown potential for the control of foliar pathogens of different crops. Among these strains, B. subtilis QST 713, the principal component of commercial bio-fungicide, Serenade, is a biological control agent of several crops to treat a variety of foliar bacterial and fungal plant diseases (Abbasi and Weselowski 2014; Abbasi and Weselowski 2015; Paçe et al. 2016; Fousia, et al. 2016; Ibrahim et al. 2016). This antagonism can be achieved through nutrient competition, site exclusion, colonization, and attachment of the bacteria to the pathogen. In addition, the B. subtilis strain QST 713 has been shown to induce plants’ natural systemic resistance or systemic acquired resistance (SAR) against bacterial pathogens (Haas and Defago 2005; Borriss 2011).
Until now, there is no scientific data on the efficacy of B. subtilis QST 713, either alone or in mixed applications with copper compounds to control CBB disease. This study is the first time in vivo conditions were included in the experiment to assess the effectiveness of foliar sprays of B. subtilis QST 713 against CBB in a susceptible dry bean cultivar in comparison with copper hydroxide and their mixes.
Materials and methods
Treatment formulations and applications
The biological control agent B. subtilis QST 713 is very important for treating a variety of diseases on different crops. For this research, B. subtilis QST 713 was purchased in the form of an aqueous suspension as an active ingredient of Serenade Max (Bayer CropScience).
In order to evaluate the effectiveness of foliar sprays of B. subtilis str. QST713 against Xap in comparison with copper hydroxide and their mixes, a susceptible bean cultivar Aras 98 was grown in pots containing sterilized soil and peat with completely randomized design (CRD) replicated three times. Six seeds were planted for each pot and finally two of the seedlings were tinned out so that four plants remained in each pot with a total of 12 plants for each treatment. When the plants reached the age of 16–21 days (3–4 leaf stage), each foliar spray treatment was applied with hand sprayer onto the aerial part of bean leaves. In each experiment, the foliar spray was applied three times per week. Each plant received approximately 30 mL of treatment formulations. The first treatment (B. subtilis str. QST 713) was prepared by diluting 20 mL of aqueous suspension of B. subtilis str. QST 713 in 1 L of sterile distilled water (SDW) at a concentration of 107 CFU/mL, 2.5 g of copper hydroxide (Kocide 2000) was diluted in 1 L of SDW as a second treatment and third treatment was formulated by combination of first and second treatment (equal mixes). From each treatment suspensions, 30 ml was sprayed per plant. Control plants were sprayed with SDW and considered as the fourth (control) treatment.
After three days of first spray treatment applications, all plants were inoculated by preparing Xap inoculum. The bacterial mix isolates used to inoculate beans were 120-X, 145-X and Xp321, originally from Turkey. Two-day-old cultures of Xap were harvested from NA medium plates, suspended in sterile deionized water, and adjusted to a concentration of 108 CFU/mL by using bio-photometer (OD650nm = 0.17) and 21-day-old plants were inoculated under 26–28 °C with a 14 h/10 hours light/dark conditions and the plots were covered by polyethylene bags for 48–72 hours. The second- and third-time foliar spray treatment applications were applied again at the same concentration every 7 days after bacterial inoculation. This experiment was repeated twice.
Collected data
Disease incidence and severity were recorded after 10 days of Xap inoculation and continued every 7 days four times (10, 17, 24, 31 days after inoculation). Disease severity score on bean leaves was assessed as modified CIAT (1998) and Ararsa et al. (2018) 0–9 disease scale.
Disease incidence (DI) on bean leaves was determined and calculated on four plants where total number of leaves was counted and express as a proportion of leaves showing CBB symptoms. The following formula was used to determine disease incidence (%);
To calculate disease severity index (DSI) or present severity index (PSI), the severity score scales were converted and expressed as percentages by the formula of Wheeler (1969).
where, Snr = the sum of numerical ratings (total number of ratings); Npr = number of plants rated (total plants) and Mss = the maximum score of the scale.
