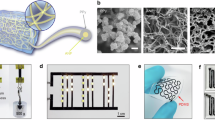
Abstract
Elastic conductors play a crucial role in the fabrication of wearable electronic devices and human–computer interaction devices. Among the various candidates for elastic conductors, hydrogels, featuring 3-D swollen macromolecular networks, exhibit exceptional stretchability and biocompatibility. Notably, physical hydrogels based on poly (vinyl alcohol) (PVA), which contains a substantial number of reactive groups (-OH groups), stand out due to their remarkable biocompatibility, superior mechanical properties, and chemical stability. This review focuses on recent advancements in the composite strategy, preparation, and current applications of PVA-based conductive composite hydrogels. Firstly, PVA-based conductive hydrogels are classified based on various conductive treatments: (i) introduction of conductive fillers to the PVA with a single network structure; (ii) introduction of conductive fillers to the PVA with double/multiple network structures (e.g., PVA/carboxymethylcellulose, PVA/poly(acrylamide)); (iii) creation of double-network PVA hydrogel combined with conductive polymers including poly(3,4-ethylene-dioxythiophene)/poly(styrenesulfonate), poly(aniline), poly(pyrrole); (iv) addition of ions to a pure PVA network; (v) addition of ions to the PVA with double network structures (e.g., PVA/sodium alginate, PVA/hydroxyethylcellulose). This review includes a comparative analysis of different conductive hydrogel systems. Secondly, PVA-based conductive hydrogels with diverse functions, such as strain sensing, shape memory, antifreeze properties, transparency, and pH response, are thoroughly reviewed. Thirdly, the latest advancements in the applications of PVA-based conductive hydrogels are demonstrated, including flexible super-capacitors, human–computer interaction devices, and triboelectric nanogenerator sensors. Finally, a summary of the current state of development and critical issues with PVA conductive hydrogels is provided, along with an outlook on how to address each.
Graphical Abstract
Systematic review on PVA conductive hydrogels: outlines preparation strategies and applications in flexible electronic devices.

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
In recent decades, the increasing prevalence of the Internet of Things (IoT) [1] and wearable devices, the demand for the smart wearable industry, has been getting higher and higher. There is no doubt that the original complex and brittle texture of the inorganic material sensors has been unable to handle the bending, twisting, folding, and other action requirements, so flexible sensor parts show great potential for development. The flexible electronics industry has been widely used in various fields, such as human–computer interaction, human motion detection, and electronic skin [2,3,4,5,6]; the challenge remains in developing flexible sensor components that offer high sensitivity, repeatable performance, resistance to freezing, portability, biocompatibility, eco-friendliness, and cost-effectiveness. Addressing this challenge is crucial for scholars seeking to better align with market demands and facilitate industrial upgrading.
The research on polymer-based composite materials has evolved from focusing solely on the structural composite materials with mechanical properties to functional composites with multiple functionalities such as optical, electrical, catalytic, and energy storage [7,8,9,10,11,12,13,14,15,16,17,18,19,20]. The composite strategies play a crucial role in supporting the study of hydrogel composite materials. Conventional flexible sensors typically comprise two components: one being a conductive material, such as Ti3C2Tx (MXene) [21,22,23,24], carbon nanotubes (CNTs) [25,26,27], graphene [28,29,30], liquid metal (LM) [31], metal nanoparticles [32,33,34], conductive polymers [35, 36], and others [37,38,39,40,41,42,43,44]; the other is the flexible substrates such as thermoplastic polyurethane (TPU), [45, 46], polydimethylsiloxane (PDMS) [47,48,49], and rubber [50], which are capable of accommodating large strains and recovering from deformations. The two aforementioned components are combined with wires through processes such as extraction and impregnation, giving rise to the formation of a flexible sensor [51,52,53]. This sensor is capable of converting motion signals from the human body into electrical signals for detection. Currently, flexible sensors prepared through this method stand as typical representatives in the flexible sensing industry [54,55,56,57,58,59]. While this preparation method is straightforward and exhibits commendable electrical conductivity and flexibility, challenges persist. Issues such as insensitive and inaccurate signal transmission, poor mechanical properties, easy separation between conductive materials and elastomers, and low repeatability are notable shortcomings. Undoubtedly, the future development of wearable devices necessitates a new generation of flexible sensors characterized by outstanding mechanical qualities and real-time monitoring to address the aforementioned challenges.
One of the more favored flexible materials, hydrogel [60, 61], is a highly hydrophilic polymer with a three-dimensional structure [61, 62]. Under specific circumstances, it can maintain a fixed volume and shape as a solid while also diffusing and permeating solutes like a liquid, exhibiting good biocompatibility. Therefore, it is widely used in tissue engineering [63], biomedicine [64, 65], and wound dressings [65,66,67]. The pure hydrogel lacks strength, making it prone to permanent fractures. Moreover, its internal structure is simple and lacks specialized functionalities, significantly limiting its applications in the field of flexible electronic devices. However, due to the advantages of softness and flexibility, self-healing, antifreeze, transparency, etc. [68], hydrogels, after undergoing structural modification, hold immeasurable potential applications in the field of wearable devices. Building double-network [69,70,71] and multiple-network structures [57] in hydrogels is an effective means to enhance their mechanical performance.
PVA hydrogels [5, 61] have been extensively employed as hydrogel materials in the past decade owing to their water solubility, chemical stability, biodegradability, and biocompatibility, attributed to the significant quantity of -OH groups on the molecular chain [72]. PVA hydrogels can undergo cross-linking through three primary techniques: repetitive freezing and thawing to generate microcrystals [73, 74]; utilization of cross-linking agents such as borax [75]; aldehydes [76] and PVA chain reaction of -OH cross-linking [77]; ultraviolet irradiation crosslinking [78] and gamma ray irradiation crosslinking [79]. The progress in PVA-based conductive hydrogel sensors, featuring enhanced mechanical properties, electrical conductivity, close conformity to the human body, and biocompatibility, is paramount. This necessity arises from the potential of all three previously mentioned cross-linking techniques to, to some extent, contribute to the degradation of the hydrogel’s mechanical properties, introduce toxicity, and give rise to other issues.
PVA hydrogels are commonly rendered conductive by incorporating solutions of salt ions, conductive polymers, carbon compounds, or metal materials into the hydrogel system. In the realm of wearable devices, the electrical conductivity of PVA-based conductive hydrogels plays a pivotal role in boosting sensor sensitivity. Nevertheless, the introduction of conductive materials may influence the mechanical properties of hydrogels. Striking a balance between electrical conductivity and mechanical performance becomes paramount. Hydrogel is a crucial material with abundant water content, and its physicochemical properties can undergo significant changes over a wide range. Researchers have conducted extensive studies on hydrogels over the years, leading to a substantial enhancement in their performance and an expanding range of applications. In the realm of flexible wearables, PVA-based conductive hydrogel, besides the necessities of electrical conductivity and mechanical performance, also demonstrates multifunctionality, such as anti-freezing, anti-drying, and biocompatible. Due to the flexibility and biocompatibility of hydrogels, they demonstrate promising prospects in the field of wearables. They have the potential to become crucial components in sensors, smart adhesive devices, etc., for monitoring physiological parameters and enabling human–machine interaction. However, before their widespread adoption, there are still many issues that need further research. Figure 1 reviews the categorization and potential applications of PVA-based conductive hydrogels.
