Abstract
γ-rays irradiation, is a powerful method for the preparation of colloidal silver nanoparticles (AgNPs). Three different AgNPs with average particle size of 5, 10 and 15 nm were synthesized and stabilized by chitosan (1%), and demonstrated a significant in vitro activities against Phytophthora capsici with 62.5, 84.3 and 100%, inhibition efficacy, respectively. An improved antifungal activity of 10 nm size AgNP was noticed by increasing the initial silver concentration with maximum inhibition efficiency of 92.9% at 100 ppm. The in vivo study performed for fungicidal effect of AgNPs in pepper plants showed that pretreatment of 1–10 ppm AgNPs effectively reduced P. capsici infection leading to enhanced rate of non-disease-outbreak of 53.3 and 95.0%, respectively. On the other hand plants treated with AgNPs after fungus infection displayed 60.0 and 91.7% non-disease-outbreak after the treatment at 10 and 50 ppm concentration, respectively. The results specify the potential role of γ-irradiation synthesized AgNPs for controlling the pathogen P. capsici on black peppers. Moreover, the environmental friendly production of AgNPs and its high effectiveness against fungi prove it a safe and alternative against the conventional antifungal agents.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Black pepper, commonly known as the “King of Spices”, a perennial crop in the tropical areas, is economically important and the widest used spice over the world. Recently, Vietnam has been recorded as the top country for pepper export. The pepper plant is vulnerable for the several diseases caused by fungi, bacteria, viruses and mycoplasma. Among them, the devastation of the dreaded disease “foot rot” caused by Phytophthora fungi is the most serious concern for pepper in Vietnam, India, Indonesia and other countries (Nair 2004). To minimize the effects caused by P. capsici, breeders used several chemical fungicides that may cause a reduction in the quality and quantity of pepper products and are also responsible for environmental pollution. Due to these reason, it is important to find a new natural product with high antifungal activity and less toxicity.
Chitosan, the second most abundant natural polysaccharide on the earth after cellulose, has been widely used in agriculture for agro-product preservation, seed coating, fertilizer and artificial seeds production, owing to its antimicrobial activity (Tay et al. 1993; Vasyokova et al. 2001; Kume et al. 2002; Luan et al. 2005) and plant growth stimulation properties (Kumar 2001). Xu et al. (2007) also reported the in vitro antifungal effect of degraded chitosan against several plant pathogenic fungi such as P. capsici, Alternaria solani, Botrytis cinerea, Colletotrichum orbiculare, Exserohilum turcicum, Fusarium oxysporum and Pyricularia oryzae. A novel feature reported for chitosan is the ability to induce phytoalexin to prevent the infection of fungal diseases in plant (Warker-Shimmons et al. 1983; Albersheim and Darvill 1985).
On the other hand, applications of nanomaterials showed beneficial effects for seed germination, plant production and protection with a reduction of environmental impact as compared to traditional approaches (Khot et al. 2012). Silver nanoparticles (AgNPs) have been suggested as a new generation of antimicrobials due to their novel properties and wide applications (Rai et al. 2009). These particles proved to have strong bactericidal effect against several bacteria including multi-resistant strains (Morones et al. 2005; Panacek et al. 2006). The studies of Panacek et al. (2006) and Shrivastava et al. (2007) on bactericidal activity of AgNPs proved that these products can eliminate bacteria at very low concentrations that do not cause any acute toxic effect for human cells (Carlson et al. 2008; Asharani et al. 2009). A few reports on the application of AgNPs for plants have also been published recently. An et al. (2008) demonstrated an effect of AgNPs on extending maintenance period of leaves (from 2 to 21 days) in asparagus plants; in addition, during this period the amounts of ascorbat, chlorophyll and fiber were increased in treated leaves. Park et al. (2006) reported hybrid nano-size composition made up of silica and silver as a new product that can control several plant diseases. Phu et al. (2010) and Lamsal et al. (2011) showed that AgNPs have a strong inhibition effect on the growth of Corticium salmonicolor causing the rubber pink-disease and Colletotrichum species causing the pepper anthracnose disease, respectively. Seif et al. (2011) reported that the treatment with 20–60 ppm AgNPs enhanced the seed yield and reduced the seed abscission in borage (Borago officinalis L.) but the increasing level of AgNPs resulted in the reduction of polyphenols compounds content in the seed. Pokhrel and Dubey (2013) also reported that the toxicity of AgNPs on seed germination and root elongation on maize and cabbage was much lower than that due to silver ions when used at the same concentration.