Subsequently, disease ratings were plotted over time to generate disease progress curve. The area under the disease progress curve (AUDPC) was calculated from the PSI values using the following formula (Shanery and Finney 1977).
Xi is the percent severity index expressed as a proportion at the ith observation, ti is the time (days after planting/inoculation) at the ith observation and n is the total number of observations. Its values were expressed in the form of %-days (Shanery and Finney 1977).
DI and PSI percent increase or decrease over control was calculated using the following formula:
The population size of Xap on bean leaves after each treatment application was determined by collecting 1 g of leaf (from bottom, middle and top parts of four plants after 24 days of Xap inoculation). A 10-fold dilution series was prepared and placed on NA and incubated at 28 °C for 48 hours with three replications. Finally, the number of CFU/g fresh weight of leaf was counted (Fousia et al. 2016).
In addition, some agronomic parameters like plant height (PH), number of leaves per plant (NLPP) (at six and eight weeks old) and number of primary branches per plant (NPBPP) was recorded. The experiment was repeated twice.
Statistical Analysis
All the measured values (disease incidence and severity, PSI, AUDPC, population size of bacteria and the agronomy parameters) were subjected to analysis of variance (ANOVA) using PROC ANOVA of SAS software version 9.1.3 to assess the differences among the tested treatments. A combined analysis was performed for each repeated test and the combined results were presented. Mean separation and grouping by letters were carried out using Duncan’s Multiple Range Test (DMRT) at 0.99 level of confidence.
Results
Effects of treatments on CBB disease development
Disease severity and incidence recording started 10 days after Xap inoculation (Figs. 1 and 2). In this study, the application of treatments (str. QST713, CH and their mixes) showed statistically significant differences (P ≤ 0.01) compared to the control treatment. The disease infection rate, AUDPC and disease incidence were significantly reduced on treated experiments (Table 1). In other words, the effect of foliar spray of the different treatments significantly reduced the disease index and incidence (P ≤ 0.01) on all dates of disease assessment (Table 2; Fig. 2). The lowest values of disease score (3.38), PSI (37.50%), disease incidence (35.07%) and AUDPC (586.60%-days) were observed from plants treated by mixes of str. QST713 and CH followed by CH and str. QST713 alone (Table 1). Str. QST 713 application also significantly reduced CBB severity and incidence, but it was less effective than the combination of str. QST 713 and copper hydroxide. However, in all disease assessment (severity score, infection rate, disease incidence and disease progress rate) were higher by far in control treatment. The disease infection rate and disease incidence in control treatment was 76.86 and 74.60%, respectively (Table 1).
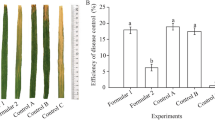
In both AUDPC and disease incidence, there was no significant difference among experiments which were treated by str. QST 713, CH and mixes. Similarly, plants treated by CH and mixes have no significant difference from each other. The experiment treated by a combination of str. QST 713 and CH showed lowest disease severity and incidence. The lowest AUDPC was also recorded from this treatment. In the same manner, the application of tank mixes was the most effective treatment against Xap, as it reduced the AUDPC twofold compared to control treatment (Table 1; Fig. 3). In addition, str. QST 713 and CH reduced significantly the AUDPC almost twofold as compared to untreated plants. Plants that are treated by the mixture of CH and str. QST 713 reduced the CBB severity on bean leaves (by 52.91% based on the AUDPC value) as compared to control treatment (Table 1)
Except the first disease assessment period (10 DAI), in all consecutive periods, the lowest disease severity index was recorded also from plants treated by mixes (Table 2). However, during the initial period of disease recording, the lowest value was recorded from plants treated with str. QST 713. In contrast, the rate of disease progress in the unsprayed plots was higher in all scoring time than the sprayed experiments. Both disease progress rate and incidence started similarly after 24 days of inoculations (Table 2; Fig. 2).