This review delves into PVA-based conductive hydrogel systems, providing an overview of strategies and preparation methods for constructing composite hydrogel systems. These include introducing conductive components into the PVA matrix to achieve high electrical conductivity, incorporating additional components to build dual or multi-network structures for enhanced mechanical properties, and utilizing composites to achieve multifunctionality such as anti-freezing, self-healing, and fatigue resistance. The review also highlights the latest advancements in the applications of PVA-based conductive hydrogels in areas such as human–machine interfaces, flexible electronic devices, and friction-based nanogenerators.
To achieve conductivity, hydrogel materials can be doped with conductive particles, including carbon nanotubes, carbon black, and nano-silver wires. Additionally, introducing conductive polymers into PVA can generate a dual network structure for intrinsic conductivity. Alternatively, submerging it in ionic solutions allows the hydrogel to achieve conductivity through the movement of ions. This paper focuses on the application of PVA-based conductive hydrogels as strain sensors, highlighting their sensing capability for strain. It also introduces various functionalities such as transparency, anti-freezing, pH responsiveness, humidity responsiveness, and shape memory. The subsequent sections of the article delve into the applications of PVA-based conductive hydrogels in flexible devices, including intelligent clothing, flexible supercapacitors, human–computer interfaces, and self-powered flexible sensing. The conclusion of the article provides an overview of the current research directions of PVA-based conductive hydrogels and analyzes the challenges and opportunities they may encounter in fields such as smart wearables.
2 Design and preparation of PVA conductive hydrogels
PVA conductive hydrogels typically comprise two main components: the hydrogel matrix and the conductive materials. Based on the diverse conductive materials used, we can broadly categorize them into the following three groups: (1) hydrogels with conductive particles; (2) hydrogels containing conducting polymers; and (3) hydrogels that conduct ions. This section discusses and summarizes the progress in research on PVA-based conductive hydrogels. It primarily introduces the design and preparation of one of the most commonly used PVA-based conductive hydrogels, along with the electronic characteristics of conductive materials for classification. The schematic representation of the classification of PVA-based conductive hydrogels is presented in Fig. 2.
2.1 Filler-type PVA hydrogel
The two main categories of fillers used in the preparation of PVA-based conductive hydrogels are carbon nanomaterials, such as carbon black (CB) [80], CNTs [81,82,83], graphene [84], and metal materials, such as silver nanowires (AgNWs) [85, 86], gold nanoparticles (AuNPs) [87], and copper nanowires, among others.
Due to its biocompatibility, ease of surface functionalization, high chemical stability, high electrical conductivity, significant surface area, and substantial mechanical strength, carbon nanofillers are among the most commonly used materials in the field of flexible sensing [88, 89]. Although conductive fillers can significantly enhance electrical conductivity, they often lead to reduced stretchability and inferior mechanical properties in conductive hydrogels.
To address the aforementioned issues, scholars have approached the problem from various angles. Analyzing the composition of hydrogel, it exhibits hydrophilic and self-healing abilities owing to its abundant -OH content. Building on this, Yue Zhang et al. [90] proposed a hydrogel with an island-bridge structure by combining PVA/CNT hydrogel and PVA/graphene conductive hydrogel (Fig. 3a), where PVA/graphene hydrogel serves as the “island” and PVA/CNT hydrogel acts as the entire “bridge.” This unique structure demonstrates outstanding electrical conductivity, remarkably high elastic strain (up to 900%), and robust mechanical strength (up to 10 kPa). It also exhibits good tensile strain sensitivity (with a measurement factor of 152.6 in the strain range of 316–600%) and a broad detection operating range (1–600%).

Reproduced with permission from Ref. [90]. Copyright 2021 RSC. b Schematic molecular structure of PVA/Gly/CNTs/CB hydrogel and its application in human physiological signal detection. Reproduced with permission from Ref. [91]. Copyright 2020 ACS
a Schematic diagram of the preparation process and formation mechanism of PVA/CNTs/hraphene hydrogel.
Jianfeng Gu et al. [91] prepared a conductive organic hydrogel with a three-dimensional honeycomb structure by adding CNTs and CB to PVA/glycerol (Gly) organic hydrogel (Fig. 3b), which had enhanced tensile strength (4.8 MPa), tenacity (15.93 MJm−3), elongation at break (640%), and sensitivity factor (GF) of 2.1 due to its special structure. The sensor can detect the full range of human physiological signals and respond to changes in temperature, and can be applied in the field of multifunctional wearable electronics.
In order to reduce the impact of the addition of conductive fillers on the mechanical properties of hydrogels, some scholars have started to look at the dispersion of conductive fillers in the hydrogel system. Zhengqiang Guo et al. [3] used fast oxidizing cellulose nanomaterials (TOCNF) as an effective dispersant for multi-walled carbon nanotubes (MWCNTs), and then combined PVA hydrogels and TOCNF-MWCNT dispersions were combined and the conductive hydrogels were prepared by a simple one-pot method and freeze–thaw cycle physical cross-linking method. The obtained hydrogels have high water content (~ 88.8%), mechanical properties (ultimate stress of 1.1 MPa and strain of 336%), and electrical conductivity (0.57S/m). The hydrogels have excellent comprehensive properties, which provide inspiration and potential for the preparation and application of wearable strain sensors.
In addition to the addition of a dispersant for carbon materials, the distribution of carbon materials in the hydrogel according to a certain structure can also solve the above problem. Sajad Abolpour Moshizi et al. [92] were inspired by the biological hair cells in the human vestibular system to develop a labyrinthine hydrogel nanocomposite based on PVA and vertically grown graphene nano-sheets (VGNs) network structured conductive hydrogel (Fig. 4a). Unlike the current common hydrogel sensors, the VGNs/PVA hydrogel absorbs a large amount of water when submerged in water, making the sensor highly sensitive to small underwater stimuli. The sensor shows high sensitivity (5.755 mV (mm s−1)−1) and very low-velocity detection (0.022 mm s−1). This miniaturized hair cell sensor paves the way for the development of next-generation ultra-sensitive impedance-based sensors for biomedical applications using hydrogels.
Hadi Ahmadi et al. [93] utilized polyvinyl alcohol (PVA) and vertically aligned graphene nanosheets (VGNs) to fabricate a highly flexible and conductive artificial hair cell sensor (Fig. 4b). This hydrogel sensor demonstrated excellent performance in simulating biological hair cells, exhibiting piezoresistive responses to sound vibrations in the auditory range from 60 Hz to 20 kHz. This work validates the role of PVA/VGN sensors as cochlear hair cells, providing a conceptual framework for a new generation of biocompatible artificial hair cell sensors that mimic the behavior of auditory hair cells within the cochlea.