Taken all the above into consideration, a promising effect is suggested if a new biological fungicide is prepared using the combination of chitosan and AgNPs. The present study is aimed to prepare AgNPs stabilized by chitosan using γ-irradiation method and to further investigate its in vitro and in vivo effect against P. capsici, causing the most serious disease on black pepper plants.
Materials and methods
AgNO3, lactic acid, NaOH, deionized water and carrot agar (CRA) media were purchased from Merck (Germany). Chitosan 8B with a deacetylation degree nearly 80% was supplied by Funakoshi (Tokyo, Japan). Gamma Co-60 with a dose rate about 3 kGy/h used for irradiation was obtained from India (BRIT-5000). The fungal strain Phytophthora capsici was supplied by Agriculture and Forestry University, Ho Chi Minh City, Vietnam.
Preparation of silver nanoparticles by γ-rays irradiation
In a typical preparation of the chitosan solution, 2 g chitosan was dissolved in 80 ml lactic acid (ca. pH 3) using a magnetic stirrer for complete dissolution. For the preparation of silver nanoparticles (AgNPs) with the average diameter approximately 15 nm, 50 ml of 20 mM AgNO3 solution was mixed with 50 ml chitosan solution and bubbled with N2 for 15 min for deaeration. For preparation of AgNPs with the average diameters approximately 5 and 10 nm, 50 ml of chitosan solution was adjusted to pH 6 and subsequently added with 50 ml of 2 and 20 mM AgNO3 solutions, respectively. The solutions were then bubbled with N2 for deaeration. All the three samples were then irradiated at doses in range 8–32 kGy by a gamma cell with a dose rate of 3 kGy/h for the synthesis of colloidal AgNPs.
Characterization of AgNPs
For characterization of AgNPs, absorbance was recorded using spectrophotometer UV-2401PC (Shimadzu, Japan) at the range of 200–800 nm. Size and morphology of formed AgNPs were determined by transmission electron microscope (TEM) (JEM 1010, JEOL, Japan) imaging. The size and distribution was then calculated using Photoshop software (Du et al. 2008).
In vitro antifungal effect of AgNPs against P. capsici
The in vitro antifungal activity of AgNPs against P. capsici was tested by using a culture medium toxicity method (Cho et al. 2005; Sanpui et al. 2008). For investigation of the particle size effect, the AgNPs with average sizes of 5, 10 and 15 nm were added to carrot agar (CRA) media (Stelfox and Herbut 1979) with the final Ag concentration of 60 ppm. For the concentration dependent study, AgNPs with a particle size of 10 nm were used at various concentrations in the range of 20 to 100 ppm. For each particle size and concentration test, 15 ml sterilized CRA media containing the desired AgNPs was poured into petri dish and incubated at room temperature for 48 h. After incubation each petri dish was inoculated with 8 mm diameter agar plug containing fungus and incubated at 28 ± 2 °C. The diameter of colony growth was measured after incubating for 60 h. All the tests were performed in triplicates and the antifungal activity was determined as follows:
where d0 and d are the fungal growth diameter (mm) in the control and on AgNPs added plates, respectively.
In vivo fungicidal effect of AgNPs against P. capsici on pepper plants
Six month old disease-free pepper plants were grown in pots and maintained in greenhouse. To test the fungicidal effect, the pots were divided into two groups based on the AgNPs treatment before and after infection by P. capsici according to the method suggested by Drenth and Guest (2004). Each group consisting of 300 plants (60 plants for each treatment) were used for the defence test, using Ag concentrations of 1, 3, 5 and 10 ppm. Each plant was treated with 50 ml of the specific AgNPs concentration by foliar spraying and 50 ml through soil, irrigating every week up to 3 weeks. The control plants were similarly treated with distilled water. After treatment, infection of P. capsici (300 ml) containing 104 fungal spores/ml was done by foliar spraying (150 ml) and soil irrigation (150 ml).
The second group included 300 plants (60 plants for each treatment), which were used for testing the elimination effect of AgNP on P. capsici. Each plant was first infected with 300 ml P. capsici containing 104 fungal spores/ml by foliar spraying (150 ml) and soil irrigation (150 ml). Three days after pathogen infection, the AgNPs solution at five different concentrations of 10, 20, 30, 40 and 50 ppm was sprayed as described above for the first group. The disease outbreak on plants was determined after 35 days of treatment and the diseased symptoms for foot rot disease were recognized as described by Nair (2004). Each experiment was carried out in triplicates and data were statistically analyzed using the analysis of variance (ANOVA) test. The mean values were compared using the Duncan’s multiple range test at a probability level below 0.5%.