In plants treated with mixes, the disease severity remained at substantially low levels throughout the experimental period, being not more than 37% at 31 DAI (Fig. 1). In addition, the 53% of the mixes treated plants remained symptomless (Table 3). There was a big difference in the physical symptom observations among the treated and untreated pants in Fig. 4. Similarly, plants treated with str. QST 713 showed a reduced disease incidence by 52.25% which was similar to the reduction percentage by the mixes (53%) (Table 3). Furthermore, the mix treatment reduced the infection rate by 51.21% over the control treatment. Generally, the aqueous suspension of bio-bactericide alone or in mixes with CH reduced the severity of CBB on bean leaves by 40–51% compared to the control treatment (Table 3). In this trial, str. QST 713 and CH and alone and as tank mixes reduced the severity of CBB on bean leaves by 40.4, 48.8 and 51.2%, respectively compared to the control treatment (Table 3). The PSI index and disease incidence were directly proportional to AUDPC. The higher the infection rate the higher AUDPC. Similarly, with increasing of disease incidence, there was an increment of AUDPC. Finally, as a consequence of the PSI, disease incidence and AUDPC observations, statistical analysis indicated that mix treatment (str. QST713 + CH) was more effective than str. QST 713 and CH alone.
Effect of str. QST 713, CH and their mixes on population size of Xap on bean leaves
The population size of Xap on bean leaf surface was also affected by the different treatments that were used in this study. The lowest bacterial population (1.38 × 105 CFU/g of fresh weight of bean leaves) was observed in plants that have been treated by str. QST713 and CH mixes which was followed by str. QST713 and CH2.84 × 105 and 3.35 × 105, respectively. This treatment reduced the Xap population on the surfaces of bean leaves by 90.72% (Table 4). Similarly, the population size of Xap was reduced by 80% when plants treated with str. QST 713. In non-sprayed plants, the Xap population size being almost two times higher than in the mix and str. QST713 treated plants. From the re-isolated Xap on Petri plates, the most crowding bacterial colonies were observed in bean leaves which were isolated from untreated plant samples (Fig. 5).
Effect of treatments on some agronomic parameters
Data on plant height, number of primary branches per plant and number of leaves per plant (at 6 and 8 weeks old) were recorded in Table 5. In this regard, significant differences were observed in plant height, number of branches per plant and number of leaves per plant. The plant height (87 cm), number of primary branches per plant (3.67) and number of leaves per plant (21.40 and 29.42 at 6 and 8 weeks old, respectively) were higher in plants treated with str. QST713 than all other treatments (Table 5). In our experiment, the foliar application of str. QST713 and tank mixes of str. QST713 and CH showed good progress on the measured agronomic parameters by increasing plant height, primary branches per plant and leaves per plant as compared to control and monk plants (plants were sprayed to runoff with SDW). However, plants treated with CH and mixes of str. QST 713 and CH do not show significant difference on number of primary branches per plant and number of leaves per plant (at 6 weeks old) to each other and monk plants but significantly different from the control treatment (Table 5).
The str. QST 713 treatment increased the PH, NPBPP, NLPP at 6 weeks old and NLPP at 8 weeks old (by 20.65, 38.69, 35.51 and 38.24%, respectively) as compared with the control treatment but 11.00, 18.26, 18.69 and 15.02%, respectively when compared to plants that were treated with SDW (monk plants) (Table 5). Generally, all the agronomic parameters were comparatively higher in foliar sprays of Serenade bio-bactericide (str. QST 713).
Discussion
To reduce the negative effects of synthetic chemicals on human health and the environment and to suppress Xap populations, an in vivo study was performed to evaluate the effect of bio-fungicide ‘B. subtilis str. QST 713’ alone and its mix with CH on CBB disease control for the first time. In our greenhouse study, dry bean cv. Aras 98 plants treated by str. QST 713, CH and their mixes gave marked reduction in disease severity as compared to control treatments. Statistically, the most effective treatment was achieved from the combination of str. QST 713 with CH. The production cost of copper compounds in combination with biological agents (bio-bactericides) may increase; however sometimes it is more effective, environmentally reliable and healthier than a CH dependent management program.