There are also some scholars from the perspective of the carbon material itself, in addition to the currently known common carbon materials, the extraction of new carbon materials from nature as added to the hydrogel as a conductive material, and the result of some specific means of treatment; so as to solve the above problem, Dinesh K. Patel et al. [94] PVA/PDA@CD conductive hydrogel was prepared, which obtained a new carbon material (CDs) from cucumber rind by one-pot heating treatment process and then synthesized PDA@CDs by polymerization process through pH-induced polymerization process, and made conductive hydrogel by compounding with PVA phase. The addition of PDA@CDs improves the adhesion and conductivity of the PVA matrix and also has superior mechanical and rheological properties.
Among the methods to improve the mechanical properties of hydrogels, the construction of multiple network structure structures is also the choice of many scholars. Wu et al. [95] prepared multiple network composite hydrogels based on PVA, CMCS, sodium oxidized alginate (OSA), and oxidized multi-walled carbon nanotubes (OMWCNTs). With the help of OMWCNTs, the fracture strength of the hydrogels was increased to about 0.8 MPa, which is about 2.5 times higher than that without OMWCNTs. Meanwhile, a new conductive network was constructed within the hydrogel using OMWCNTs, which increased the electrical conductivity of the hydrogel from 1.75 × 10−4 to 7.02 × 10−4S/cm, thus allowing feedback signals to be generated for small human movements.
Besides carbon materials, metal nanomaterials are also widely used in the field of flexible sensing because of their biocompatibility, chemical stability, strong electrical conductivity, good ductility, and easy surface modification. As a one-dimensional metal material, silver nanowires not only have the excellent electrical conductivity and mechanical stability of traditional metallic silver but also have the unique surface effect and quantum size effect of nanomaterials due to their nano size, which is widely used in the field of electronics. In order to ensure that the silver nanowire action is not affected in the hydrogel matrix, Shohreh Azadi et al. [96] proposed a biocompatible hydrogel sensor with a substrate prepared with a highly concentrated PVA solution, and a thin conductive layer of AgNWs was deposited on the PVA substrate, while the top layer consisted of a dilute PVA solution, so that the high content of AgNWs could be dispersed to achieve high conductivity (Fig. 5a). With high tensile properties up to 500% strain, the high mechanical strength of 900 kPa, electrical conductivity (1.85 S m−1), good durability under cyclic loading, low hysteresis of 7%, fast response time (0.32 s), and biocompatibilit, this novel sensor offers great potential as a wearable sensor for epidermal sensing applications.

Copyright 2020 Adv.Mater. b Preparation process diagram and sensing performance characterization of PVA/OP/borax hydrogen; reproduced with permission from Ref. [71]. Copyright 2022 Elsevier
a The schematic diagram of the structure design for a two-layer sensor and the curves depicting the relative resistance values over time under different frequencies and multiple cycles; reproduced with permission from Ref. [96].
Yinghui Ma et al. [71] also used silver nanowires as a conductive material to prepare a double-network structured stretchable self-healing strain hydrogel sensor consisting of PVA, okra polysaccharide (OP), borax, and silver nanowire conductive layer (Fig. 5b). The synthesized hydrogel strain sensor has high sensitivity (measurement factor = 6.34), a short response time (~ 20 ms), and good operational stability. On the one hand, the addition of silver nanowires greatly improved the conductivity of the hydrogel, and on the other hand, it also provided an innovative idea for developing the application of bio-polysaccharide hydrogels in the sensor field.
In order to make the conductive metal network more uniformly distributed in the three-dimensional hydrogel, metals can be prepared as nanomaterials, in addition to some scholars add liquid metal (LM) to PVA hydrogels, which can solve the problem of uneven dispersion of metal nanomaterials on the one hand; on the other hand, it plays a positive role in the self-healing of hydrogel materials. Koki Murakami et al. [97] utilized a PVA film to transfer liquid metal (LM) onto a PAM/PVA hydrogel material, achieving wiring along the three-dimensional (3D) substrate shape (Fig. 6a), thereby developing a system composed of solid-state electronic components and LM on the hydrogel. With the three-dimensional interconnection technique mentioned above, multiple systems can be integrated within a single device through the insulating layer of the gel. This fundamental manufacturing and system integration technology can be applied to wearable devices with higher flexibility and implantable devices and gel actuators capable of measuring bodily health states.

Copyright 2022 ACS. b Schematic diagram of the preparation mechanism of PVA-LMPs hydrogels; reproduced with permission from Ref. [98]. Copyright 2019 ACS
a The preparation process of LM paste patterning and the application instances of LM circuits in temperature testing and on the surface of hydrogel materials; reproduced with permission from Ref. [97].
Meihong Liao et al. [98] reported PVA-stabilized liquid metal particle (LMPs) (PVA-LMPs) hydrogel with excellent self-healing properties and soluble wearable epidermal sensors, constructed by dispersing eutectic gallium and indium (EGaIn) into a PVA polymer network of borate gels (Fig. 6b), where dynamic cross-linking bonds between the hydrogel and LMPs can reversibly break or bond in the network, enabling the hydrogel to conduct electricity while being self-healing.
In addition to the two single-component conductive particle networks previously discussed, certain investigators have discovered methods to combine metal nanomaterials and carbon nanomaterials as conductive particles. Such approaches preserve the unique features of carbon nanomaterials while also being compatible with the positive aspects of metal nanomaterials, which are often expensive, cannot be added in large quantities, and on rare occasions have a structure that is biomimetic. Lu Zhang [99] et al., inspired by human neurons, combined ultra-long silver nanowires with modified carbon black nanoparticles and combined composite conductive materials (AgNWs/CB-OH) with PVA/tannic acid(TA)/PAM composite hydrogels to improve the electrical conductivity of composite conductive hydrogels and the sensitivity of flexible sensors based on conductive hydrogels by mimicking the structure of neurons. When a small amount of composite conductive material (~ 2.65 wt%) was added to the hydrogel (Fig. 7a), the conductivity of the composite conductive hydrogel was significantly improved with high sensitivity (GFmax = 68.64, Smax = 0.229 kPa−1), ability to cycle stably (~ 300 cycles), and can meet the demand for long-term detection of human motion.
Other scholars are chemically combining carbon nanomaterials with metallic materials. Jiaming Zhang [100] loaded silver nanoparticles (AgNPs) onto reduced graphene oxide (rGO) to form composite sheets (Ag@rGO) (Fig. 7b), and then Ag@rGO was further doped into PVA-PAAm double network hydrogels to obtain conductive hydrogels with significant antibacterial properties and high electrical conductivity.