Results and discussion
Characterization of AgNPs prepared by irradiation
For the preparation of AgNPs by γ-irradiation method, chitosan is an effective agent used for reducing and stabilizing, due to its property of scavenging OH radicals generated from radiolysis of water with the abstraction of hydrogen and formation of the new chitosan radicle that itself can reduce Ag+ to Ag0 (Chen et al. 2007; Long et al. 2007). In addition, the stabilization mechanism of chitosan for AgNPs is due to their interaction with amino (-NH2) groups in chitosan molecule and the AgNPs are covered by the fragments of chitosan. Beside this, in solution -NH2 groups of chitosan shell are protonated and form NH3+ ions, which protect AgNPs from the agglomeration through static repulsions between each other (Chen et al. 2007). For this reason, we used chitosan for preparation of the colloidal AgNPs solution.
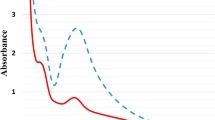
The OD value of colloidal AgNPs solution is 1.057 at the irradiation dose of 8 kGy, with the λmax 395 nm, and 5 nm average particle size (Fig. 1). It has been shown previously by Phu et al. (2010) that the dose of 8 kGy is enough to saturate conversion for Ag+→Ago completely.
Results depicted in the Fig. 2 pointed out that the dose of 28 kGy generated the colloidal AgNPs solution with OD value of 0.847, λmax 404 nm and average particle size of about 10 nm.
The results for preparation of AgNPs in Fig. 3 showed that the colloidal AgNPs solution synthesized by irradiating the low pH solution of 5 mM AgNO3 at 16 kGy had OD value of 0.754, λmax value of 412 nm and average particle size of about 15 nm. The reason for the fact that the size of AgNPs in this experiment was bigger than that of AgNPs size prepared from the solution containing 10 mM Ag may be explained as the reduction reaction of Ag+ into Ag0 could not be favorable for the formation of small size AgNPs in an acidic medium that contains a higher H+ concentration (Phu et al. 2010). Moreover, Sun et al. (2008) also confirmed that in an acidic aqueous solution the chains of chitosan were easily broken leading to a reduced stabilization function.
In summary, the characteristics of all three prepared colloidal AgNPs solutions were presented in Table 1.
The antifungal effect of AgNPs against P. capsici in vitro
The antibacterial effect of AgNPs has been studied on several bacterial strains (Cho et al. 2005) and the antimicrobial activity of AgNPs was well associated with the particles size. Results of antifungal activity of AgNPs with average size of 5, 10 and 15 nm are presented in Fig. 4 and Table 2. It can be seen that the AgNPs with sizes from 5 to 15 nm inhibited the growth of the causative fungus of pepper foot rot disease and antifungal effect was inversely proportional to the particle size. In particular, the supplementation of 60 ppm of 5 nm AgNPs completely inhibited the growth of test fungus (the inhibition efficiency was almost 100%) while the inhibition efficiency decreased to 84.3 and 62.8% using 10 nm and 15 nm AgNPs, respectively. Although the 5 nm AgNPs displayed the highest antifungal effect, the Ag concentration was rather low (1 mM) and it was considered to be not convenient for production and high cost for application. The 10 nm AgNPs also strongly inhibited by 84.3% the growth of tested fungus but the Ag concentration in this AgNPs solution was 10 folds higher (10 mM). Therefore, the 10 nm AgNPs solution was selected for the further experiments.
As depicted in Fig. 5 and Table 3 the growth of P. capsici on CRA medium was inhibited to 22.6% by the addition of 20 ppm AgNPs (10 nm) and the inhibition efficiency was improved by the increasing of AgNP concentration. At the concentration of 100 ppm, AgNPs completely inhibited the growth of this fungi. These results are in agreement with those of Phu et al. (2010) on C. salmonicolor, a fungus strain causing the serious pink-disease on rubber plant.