By far, disease incidence and severity of several plant pathogens were suppressed by copper compounds mixed with QST 713. Ibrahim et al. (2016) reported that the mix of QST 713 with copper hydroxide also consistently reduced the development of citrus bacterial canker disease. They also indicated that about 87% disease control was achieved from this treatment. Similarly, B. subtilis str. QST 713 in tank mix with copper hydroxide reduced disease severity of bacterial spot of tomato in all four years followed by str. QST 713 alone (Abbasi and Weselowski 2015). This indicates that copper hydroxide and biological control agents are compatible. This idea is also supported by Valarmathi et al. (2013), chemical fungicides/bactericides that are active against a narrow spectrum of plant pathogens but not against biological agents. B. subtilis was compatible with copper hydroxide (Kocide 3000) even at a high concentration at in vitro conditions (Valarmathi et al. 2013).
Weekly sprays of B. subtilis str. QST 713 as a tank mix with copper hydroxide consistently reduced disease severity of early blight of tomato even if disease pressure was high in plots (Abbasi and Weselowski 2014). In parallel, B. subtilis str. QST 713 in combination with azoxystrobin gave the best strategy for controlling of powdery mildew disease in zucchini (Gilardi et al. 2008).
In other studies, str. QST 713 alone showed a significant reduction of disease severity and incidence. In a study by Fousia et al. (2016), the bacterial speck disease severity and incidence were significantly reduced when tomato plants treated with str. QST 713 compared to control treatment. They also reported that about 60% of B. subtilis str. QST 713 treated plants remained symptomless, which was in agreement with our study. Similarly, the application of Serenade was control over 90% of disease development in clubroot of canola (Lahlali et al. 2011). According to Baker et al. (1983), the application of B. subtilis str. APPL-1 to bean leaves decreased the incidence of rust (Uromyces phaseoli) disease by 75% which was equivalent to weekly application of mancozeb.
The application of str. QST 713 on Pseudomonas and Xanthomonas pathogens of tomato under greenhouse conditions (Roberts et al. 2008; Gilardi et al. 2010) and E. amylovora on apple trees (Sundin et al. 2009) was effective in controlling the development of disease. In our studies, foliar applications of str. QST 713 alone and copper hydroxide alone significantly decreased the disease severity and incidence compared to control treatments, but the results showed that the mixture applications (str. QST 713 + CH) were better than separately applications. This is combined copper hydroxide with str. QST 713. It has been confirmed that the applications of str. QST 713 integrated with CH provides consistent results. However, further studies are needed to verify the consistency of this phenomenon. Because the inclusion of bio-control agents in integrated disease management strategies is currently one of the challenges facing researchers, extension services and manufacturers because of effects of environmental conditions on microorganisms (Gilardi et al. 2008). This is described by Abbasi and Weselowski (2014), Abbasi and Weselowski, (2015), and by Pache et al. (2016) and Ibrahim et al. (2016). However, Zitter et al. (2005) found that alone or with mixed copper integrated bio-fungicide Serenade, leaves did not adequately control the early burning of tomato in field conditions.
Application of B. subtilis and other Bacillus spp. through foliar spray, fruit spray, seed treatment and etc. were very important for growth and developments of crop plants in addition to pest and disease control (Nakkeeran et al. 2005). In our experiment, PH, NPBPP and NLPP were significantly increased when bean plants are treated with B. subtilis str. QST 713 and mixes of str. QST713 and CH compared to control and monk plants. Similar trends have been reported by Fousia et al. (2016) significant increase in PH was observed when plants sprayed with str. QST 713 and CH treatments separately. The weekly spray of aqueous suspension of Serenade with copper hydroxide significantly increased the fruit yield of tomato (Abbasi and Weselowski 2014). A significant difference was observed in total number of branches over time when the blackberry plant treated by different bacterial strains including B. subtilis QST 713 (Robledo-Buriticá et al. 2018).