New two-dimensional material Mxene [101, 102], because of its excellent electronic, optical, mechanical, and thermal properties, with a variety of transition metals and surface chemistry, has shown promise in many applications such as energy storage, electromagnetic interference shielding, transparent electrodes, sensors, catalysis, and photothermal therapy. Scholars have also used MXene extensively in hydrogel systems because of its excellent electrical properties. Jiaqi Zhang et al. [103] developed a highly stretchable and self-healing MXene/PVA hydrogel electrode for capacitive strain sensors of electronic skin. The doping of MXene into PVA improves the conductivity and self-healing properties of the hydrogel. The electrodes exhibited high tensile capacity (≈1200%) and instantaneous self-healing (healing time≈0.15s) upon fracture. The capacitive sensor based on these electrodes shows high linearity of up to 200%, low hysteresis, the sensitivity ≈0.40, and good mechanical durability (5.8% reduction in relative capacitance change after 10,000 cycles). Furthermore, the sensor maintains its performance after self-healing tests, demonstrating its potential for monitoring human motion.
Several researchers have improved the sensing and mechanical properties of PVA-based hydrogels by simply co-blending carbon nanomaterials with them in order to enable the enhancement of the conductivity of hydrogel sensors, taking advantage of the unique strain dependence exerted by Mxene. Yi Zhao et al. [104] designed a unique strain-dependent micro-crack structure design using carbon nanomaterials in synergy with MXene, and the flexible sensor designed by them has excellent strain sensing properties, including ultra-low detection limit (0.001% strain), ultra-high sensitivity (measurement factor, GF = 3.92 × 107), ultra-fast response time (5 ms), and superior durability and stability (> 45,000 cycles).
Filler-based conductive PVA-based hydrogels considering their simplicity of preparation are of interest. Table 1 lists both the advantages and disadvantages associated with different conductive fillers and conductive components.
PVA hydrogels are given electrical conductivity and sensitivity by the incorporation of conductive fillers, together with a few enhancements in their mechanical properties occasionally the addition of special capabilities including shape memory. The non-uniform dispersion of nano-fillers in the hydrogels’ three-dimensional matrix and the emergence of incompatibility are two issues that have yet to be resolved with the addition of nano-fillers. Therefore, the primary problem which needs immediate attention is the process method for achieving the conductive function while simultaneously guaranteeing that it is uniformly distributed.
2.2 Conductive polymer-type PVA hydrogel
Conductive polymers [35], generally, are polymeric materials that are intrinsically conductive, due to the presence of alternating single and double bonds in their backbone, thus forming large conjugated π-bonds, which conduct electricity through the flow of electrons, and can be doped to reach the conductive state. Conductive polymers can be in situ polymerized in a PVA hydrogel matrix compared to filler-type conductive particles and therefore have better dispersion properties, which gives them an advantage in the fabrication of flexible sensing. The conductive polymers commonly used in hydrogels are poly 3,4-ethylene-dioxythiophene (PEDOT), PAN, PPy, etc.
PEDOT [118] is one of the most widely studied conductive polymers with the advantages of good flexibility, high transparency in the visible region, adjustable conductivity, etc. The double-network structure hydrogel prepared by co-preparing it with PVA has been positively altered in terms of improved conductivity biocompatibility and high mechanical strength at same time. Yinjie Peng et al. [119] developed a multifunctional hydrogel sensor based on PVA with PEDOT as the conductive filler and glycerol/water fraction solvent as the dispersion medium. The best sample obtained has high tensile stress (~ 1.0 MPa), large elongation (~ 400%), good conductivity (~ 3.5 S m−1), and the glycerol/water solvent give the hydrogel good anti-freezing and moisturizing properties, which can provide applications in a wide range of wearable fields.
PPEDOT is oxidized by the oxidation of dithionite and catalyzed by Fe3+ to form a strong electrostatic force, resulting in PEDOT: PSS with high electrical conductivity, stability and transparency, and excellent processability in aqueous solutions [120]. Jagan Mohan Dodda et al. [121] combined chitosan, starch/cellulose, PVA, and PEDOT: PSS synthesizing hydrogels by one-pot synthesis (Fig. 8a). The dissolution rates of the cellulose-based hydrogels ranged from 121 to 156%, while those of the starch-based hydrogels ranged from 234 to 280%; and tensile tests to test their mechanical properties illustrated that the addition of starch to the hydrogels resulted in a significant increase in stretchability (strain at break > 300%), and the combination with cellulose resulted in the formation of spiky hydrogels (elastic modulus of 3.9–6.6 MPa). The ultimate tensile strengths of the two hydrogels were similar (2.8–3.9 MPa). The hydrogel materials prepared by Jagan Mohan Dodda et al. mentioned above possess good biocompatibility and mechanical strength along with electrical conductivity, which broadens the due field of PVA-based conductive hydrogels.

Copyright 2022. CELLULOSE. b The schematic diagram of the preparation process and the sensing performance testing of PEDOT: PSS/PVA hydrogel sensors; reproduced with permission from Ref. [122]. Copyright 2022 ACS
a Chitosan, starch/cellulose, PVA, and PEDOT: PSS hydrogel preparation process and mechanical performance testing; reproduced with permission from Ref. [121].
In the pursuit of mechanical properties and sensing performance, the service life of the main parameters of the sensor has become a subject of interest for scholars. General hydrogels often face the problem of losing water and drying out under normal atmospheric conditions and losing mechanical properties, and among current researchers, this problem is often solved by encapsulating them. However, Wanhui Shi [122] achieved resistance to extreme environments by adding GEL in a flexible hydrogel PEDOT: PSS/PVA (Fig. 8b). The results show that the prepared sensor has good flexibility and mechanical strength, and its ultimate tensile strength can reach 13.76 Mpa when the elongation at break is 519.9%; the hydrogel sensor also has good water retention and low-temperature resistance, and it still shows good flexibility after 1 year in the atmospheric environment, and at a low temperature of − 60 °C. The sensor can withstand 1000 repetitions of stretching and shrinking (10% elongation) stably; it can also detect very small strains down to 0.01%. The results demonstrate that the encapsulation-free hydrogel sensor can perfectly monitor complex human movements such as knuckle flexion, vocalization, and pulsation.
PAN [123] has been extensively studied for its easy processing and adjustable conductivity for mortal able reasons. Chengxin Hu et al. [124] planned to give more functions to conductive polymer-based hydrogels; a conductive hydrogel polyvinyl alcohol/polyethylene glycol/polyaniline (PGA) was prepared using an organic combination of low-cost PVA, PAN, and glycerol, which has high sensitivity (GF = 2.14), fast response time (230 ms), and can be cycled many times (~ 540 cycles). In addition, the PGA conductive gel maintains good electrical conductivity (0.32 S/m) and mechanical properties even at − 20 °C. This makes the hydrogel not only have excellent sensing function but also have the ability to resist freezing, realizing multifunctional hydrogel.
Conductive polymer-based PVA hydrogel sensors are gradually becoming a focus of researchers in addition to the need for high performance and multifunctionality. Ppy hydrogels [125,126,127] have been widely used in electrochemical and biomedical fields due to their non-toxic, controlled conductivity, thermal stability, and environmental stability. Ayinuer Abodurexiti et al. [128] prepared an antifreeze conductive hydrogel sensor based onPVA, silk cellulose (SF), and PPy. The hydrogel has good electrical conductivity, considerable stretchability (860%), and compressibility. It can achieve good self-healing properties under environmental conditions. In addition, it can accurately capture changes in the temperature adjustment range of − 10 to 50 °C and can be further developed into a flexible temperature sensor, and a wearable strain/pressure sensor was prepared using the 3D printing method.