The fungicidal effect against P. capsici in vivo
There are several reports suggesting the antimicrobial effect of AgNPs against bacteria and fungi (Cho et al. 2005; Du et al. 2008; Jo et al. 2009; Phu et al. 2010), however, the in vivo studies on plants are still limited. In the present study, the antifungal effect of AgNPs against P. capsici was investigated directly on black pepper plants. Since the infection occurred aerially as well as through soil, the AgNPs with low Ag concentrations (1–10 ppm) were applied on the tested pepper plants via both foliar spraying and soil irrigation before being infected by fungi, in order to evaluate the efficiency of prevention. After 35 days since fungal infection, the disease-outbreak plants were determined by the specific symptoms described by Nair (2004) as follows. Once there is a foliar infection, one to many dark spots are produced, consisting of characteristic fimbriate margins, which eventually leads to defoliation before the lesions are scattered to the whole lamina. On tender shoots, the fungi freely develop and then form a white covering. When the stem feels the infection, the entire plant will come to the stage of abrupt wilting. In case the infection occurs underground on roots, rotting and degeneration appears, which leads to yellowing, defoliation, and drying up the whole plants. The main roots are the route that feeder roots infection takes place, causing the characteristic foot rot.
The results depicted in Table 4 revealed clearly that the preventive application of AgNPs at concentrations from 1 to 10 ppm significantly decreased the number of diseased plants and the ratio of non-diseased plants was increased from 53.3% by the treatment of 1 ppm AgNP to about 95% by the treatment of 10 ppm AgNPs. It can be hypothesized that, after spraying, plant cells could retain AgNPs, which may kill the infecting pathogen fungus (Panacek et al. 2006; Shrivastava et al. 2007). In addition, the chitosan used for stabilizing AgNPs also can elicit the multiple defense responses in the treated plants (Xu et al. 2007).
Alternatively, the AgNP also proved efficient in controlling the disease when sprayed after the infection caused by fungi. The results from Table 5 disclosed that the damages of pepper plants infected by P. capsici fungus could be minimized by AgNP. In particular, the ratio of non-diseased plant was increased from 60 to 91.7% by treating AgNPs with the Ag concentrations from 10 to 50 ppm, respectively. This may happen because the cell density of fungi in the tested plants are higher after infection and thus a higher number of AgNPs are required for killing them. It is evident from Tables 4 and 5 that the preventive application of AgNP can reduce its quantity from 5 to 10 folds as compared to that for recovery treatment after infection of pepper plants by P. capsici. These rsults are in agreement with the previous findings on Colletotrichum species causing the pepper anthracnose disease (Lamsal et al. 2011). Furthermore, an interesting point inferred from Fig. 6 is that the development of roots in the pepper plant treated with AgNPs (3 ppm) before fungi infection was much better than that of the plant treated with AgNPs (30 ppm) after infection.
Black pepper plants after cultivating in a greenhouse for 35 days. a a control plant artificially-infested by the pathogenic fungus and without treatment by AgNPs, b a plant artificially-infested by the pathogenic fungus and then treated with 20 ppm AgNPs and c a plant treated with 3 ppm AgNPs before artificial inoculation with the pathogenic fungus
The study for application of AgNPs in agriculture is still limited. According to Scheckel et al. (2010), the aging of AgNPs released into the environment may cause the AgNPs to undergo physical or chemical changes, including changes in size, shape, agglomeration status and surface chemistry, that may cause toxicological and environmental negative effects. More studies are needed to understand the toxicological and ecological risks associated with the use of AgNPs. AgNPs have been found to exhibit higher toxicity to microorganisms while it exhibits lower toxicity to mammalian cells (Zhao and Stevens 1998). According to Maneewattanapinyo et al. (2011) the LD50 of colloidal AgNPs is greater than 5,000 mg/kg body weight. All these toxicity tests suggested that colloidal AgNPs could be relatively safe when administered to oral, eye and skin of the animal models for short periods of time. Babu et al. (2014) also reported both larvicidal and bactericidal activities of AgNPs in their research. The treatment with AgNPs significantly increased the mortality of the larvae of Lepidiota mansueta (Burmeister), but the maximum LC50 and LC90 values with III-instar larvae were found at rather high concentrations (4,529 and 9,580 mg/l, respectively). Even so, World Health Organisation’s guidelines for drinking water quality indicated a limit of 0.1 ppm for the concentration of silver ions in drinking water and a total lifetime oral intake of about 10 g of silver can be considered as the human NOAEL (No-observed-adverse-effect level) (WHO 2011). In addition, Tran et al. (2013) mentioned that AgNPs can be promisingly used as a new product for treating infectious pathogens and preventing microbial infections and for advanced environmental treatments (air disinfection, water disinfection, surface disinfection and personal hygiene). However, its application needs to determine how to safely design, use, and dispose of products containing silver without creating a new risk to humans or the environment (including the kill of non-targeted microbials). Due to the above reasons, the AgNPs products are not approved as a fungicides for agriculture world-wide. In Vietnam, only two products containing AgNPs have been approved as fungicides for rice crop by the Ministry of Agriculture and Rural Development.