Similar to the reduction in disease incidence, PSI and AUDPC in the B. subtilis str. QST 713 integrated with CH as a mix and str. QST 713 treated plants have shown a suppressive activity of tank mixes and str. QST 713 on the bean leaf population size of Xap (Table 5). This finding can be in agreement with the results of Fousia et al. (2016) study that B. subtilis str. QST 713 on tomato leaf reduced or suppressed the development and population size of Pseudomonas syringae pv. tomato. The reduction of Xap population on the surface of bean leaves is due to the antagonistic effect of both bio and chemical fungicide. According to Chen et al. (2009), B. subtilis str. QST 713 produces various antibiotics. The different types of polyketide compounds are produced from B. subtilis str. QST 713 and act as an antagonistic for both gram-positive and gram-negative bacteria (Chen et al. 2006, 2009; Kinsella et al. 2009) by inhibiting protein synthesis (Zweerink and Edison 1987).
In conclusion, there was a significant control of disease infection rate and incidence by all treatments (B. subtilis str. QST713, Copper Hydroxide, B. subtilis str. QST713 + Copper Hydroxide) as compared to the untreated plants. Plants which were treated with mixes and str. QST713 reduced the infection rate by 51.21 and 40.36%, and the disease incidence by 53 and 52.25%, respectively. This clearly indicates that the bean growers should mainly focus on integrated disease management practice to control or decrease disease incidence. To minimize the impact of CBB disease on beans, growers must carefully integrate recommended strategies such as sanitation, crop rotation, use of treated or healthy seeds, tolerant or resistant varieties, and proper bio-bactericides and chemical compound. In addition to reduction of disease incidence, the integrated approaches reduce or delay disease severity during the critical periods of vegetative and reproductive plant growth (Belete and Bastas 2017). According to Fetene and Ayalew (2016), seed treatment integrated with bio-fumigation and foliar sprays reduced CBB disease severity up to 66.5% and increased seed yield gain up to 67.61%. The lowest bacterial populations per gram of fresh weight of leaves were also recorded in plants that have been treated by mixes and str. QST713 treatments. These results suggest that the application of str. QST 713 alone may not be enough for reducing the disease. However, it may be more successful in combination with copper hydroxide and may also reduce the rate of CH application. Since copper hydroxide, which is incessantly used on beans to manage CBB disease, has caused serious damage to natural ecosystems and has shown frequent failure for controlling of CBB, a reduction of CH application would result in more sustainable CBB disease management. In addition, reduction in copper use would be beneficial to reduce the effect on the environment and to lower copper accumulation in the soil. A combination of bio-bactericides and copper-based bactericides can offer effective disease management strategy for foliar bacterial diseases in organic agriculture. Integrated disease management is the best strategy for effective control of CBB and other bacterial diseases in sustainable and organic production system (Belachew et al. 2015). On the other hand, the intensive use of copper-based compounds to manage bacterial pathogens has a number of negative impacts such as phytotoxicity and on soil biota (Lamichhane et al. 2018). The development of copper resistance is also a serious problem mainly in certain crop diseases caused by Xanthomonas. According to Horvath et al. (2012), Xanthomonas perforans strains isolated from bacterial spots of tomato lesions in the tomato production areas were found to be resistant to copper-based compounds. Sundin et al. (1989) reported that when a bacterial strain has acquired copper resistance, the continuous selection pressure gradually increases the frequency of the resistant pathogen population and compromises the efficacy of copper. The development of resistance by Xap to copper-based chemical compounds raises a serious problem, thereby compromising the sustainability of beans protection systems. Therefore, integrated CBB management is a preferred strategy because of an increased awareness of the residual effects of copper-based compounds on non-target organisms and environment, and on the development of copper-resistant bacterial strains as well as the limitation of a single alternative management option to achieve the same level of control and reliability as that of chemicals.