Huiyu Bai et al. [129] prepared a polyvinyl pyridyl (PVA-SbQ)/FeCl3 hydrogel sensor, which with a unique double cross-linked single network was prepared by UV cross-linking and ionic coordination. PVA-SbQ/FeCl3 hydrogel has remarkable stretchability (~ 858%), good tensile stress (~ 0.3 MPa), high ionic conductivity (0.29 S/m), high sensitivity, and good water retention capacity due to photo-crosslinking, physical crosslinking, and hydrogen bonding phase association as well as the introduction of Fe3+ ions, and these properties give PVA-SbQ/FeCl3 hydrogels the potential for practical application as flexible electronic components.
Table 2 compares PVA hydrogels, double-network hydrogels, and multi-network conductive hydrogels created from conductive polymers in terms of electrical conductivity and their mechanical properties.
Although the experimental steps for incorporating conductive polymers into the original PVA hydrogels are easy to understand some conductive polymer monomers can be dispersed more uniformly in the PVA matrix through in situ polymerization. A dual molecular chain structure will also allow the hydrogel mechanical properties, such as increased toughness. Overall, conductive polymers have advantages when it comes to the preparation of PVA-based conductive hydrogels. However, their incorporation leads to opacity, which significantly restricts their use in visualization and other aspects; some conductive polymers are not bio-friendly materials, making them limited in use, which are obstacles to the preparation of multifunctional conductive hydrogels; therefore, the preparation of PVA-based conductive hydrogels with visualization capabilities and environmentally friendly and bio-friendly still needs to look for better conductive methods.
2.3 Ionic PVA conductive hydrogels
Due to their distinct advantages over filler-type PVA-based hydrogels and conductive polymer PVA-based hydrogels, such as transparency, visibility, ease of control, and good mechanical properties, ion-conductive PVA-based hydrogels are more frequently used in the creation of PVA-based conductive hydrogel sensors. In ion-conductive hydrogels, ions can occasionally perform more than just act as conductors; some ions can also act as ligand sites, cross-linking the hydrogel matrix and improving its mechanical properties. There will not be any filler-type conductive particles in the PVA matrix dispersion of the problem either because of the ionic conductivity [139], which can pierce into the hydrogel’s pore-rich structure. Common ionic solutions consist of NaCl, MgCl2, CaCl2, and others. The formed hydrogel may also be divided into a suitable dimension and soaked in a specific concentration of ionic solutions, or the ionic solution can be added to the hydrogel precursor and prepared using the one-pot method.
Xiang Di et al. [140] developed a high-strength, relatively high-sensitivity ion-conductive hydrogel with excellent plasticity and excellent mechanical properties (8.03 MPa), high toughness (28.7 MJ m−3), and high elastic modulus (1 MPa) using fully physically cross-linked polyvinyl alcohol (PVA) impregnated with inorganic salt solution (NaCl) (Fig. 9a). By integrating PVA-NaCl gel into a flexible strain sensor, the sensor shows high conductivity (7.14 S/m) at room temperature and zero temperature, high accuracy (GF = 0.989), sensitive strain responsiveness (0.2–400%) over a wide strain detection window, and can be used in medical applications for human health detection due to its good biocompatibility and antibacterial properties. It can be used in medical applications for human health testing due to its biocompatibility and antibacterial properties.

Copyright 2021 RSC. b Schematic diagram of the preparation process of M-PVA hydrogels and the demonstration of the hydrogel’s elasticity, compressibility, and stretchability; reproduced with permission from Ref. [141]. Copyright 2020 RSC
a Schematic diagram of the preparation process of PVA-N hydrogels and the demonstration of the hydrogel’s flexibility; reproduced with permission from Ref. [140].
The addition of ionic solutions sometimes not only provides the conductive properties of hydrogels but also synergizes with the -OH in the molecular chains of PVA hydrogels to improve their mechanical properties. Yafei Gao et al. [141] constructed a highly mechanical, freeze-resistant, ion-conductive M-PVA hydrogel by immersion treatment in iron sulfate solution to introduce chain entanglement and complexation between Fe3+ ions and -OH into the microcrystalline network (Fig. 9b). Due to the synergistic effect of multiple physical cross-linking, the M-PVA hydrogels exhibit significant deformability in elongation (1120%) and compression (98%), high strength, high toughness, fast self-recovery, and fatigue resistance. The incorporation of Fe3+ and SO42− gives the M-PVA hydrogels good electrical conductivity and significant frost resistance (down to − 50 °C), which are assembled into hydrogel sensors. Capable of detecting a wide range of elongation (~ 900%), compression (~ 70%), bending, and contact pressure (up to 4.60 MPa) with cyclic stability and durability for continuous monitoring in extreme weather (Fig. 10b–j), the gel is a promising flexible sensing material for a wide range of applications in ionic skin, motion recognition, and smart wearables.

Copyright 2020 Elsevier. b Preparation scheme and photographs of SPP hydrogel; photographs of unknotted, knotted, stretched, and recovered SPP hydrogel; photographs of compression process of SPP hydrogel; reproduced with permission from Ref. [148]. Copyright 2018 ACS
a Demonstration of the mechanical properties and schematic diagram of the preparation process of PVA/PAA/Fe3+ hydrogels; reproduced with permission from Ref. [147].
Although pure PVA-based hydrogels have been studied by scholars and their mechanical properties such as strength have been improved, they sometimes still cannot meet the needs of large strains. Since Jian Ping Gong [142] discovered the dual network structure, both a layer of rigid network structure acts as the basic skeleton and a layer of flexible network structure forms the conductive network; both tensile strength and fracture strength, toughness, and other properties have been greatly improved. Scholars have constructed different double network structures by introducing different heavy networks, so that the originally brittle hydrogel presents mechanical properties such as toughness, thus broadening the proper field of hydrogel.
Hydrogels based on cellulose nanocrystals (CNCs) [143, 144] have become important materials in the field of human sensing because of their low toxicity, biocompatibility, biodegradability, and excellent mechanical stability. Huiqiang Wang et al. [145] proposed a simple strategy to prepare hydrogels by combining CNCs with PVA to achieve enhanced toughness of PVA hydrogels while safeguarding bio-compatibility. PVA/CNC hydrogels have an electrical conductivity of 0.021 S/cm at room temperature and maintain conductivity at − 70 °C (0.014 S/cm); they also have excellent mechanical properties (stress about 1.4 MPa, strain 1018%), excellent transparency, frost resistance (− 70 °C), and other comprehensive properties. It shows good responsiveness and stability in use for monitoring human movement and has a transparent texture with frost resistance.