In conclusion, synthesized AgNP has a great potential to be used as control agent against plant pathogenic fungi. Moreover, its low toxicity to environment makes it a suitable candidate as a commercial fungicide after sufficient field trials.
References
Albersheim P, Darvill AG (1985) Oligosaccharins. Sci Am 253:44–50
An J, Zhang M, Wang SH, Tang J (2008) Physical, chemical and microbiological change in stored green asparagus spears as affected by coating of silver nanoparticle-PVP. LWT Food Sci Technol 41:1100–1107
Asharani PV, Mun GLK, Hande MP, Valiyaveettil S (2009) Cytotoxicity and genotoxicity of silver nanoparticles in human cells. ACS Nano 3:279–290
Babu MY, Devi JV, Ramakritinan CM, Umarani R, Taredahalli N, Kumaraguru AK (2014) Application of biosynthesized silver nanoparticles in agricultural and marine Pest control. Curr Nanosci 14:374–381
Carlson C, Hussain SM, Schrand AM, Braydich-Stolle LK, Hess KL, Jones RL (2008) Unique cellular interaction of silver nanoparticles: size-dependent generation of reactive oxygen species. The Journal of Physical Chemistry (Part B) 112:130608–130619
Chen P, Song L, Lui Y, Fang Y (2007) Synthesis of silver nanoparticles by γ – ray irradiation in acetic water solution containting chitosan. Radiat Phys Chem 76:1165–1168
Cho KH, Park JE, Osaka T, Park SG (2005) The study of antimicrobial activity and preservative effects of nanosilver ingredient. Electrochim Acta 51:956–960
Drenth A, Guest DI (2004) Diversity and Management of Phytophthora in Southeast Asia. BPA Print Group Pty Ltd, Melbourne
Du BD, Phu DV, Duy NN, Lan NTK, Lang VTK, Thanh NVK, Phong NTP, Hien NQ (2008) Preparation of colloidal silver nanoparticles in poly (N- vinylpyrrolidone) by γ- irradiation. J Exp Nanosci 3:207–213
Jo YK, Kim BH, Jung G (2009) Antifungal activity of silver ions and nanoparticles on Phytopathogenic fungi. Plant Disease Journal 10:1037–1043
Khot LR, Sankaran S, Maja JM, Ehsani R, Schuster EW (2012) Applications of nanomaterials in agricultural production and crop protection: a review. Crop Prot 35:64–70
Kumar MNVR (2001) A review of chitin and chitosan applications. React Funct Polym 46:1–27
Kume T, Nagasawa N, Yoshii F (2002) Utilization of carbohydrates by radiation processing. Radiat Phys Chem 63:625–627
Lamsal K, Kim SW, Jung JH, Kim YS, Kim KS, Lee YS (2011) Application of silver nanoparticles for the control of Colletotrichum species in vitro and pepper anthracnose disease in field. Mycobiology 39:194–199
Long D, Wu G, Chen S (2007) Preparation of oligochitosan stabilized silver nanoparticles by gamma irradiation. Radiat Phys Chem 76:1126–1131
Luan LQ, Ha VTT, Nagasawa N, Kume T, Yoshii F, Nakanishi TM (2005) Enhancement of plant growth activity of irradiated chitosan by molecular weight fractionation. Biotechnol Appl Biochem 41:49–57
Maneewattanapinyo P, Banlunara W, Thammacharoen C, Maneewattanapinyo P, Banlunara W, Thammacharoen C, Ekgasit S, Kaewamatawong T (2011) An evaluation of acute toxicity of colloidal silver nanoparticles. J Vet Med Sci 73:1417–1423
Morones JR, Elechiguerra JL, Camacho A, Holt K, Kouri JB, Ramirez JT (2005) The bactericidal effect of silver nanoparticles. Nanotechnology 16:2346–2353
Nair KPP (2004) The agronomy and economy of black pepper (Piper nigrum L) - the “king of spices”. Academic Press, New Delhi
Panacek A, Kvítek L, Prucek R, Kolar M, Vecerova R, Pizúrova N, Sharma VK, Nevecna T, Zboril R (2006) Silver colloid nanoparticles: synthesis, characterization, and their antibacterial activity. J Phys Chem 110:16248–16253