In general, it is considered that integrated management with biocontrol agents is the best strategy for effective control of CBB in sustainable and organic agriculture. Since this research is the first report on CBB disease using B. subtilis str. QST 713 and combination of B. subtilis str. QST 713 and copper hydroxide, further studies are needed to evaluate the effectiveness of this combination on large-scale applications to control CBB with additional yield and yield related parameters. In addition, further research should be conducted to investigate the molecular characterization of the plants’ defense-related gene expression before and after application of these treatments upon Xap and synergistic effect and compatibility between B. subtilis str. QST 713 and copper hydroxide.
Data availability
All materials and data are available and have been presented in the manuscript.
References
Abbasi PA, Weselowski B (2014) Influence of foliar sprays of Bacillus subtilis QST 713 on development of early blight disease and yield of field tomatoes in Ontario. Can J Plant Pathol 36(2):170–178
Abbasi PA, Weselowski B (2015) Efficacy of Bacillus subtilis QST 713 formulations, copper hydroxide, and their tank mixes on bacterial spot of tomato. Crop Prot 74:70–76
Allen DJ, Lenne JM (1998) Disease as constraints to production of legumes in agriculture. In: Allen DJ, Lenne JM (eds) The pathology of food and pasture legumes. CAB International, Wallingford, pp 1–61
Ararsa L, Fikre L, Getachew A (2018) Evaluation of integrated management of common bacterial blight of common bean in Central Rift Valley of Ethiopia. Am J Phytomed Clin Ther 6:1:3
Baker SC, Stavely JR, Thomas CA, Sasser M, Mac. Fall SJ (1983) Inhibitory effect of Bacillus subtilis on Uromyces phaseoli and on development of rust pustules on bean leaves. Phytopathol 73:1148–1152
Balestra GM, Antonelli MG, Fabi A, Varvaro L (1999) Effectiveness of natural products for in vitro and in vivo control of epiphytic population of Pseudomonas syringae pv. tomato on tomato plants. J Plant Pathol 80(3):251
Balestra GM, Heydari A, Ceccarelli D, Ovidi E, Quattrucci A (2009) Antibacterial effect of Allium sativum and Ficus carica extracts on tomato bacterial pathogens. Crop Prot 28(10):807–811
Belachew K, Gebremariam M, Alemu K (2015) Integrated management of common bacterial blight (Xanthomonas axonopodis pv. Phaseoli) of common bean (Phaseolus vulgaries) in Kaffa, Southwest Ethiopia. Malays J Med Biol Res 2:147–152
Belete T, Bastas KK (2017) Common bacterial blight (Xanthomonas axonopodis pv. phaseoli) of beans with special focus on ethiopian condition. J Plant Pathol Microbiol 8:403
Borriss R (2011) Use of plant-associated Bacillus strains as biofertilizers and biocontrol agents in agriculture. In: Maheshwari D (ed) Bacteria in agrobiology: plant growth responses. Springer, Berlin, pp 41–76
Chen XH, Vater J, Piel J, Franke P, Scholz R, Schneider K, Koumoutsi A, Hitzeroth G, Gramme N, Strittmatter AW, Gottschalk G et al (2006) Structural and functional characterization of three polyketide synthase gene clusters in Bacillus amyloliquefaciens FZB42. J Bacteriol 188:4024–4036
Chen XH, Koumoutsi A, Scholz R, Schneider K, Vater J, Sussmuth R, Piel J, Borriss R (2009) Genome analysis of Bacillus amyloliquefaciens FZB42 reveals its potential for biocontrol of plant pathogens. J Biotechnol 140:27–37
CIAT (Centro International de Agricultura Tropical) (1998) Annual report. International centre for Tropical Agriculture, CIAT, Cali, Colombia, 39 pp