Polyacrylic acid (PAA) [146] is a non-toxic, biocompatible, and biodegradable polymer that has attracted wide interest in recent years. Yongzhi Liang et al. [147] prepared new fully physically cross-linked PVA/PAA/Fe3+ hydrogels (Fig. 10a) with stretchable, tough, electrically conductive, and self-healing properties by one-pot polymerization at room temperature with high tensile strength (~ 1.03 MPa), high stretchability (~ 1370%), high toughness (~ 6.37 MJ/m3), and excellent self-healing properties (self-healing efficiency can reach 97.34%), as well as fast self-healing performance (recovery rate of 0.83 after waiting for 15 min). In addition, the conductivity of the hydrogel can reach 2.67 S/m, and the conductivity of the hydrogel varies accordingly, and the hydrogel also has a high measurement factor (GF = 3.79) and exhibits a high sensitivity over a wide strain range (0–800%), enabling real-time human detection.
By chemically modifying the carboxyl group of PAA, PAA derivatives with better chemical properties than PAA can be obtained. Mei Xiang Wang et al. [148] use hydrogen-bonded sulfuric acid to modify hydrogels and obtain hydrogen-bonded sulfuric PAA/PVA (SPP) physically cross-linked hydrogels (Fig. 10b). The addition of sulfuric acid not only provides conductive ions to promote the conductivity of the hydrogel, but also inhibits the ionization of carboxyl groups from PAA chains and forms more hydrogen bonds to enable the hydrogel to obtain comprehensive mechanical properties, and the designed hydrogel has high fracture strength (3.1 MPa) and high toughness (18.7 MJ m−3). Wang et al. [148] provide the new idea to prepare such hydrogels that can exhibit stable operation after repeated loading, which indicates that the mechanical properties of hydrogels are greatly improved and more favorable to be used in the field of flexible electronic devices.
Ruixue Liu et al. [149], by introducing methacrylic acid-modified dopamine into the sodium polyacrylate crosslinking network, obtained a multifunctional hydrogel with poly(sodium acrylate-dopamine-based meth acrylamide) (P(AAS-DAAM)/PVA, which with poly-stretchable, self-adhesive, conductive, and anti-freeze properties. The hydrogels have good stretchability (> 1000%), high conductivity (0.038 ± 0.003 S⋅cm−1), good frost resistance (− 30 °C), and good adhesion (419.3 ± 5.95 N/m), and remarkable biocompatibility (relative cell viability up to 132 ± 2.63%), high sensitivity (GF = 1.65), and stable, accurate monitoring of large joint flexions (fingers, wrists, elbows, knees) or small body (swallowing, speaking) activities.
SA [150] is a biodegradable and biocompatible natural electrolyte that provides a large number of conductive ions, ensures high sensitivity of ionic hydrogels, and can form a double interpenetrating network structure with PVA hydrogels. Yiwei Zhan et al. [151] successfully developed comfortable and safe conductive hydrogels with SA/PVA conductive and non- or weakly conductive layers, which also have good mechanical properties and thickness tunability. The conductivity of the strongly conductive alginate layer is strain responsive and its good mechanical properties allow the hydrogel to be used as a strain sensor to sense joint movements in different body parts of the human body, such as fingers, wrists, elbows, and knees, where the weakly conductive layer acts as an isolation layer that facilitates its safe application and has great potential as a strain sensor for detecting human strain.
Currently prepared hydrogels need to consider not only the mechanical properties but also the electrical conductivity is very important. Yang Zhou et al. [152] demonstrated a new method to fabricate ion-conductive hydrogels: biocompatible hydroxypropyl cellulose (HPC) and PVA hydrogels were formed into a double network structure by physical cross-linking and then immersed in sodium chloride (NaCl) solution to form HPC/PVA ion-conductive hydrogels (Fig. 11a). The advantage is that the mechanical properties and ionic conductivity of HPC/PVA ion-conductive hydrogels can be easily adjusted by adjusting the content of the added HPC and the concentration of the soaked NaCl solution to meet different needs so that the hydrogels with adjustable conductivity can be more widely used in the field of flexible electronic devices.

Copyright 2018 AFM. b Structure of hydrogel network; PVA/CNF hydrogels in use; photographs of PVA/CNF hydrogels in use. Reproduced with permission from Ref. [153]. Copyright 2019 Elsevier
a Tensile curves of HPC/PVA hydrogel with different PVA wt% and pure PVA hydrogel as reference at 3 m soaking level, tensile strength, strain, Young’s modulus, and toughness of HPC/PVA16% ionic conductive hydrogel with different ionic concentrations from 1 to 5 m; stretching, knotting, and compression of HPC/PVA16% ionic conductive hydrogel; reproduced with permission from Ref. [152].
In addition to the dual network structure, some scholars have formed non-covalent phases based on the dual network structure to obtain better mechanical properties, such as electrostatic interactions, hydrogen bonding, hydrophobic bonding, and supramolecular recognition, and other structures to further provide mechanical properties. Xin Jing et al. [153] synthesized a PVA/cellulose nanofilament (CNF) hydrogel with a double cross-linked network for highly transparent, stretchable, and self-healing pressure and strain sensors (Fig. 11b). The hydrogel contains dynamic borate bonds, metal-carboxylic acid coordination bonds, and hydrogen bonds contributing to the hydrogel’s superior dimensional stability, mechanical strength and flexibility, and self-healing ability. The developed hydrogel has a moderate modulus of 11.2 kPa and a high elongation of 1900%. It can heal itself within 15s without any external stimuli, with a light transmission rate of more than 90% and good biocompatibility. Sensors developed based on PVA/CNF hydrogels show great potential not only for electronic skin, personal healthcare, and wearable devices but also may inspire the development of transparent and smart skin-like sensors.
In recent years, ionic conductive hydrogels have become a research hotspot, and the comparison of mechanical and electrical properties of conductive hydrogels with different network structures and different ionic types in Table 3 is shown.
In conclusion, the three different conductive mechanisms of PVA-based conductive hydrogels have an important effect on conductive materials in general: carbon materials, metal nanomaterials, and other filler-based conductive materials tend to be black or dark; doping as a conductive network undoubtedly impacts its translucency; and the conductive network in the hydrogel system is also uniformly dispersed and is an urgent issue for all scholars. Metal-based materials, such as metal nanomaterials and LMs, have increased conductivity and reduced stretching in the preparation of conductive hydrogels, but the prepared conductive hydrogels frequently become opaque, limiting their development in the field of visualization. Carbon-based materials, such as graphene/reduced graphene oxide and carbon nanotubes, can provide good conductivity for hydrogels, but they also face the problem of non-uniform dispersion.
The inherent stiffness and hydrophobicity of conductive polymers when used as polymer frameworks can reduce the stretchability of PVA hydrogels while increasing the mechanical strength of conductive hydrogels. Moreover, the polymerization process of some conductive polymers in PVA base is not fully controllable and sometimes faces incomplete polymerization. Most importantly, some of the conductive polymers are not biocompatible, which can lead to the preparation of PVA-based conductive hydrogels that cannot be used in human testing and other areas, greatly reducing their field of use.