Park HJ, Kim SH, Kim HJ, Choi SH (2006) A new composition of nanosized silica-silver for control of various plant diseases. The Plant Pathology Journal 22:295–302
Phu DV, Lang VTK, Lan NTK, Duy NN, Chau ND, Du BH, Cam BD, Hien NQ (2010) Synthesis and antimicrobial effects of colloidal silver nanoparticles in chitosan by γ-irradiation. J Exp Nanosci 5:169–179
Pokhrel LR, Dubey B (2013) Evaluation of developmental responses of two crop plants exposed to silver and zinc oxide nanoparticles. Sci Total Environ 452-453:321–332
Rai M, Yadav A, Gade A (2009) Silver nanoparticles as a new generation of antimicrobials. Biotechnol Adv 27:76–83
Sanpui P, Murugadoss A, Prasad PVD, Ghosh SS, Chattopadhyay A (2008) The antibacterial properties of a novel chitosan-ag-nanoparticle composite. Int J Food Microbiol 124:142–146
Scheckel KG, Luxton TP, El-Badawy AM, Impellitteri CA, Tolaymat TM (2010) Synchrotron speciation of silver and zinc oxide nanoparticles aged in a kaolin suspension. Environ Sci Technol 44:1307–1312
Seif SM, Sorooshzadeh A, Rezazadehs H, Naghdibadi HA (2011) Effect of nano silver and silver nitrate on seed yield of borage. Journal of Medicinal Plants Research 5:171–175
Shrivastava S, Bera T, Roy A, Singh G, Ramachandrarao P, Dash D (2007) Characterization of enhanced antibacterial effects of novel silver nanoparticles. Nanotechnology 18:103–112
Stelfox D, Herbut M (1979) Growth of a Phytophthora sp. on carrot agar. Can Plant Dis Surv 59:61–62
Sun C, Qu R, Chen H, Ji C, Wang C, Sun Y, Wang B (2008) Degradation behavior of chitosan chains in the ‘green’ synthesis of gold nanoparticles. Carbohydr Res 343:2595–2599
Tay LP, Khoh LK, Loh CS, Khor E (1993) A review of the application of chitin and its derivative in agriculture to modify plant-microbial interaction and improve crop yields. Biotechnol Bioeng 42:449–454
Tran QH, Nguyen VQ, Le AT (2013) Review: silver nanoparticles: synthesis, properties, toxicology, applications and perspectives. Advances in Natural Sciences. Nanosci Nanotechnol 4:033001
Vasyokova NI, Zinov'eva SV, Il'inskaya LI, Perekhod EA, Chalenko GI, Gerasimova NG, Il'ina AV, Valamov VP, Ozeretskovskaya OL (2001) Modulation of plant resistance to diseases by water-soluble chitosan. Appl Biochem Microbiol 37:103–109
Warker-Shimmons M, Hadadwiger L, Ryan CA (1983) Chitosans and pectic polysaccharides both induce the accumulation of antifungal phytoalexin pisatin in pea pods and antinutrient proteinase inhibitor in tomato leaves. Biochem Biophys Res Commun 110:194–199
World Health Organization (WHO), 2011. Guidelines for drinking water quality. 4th Ed. Geneva, Switzerland. pp 415
Xu J, Zhao X, Han X, Du Y (2007) Antifungal activity of oligochitosan against Phytophthora capsici and other plant pathogenic fungi in vitro. Pestic Biochem Physiol 87:220–228
Zhao GJ, Stevens SE (1998) Multiple parameters for the comprehensive evaluation of the susceptibility of Escherichia coli to the silver ion. Biometals 11:27–32
Acknowledgments
This research was supported by Biotechnology Center of Ho Chi Minh City (Project No. NN01/16-17).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Luan, L.Q., Xo, D.H. In vitro and in vivo fungicidal effects of γ-irradiation synthesized silver nanoparticles against Phytophthora capsici causing the foot rot disease on pepper plant. J Plant Pathol 100, 241–248 (2018). https://doi.org/10.1007/s42161-018-0064-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42161-018-0064-4