Corrêa BO, Soares VN, Sangiogo M, de Oliveira JR, Moura AB (2017) Interaction between bacterial biocontrol-agents and strains of Xanthomonas axonopodis pv. phaseoli effects on biocontrol efficacy of common blight in beans. AJMR 11(32):1294–1302
Donmez MF, Sahin F, Elkoca E (2013) Identification of bean genotypes from Turkey resistance to common bacterial blight and halo blight diseases. . Acta Sci Pol Hortorum Cultus 12(4):139–151
Dursun A, Donmez MF, Sahin F (2002) Identification of resistance to common bacterial blight disease on bean genotypes grown in Turkey. EJPP 108:811–813
Fetene S, Ayalew A (2016) Integrated management of common bacterial blight (Xanthomonas campestris pv. phaseoli) and its effect on seed yield of common bean (Phaseolus vulgaris L.). Int J Life Sci 4(3):336–348
Fortunati E, Mazzaglia A, Balestra GM (2019) Sustainable control strategies for plant protection and food packaging sectors by natural substances and novel nanotechnological approaches. J Sci Food Agric 99:986–1000
Fousia S, Paplomatas EJ, Tjamos SE (2016) Bacillus subtilis QST 713 confers protection to tomato plants against Pseudomonas syringae pv. tomato and induces plant defence-related genes. J Phytopathol 164:264–270
Gilardi G, Manker DC, Garibaldi A, Gullino ML (2008) Efficacy of the biocontrol agents Bacillus subtills and Ampelomyces quisqualis applied in combination with fungicides against powdery mildew of zucchini. JPDP 115(5):208–213
Gilardi G, Gullino ML, Garibaldi A (2010) Evaluation of spray programmes for the management of leaf spot incited by Pseudomonas syringae pv. syringae on tomato cv. Cuore di bue. Crop Prot 29:330–335
Haas D, Defago G (2005) Biological control of soil-borne pathogens by fluorescent Pseudomonas. Nat Rev Microbiol 3:307–319
Horvath DM, Stall RE, Jones JB, Pauly MH, Vallad GE, Dahlbeck D, Scott JW (2012) Transgenic resistance confers effective field level control of bacterial spot disease in tomato. PLoS One 7:1–9
Ibrahim YE, Saleh AA, El-Komy MH, Al-Saleh MA (2016) Bacillus subtilis QST 713, copper hydroxide, and their tank mixes for control of bacterial citrus canker in Saudi Arabia. J Cit Pathol 3(1):1–6
Kinsella K, Schulthess CP, Morris TF, Stuart JD (2009) Rapid quantification of Bacillus subtilis antibiotics in the rhizosphere. Soil Biol Biochem 41:374–379
Lahlali R, Peng G, McGregor L, Gossen BD, Hwang SF, McDonald M (2011) Mechanisms of the biofungicide Serenade (Bacillus subtilis QST713) in suppressing clubroot. Biocontrol Sci Technol 21(11):1351–1362
Lamichhane JR, Osdaghi E, Behlau F, Köhl J, Jones JB, Aubertot JN (2018) Thirteen decades of antimicrobial copper compounds applied in agriculture. A review. Agron Sustain Dev 38(3):28
Lugtenberg B, Kamilova F (2009) Plant-growth promoting rhizobacteria. Annu Rev Microbiol 63:541–556
Nakkeeran S, Fernando WGD, Siddiqui ZA (2005) Plant growth promoting rhizobacteria formulations and its scope in commercialization for the management of pests and diseases. In: Siddiqui ZA (ed) PGPR: Biocontrol and Biofertilization. Springer, Dordrecht, pp 257–296
Opio AF, Allen DJ, Teri JM (1996) Pathogenic variation in Xanthomonas campestris pv. phaseoli, the causal agent of common bacterial blight in Phaseolus beans. Plant Pathol 45:1126–1133
Paçe H, Vrapi H, Gixhari B (2016) Evaluation of some reduced-risk products for management of powdery mildew in greenhouse tomatoes. IJEES 4:505–508
Popovic T, Starovic M, Aleksic G, Zivkovic S, Josic D, Ignjatov M, Milovanovic P (2012) Response of different beans against common bacterial blight disease caused by Xanthomonas axonopodis pv.Phaseoli. Bulg J Agric Sci 18:701–707