It is possible to manage the conductivity of the ion-conductive hydrogels made from electrolytes while also uniformly dispersing them throughout the hydrogel. While particle conductive materials and conductive polymers typically have reduced mechanical properties due to the formation of polymer networks, ligand bonding and hydrogen bonding can improve the hydrogel’s mechanical properties, allowing the ionic conductive hydrogel to achieve excellent mechanical properties. Additionally, the addition of inorganic salt makes the conductive hydrogel more ionic and makes it biocompatible for use inside the human body.
A reference value for PVA-based conductive hydrogels in applications such as human motion detection, human–computer interaction, electronic skin, smart wearables, supercapacitor, and friction nano-generator comes from the special properties of conductive hydrogels with various conductive materials.
3 Application of PVA-based conductive hydrogel
PVA-based conductive hydrogels have good mechanical properties, such as tensile, bending, and compression, and therefore show excellent prospects for application in strain sensors. The general PVA conductive hydrogel mechanism is that the shape of the conductive hydrogel changes when stimulated by a certain external force, and at the same time the conductive pathway of the conductive hydrogel changes, and the resistance or conductivity changes accordingly, thus visualizing the external stimulus into data. Compared with the traditional flexible sensors coated with conductive materials on a flexible layer, PVA-based conductive hydrogels are biocompatible and do not have harmful effects on the human body when used in close proximity to the human body; and because most hydrogels are prepared by the one-pot method, the service life of the conductive layer will not be greatly reduced due to the peeling off of the surface, thus avoiding equipment failure when in use.
Based on the excellent physicochemical properties of PVA-based conductive hydrogels, such as anti-freeze, self-healing, anti-swelling, anti-bacterial, and transparent, they can still be sensed under some special circumstances; also because of their simple preparation, their sensing of external stress, and strain at the same time, after the functional design of adding different materials, they also have a temperature, pH value, humidity, and other sensing functions, broadening the scope of their applications. In addition, most of the sensors prepared from PVA-based hydrogels are highly sensitive and can accurately capture small human movements, such as talking, swallowing, and knee flexion, and can be widely used in human detection, human–computer interaction, and wearable. This section discusses the applications of PVA-based conductive hydrogels in different fields as well as elaborates on the current development directions.
3.1 PVA hydrogen flexible strain sensors
According to the sensing mechanism, strain sensors can be divided into four categories: resistive, capacitive, piezoelectric, and frictional. Strain sensors are devices that translate physical distortion into changes in electronic signals. The flexible resistive PVA-based strain sensors that change resistance in response to strain are the subject of this section. PVA-based hydrogel sensors must provide good accuracy, comfort, and long service life in addition to having a broad response range and a quick response time. However, it is still difficult to accomplish the aforementioned functions simultaneously in various environments for use in wearable devices, which has led many scholars to pursue this line of research.
The introduction of non-covalent bonds into hydrogel systems in order to obtain self-healing properties [162] is a common means, and hydrogen bonds and borate ester bonds are common in PVA hydrogel systems. For example, Hailin Wu [163] immersed PVA hydrogels in different concentrations of γ-polyglycolide(γ-PGA) solutions by freeze–thaw physical method; a high-strength and tough γ-PGA/PVA hydrogels were developed, attributed to the formation of hydrogen bonds between the carboxyl group of γ-PGA and the hydroxyl group of PVA to enhance the mechanical properties and stability of PVA hydrogels (Fig. 12a). After soaking in γ-PGA hydrogel for 24 h, where the concentration of γ-PGA was 20% wt, the tensile strength of γ-PGA/PVA hydrogel was the best, reaching 16.69 ± 0.43 MPa, which was 128 times higher than the tensile strength of PVA hydrogel (0.13 ± 0.01 MPa). Meanwhile, the γ-PGA/PVA hydrogel exhibited a high level of twisting, knotting, and puncture resistance, and could lift 5 kg without significant rupture. In addition, the prepared γ-PGA/PVA hydrogel strain sensors have good cytocompatibility and can provide accurate and reliable detection of subtle physiological signals, such as finger bending at different angles, swallowing, and compression pressure, which have great potential applications in carrying artificial tissues and sensors.

Copyright 2022 Wiley. b Schematic diagram of the preparation process of PBSTCE organic hydrogel; illuminated light bulb experiment of initial and self-healing PBSTCE hydrogel; reproduced with permission from Ref. [165]. Copyright 2023 Elsevier
a Schematic diagram of the preparation process of γ-PGA/PVA hydrogels; reproduced with permission from Ref. [163].
While Zhennan Chen et al. [164] prepared lignin/β-CD-PVA (LCP) self-healing conductive hydrogels consisting of cyclodextrin (β-CD) and PVA as a backbone, borax as a cross-linking agent, and alkaline lignin as a plasticizer by a one-step method, compared with PVA hydrogel, its maximum storage modulus and elongation are increased by 2.5 and 20.0 times, respectively. LCP hydrogel can maintain its self-healing ability in both high (45 °C) and low (0 °C) temperature environments, and the self-healing ability is not affected by pH value. In addition, it has good electrical conductivity, elongation at break (2000% strain at break), antimicrobial properties, thermostability, and UV resistance, which have good prospects for applications in 3D printing and wearable electronic devices.
Tao Ke et al. [165] prepared multifunctional conductive hydrogel based on the reversible borate ester bond formed by starch/PVA/tea polyphenol (TP) and borax, and multiple hydrogen bonding interactions between PVA, starch, TP, and ethylene glycol (EG), and sAgNPs and MWCNTs were used to form a conductive cross-linked network, which enabled the obtained PBSTCE hydrogels with antibacterial and conductive properties (Fig. 12b). Also due to the presence of multiple non-covalent bonds, the conductive hydrogel has high electrical conductivity (2.32S/m), sensitivity (GF = 6.85), rapid self-healing (self-healing efficiency = 96.07%), self-adhesion, significant elongation (814% elongation), freeze (− 20 °C) and moisture resistance, and antibacterial properties. The strain sensor formed by PBSTCE conductive hydrogel can not only effectively record large-scale human motion, but also accurately capture subtle changes in motion, which has considerable potential for future applications in wearable flexible devices.
Different structural features bring various functionalities to conductive hydrogels. For example, inspired by the Lorenzini ampullae (AoL) structure in shark’s snouts, Peng Sun et al. [166] designed a polymer-based hydrogel composed of a cross-linked polyvinyl alcohol (PVA) framework and polyaniline (PANI) nanoparticles (Fig. 13a). This structure achieved outstanding ion conductivity (10.8 mS cm−1) in a simulated marine environment. It demonstrated excellent selectivity for specific ions (Fe3+ and Cu2+), aiding in the real-time identification of particular substances in the environment. The AoL-inspired hydrogel exhibits several functionalities and holds significant potential applications in underwater detection instruments, such as submarines, within the field of marine exploration.