Quattrucci A, Ovidi E, Tiezzi A, Vinciguerra V, Balestra GM (2013) Biological control of tomato bacterial speck using Punica granatum fruit peel extract. Crop Prot 46:18–22
Rahman MM, Ali ME, Ali MS, Rahman MM, Islam MN (2008) Hot water thermal treatment for controlling seed-borne mycoflora of maize. Int J Sustain Crop Prod 3:5–9
Roberts PD, Momol MT, Ritchie L, Olson SM, Jones JB, Balogh B (2008) Evaluation of spray programs containing famoxadone plus cymoxanil, acibenzolar-S-methyl, and Bacillus subtilis compared to copper sprays for management of bacterial spot on tomato. Crop Prot 27(12):1519–1526
Robledo-Buriticá1 J, Aristizábal-Loaiza1 JC, Ceballos-Aguirre N, Cabra-Cendales T (2018) Blackberry (Rubus glaucus Benth. cv. thornless) growth under semi-cover and field conditions. Acta Agron 67(2):258–263
Schuster ML, Coyne DP (1981) Source of Phaseolus species resistance and leaf and pod differential reactions to common blight. Hort Sci 18(6):901–903
Shanery G, Finney RE (1977) The effect of nitrogen fertilization on the expression of slow-mildewing resistance in Knox wheat. Phytopathol 67:1051–1056
Sundin GW, Jones AL, Fulbright DW (1989) Copper resistance in Pseudomonas syringae pv. syringae from cherry orchards and its associated transfer in vitro and in planta with a plasmid. Phytopathology 79:861 865
Sundin GW, Werner NA, Yoder KS, Aldwinckle HS (2009) Field evaluation of biological control of fire blight in the eastern United States. Plant Dis 93:386–394
Valarmathi P, Pareek SK, Priya V, Rabindran R, Chandrasekar G (2013) Compatibility of copper hydroxide (Kocide 3000) with biocontrol agents. IOSR-JAVS 3(6):28–31
Wheeler BEJ (1969) An introduction to plant diseases. Wiley, London., 347 pp
Zitter TA, Drennan JL, Mutschler MA, Kim MJ (2005) Control of early blight of tomato with genetic resistance and conventional and biological sprays. Acta Hortic 695:181–190
Zweerink M, Edison A (1987) Difficidin and oxydifficidin: novel broad-spectrum antibacterial antibiotics produced by Bacillus subtilis. III. Mode of action of difficidin. J Antibiot 40:1692–1697
Acknowledgements
It is our pleasure to acknowledge Scientific Research Projects Coordination Unit of Selcuk University, Turkey for partial funding to support this research. We also sincerely thank Prof. Dr. Ercan CEYHAN, head department of Field Crop, for allowing the use of his modern greenhouse. We extend our gratefulness to the staff members of Plant Protection Department, Selcuk University for helping us during data collection.
Funding
This project was supported by Scientific Research Project Coordination Unit (No.18201104) of Selcuk University, Turkey. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
First author TB was responsible for planning, design and implementation of the experiments, analyzed and interpreted data, and write and organized the manuscript. Second author KKB conceived of the study, participated in its design, revised the paper scientifically and made suitable changes. SF and GMB accurately revised the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Code availability
Not applicable.
Rights and permissions
About this article
Cite this article
Belete, T., Bastas, K.K., Francesconi, S. et al. Biological effectiveness of Bacillus subtilis on common bean bacterial blight. J Plant Pathol 103, 249–258 (2021). https://doi.org/10.1007/s42161-020-00727-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42161-020-00727-8