Reproduced with permission from Ref. [166]. Copyright 2022 Elsevier. b Schematic diagram of a flexible sensor fabricated by inspiration of hair and mechanical receptors under the skin surface, where the inclined cylinder is similar to the hair on the skin surface; schematic diagram of the process of ionic hydrogel sensor fabrication; schematic diagram of the sensing mechanism of a pressure sensor with stable/unstable microstructure before and after applying pressure; loading and unloading of different objects (a book and a packet of tissue), the signal output of the sensor attached to the fingertip; flexible sensor for carotid pulse wave monitoring; pulse wave monitoring; radial pulse wave monitoring; fingertip artery pulse wave monitoring. Reproduced with permission from Ref. [167]. Copyright 2022 Elsevier
a Morphology and mechanism of ampullae of Lorenzini for shark and the artificial ampullae based on high conductive hydrogel jelly; unique function of detecting environmental perturbation nearby of the jelly-based system.
To detect small strain conditions in the human body, Lyuming Pan et al. [167] used a special photolithography technique to synthesize a flexible PVA-H3PO4 ion-conductive hydrogel sensor that mimics the human hair-like adjustable microstructure (Fig. 13b). High sensitivity at 0–4 kPa; 1167 kPa−1 at 4–40 kPa; and 511 kPa−1 at 40–100 kPa; a large detection limit range (down to 3.9 Pa); fast response time (37 ms); and long service life (2900 cycles). The flexible sensor allows monitoring of pulse waves and heart rate without external pressure and cuff compression and also allows monitoring of pulse waves at different static pressures to enable blood pressure measurement.
Several scholars have used the Janus layer structure [168] to design hydrogel layers with different viscosities to fit human wearables. Mingcheng Wang et al. [169] used cyclic freeze–thaw to combine viscous PVA/inositol hexakisphosphate (PA) and PVA/PANI and successfully constructed a hydrogel with one-sided viscosity Janus laminated hydrogel tape with a strong interface between the two layers with an interfacial peel force of 114.6 N/m, which has potential for wearable body wear. It is connected to a Bluetooth system to create wireless wearable sensors that can easily monitor movements such as walking, squatting, and climbing stairs. The strain sensor assembled from Janus hydrogel tape achieves low response time (tl = 60ms, tu = 62ms), high sensing with high lifetime, and high sensitivity (GF = 3.4).
Ruonan Liu et al. [85] proposed a dual-network structure multifunctional hydrogel synthesized from PVA, hydroxyethyl cellulose (HEC), glycerol, and borax, which was made in three different states (named PHG, PHG-F, and PHG-S, respectively) by different means: PHG hydrogel chemically cross-linked by borax has unique transparency, plasticity, self-healing, injectability, electrical conductivity and thermal sensitivity, which can be used to detect temperature changes with fast response time; PHG-F hydrogel physically cross-linked by PHG hydrogel by the cryogenic freezing method has strong mechanical properties and toughness (up to 0.66 MPa), which can be applied with human motion monitoring to provide good performance; PHG-S hydrogel obtained by dissolution of dried PHG hydrogel has strong mechanical properties. The strong mechanical properties and toughness (up to 1.31 MPa) of PHG-S hydrogels obtained by dissolving dried PHG hydrogels can be immersed in ionic solutions to obtain high conductivity (up to 2.3 Sm−1), making them well suited for strain sensing applications. Multifunctional hydrogels create opportunities for large-scale sensing applications and provide the technological basis for the development of flexible electronic devices, wearable devices, smart sensing, human–computer interaction, and biomedical engineering.
Some scholars have pretreated materials that are otherwise difficult to disperse in the hydrogel system to be used to build a double network structure in the hydrogel system, which leads to improved mechanical and electrical properties of the hydrogel. For example, Yin Yan et al. [170] used ternary DES (choline chloride, glycerol, and Lewis acid) to pretreat lignocellulose, and prepared DES-based PAA/PVA double network hydrogels with good mechanical properties, self-adhesive properties, and high electrochemical sensitivity using the DES solution with dissolved lignin as the medium of the hydrogel. DES and Al3+ formed a metal phenol network structure (MPN), which enhanced the dynamic coordination of the polymer network structure and enhanced its mechanical properties, while possessing electrical conductivity, thus allowing the hydrogels to maintain excellent electrical conductivity (10ms/cm) and good ion sensing in extreme sub-zero environments; when DES hydrogels are used as flexible sensing, a high sensitivity factor can be achieved (GF = 4.9). When the hydrogel is subjected to tensile stress, because the constitutive double network structure in which Al3+ forms covalent bonds with -COOH on PAA ensures the state and elasticity of the hydrogel, the long rigid chains and self-winding of PVA enhance the dense structure of the hydrogel, effectively ensuring an elongation at break of 400% and a breaking strength of 150 kPa. Meanwhile, polyphenol lignin in DES solution and carboxyl groups on PAA enhance the adhesion of the hydrogel, and choline chloride and small molecule lignin from DES solution confer good antibacterial properties and cytotoxicity to the hydrogel, which provides a great possibility for it to be a human wearable sensor.
3.2 PVA hydrogen multifunctional sensors
3.2.1 Shape memory function
In addition to human detection, more functional hydrogel sensors with sensing at the same time become the current trend. Having a shape memory function can improve the service life of hydrogel on the one hand; on the other hand, programmable shape immobilization and recovery ability will further expand its application in emerging fields such as soft robotics and information storage, but how to integrate self-healing and shape memory into hydrogel is still a great challenge, and shape memory hydrogel usually requires a permanent cross-linked network, which will hinder the internal structure of hydrogel. This will impede the flow and ion exchange within the hydrogel structure. Therefore, it is challenging to obtain hydrogels with superior healing efficiency and shape memory properties.
For example, Guifa Xiao et al. [171] by incorporating cellulose nanocrystals grafted phenylboronic acid (CNCs-ABA) and MWCNTs into PVA obtained a conductive hydrogel with good biocompatibility, fast healing, and shape memory (Fig. 14a). The pH-induced dynamic borate bonds resulted in good shape recovery and fixation values of 82.1% and 78.2% for the hydrogel; 97.1% healing efficiency was obtained within 2 min based on the significant photothermal effect and reversible micro crystallization of MWCNTs; the dual network structure simultaneously improved its tensile strength, strain modulus, and elastic modulus, reaching 227.0 kPa, 395.0%, and 9.0 kPa, respectively; the synergistic effect of MWCNTs and sodium hydroxide enhanced the electrical conductivity of the hydrogel with a value of 3.8 × 10−2 S/m. The hydrogel can be used as a strain sensor to detect human motion with superior biocompatibility and fast resistance response to the applied strain, which is suitable for human health management.

Reproduced with permission from Ref. [171]. Copyright 2021 Elsevier. b Schematic diagram of PVA/IL hydrogel preparation. Reproduced with permission from Ref. [172]. Copyright 2022 Elsevier
a Schematic diagram of hydrogel preparation; photograph of shape memory behavior of hydrogel; mechanism of the shape memory process.
And Haiyan Du et al. [172] used polyvinyl alcohol ionic solution (PVA/IL) and1-butyl-3-methylimidazolium tetrafluoroborate solution repaired multifunctional conductive hydrogels capable of spontaneous polymerization with transparent, self-curing, self-healing, and shape memory functions by a simple one-step method using PVA/ionic solution (IL) and 1-butyl-3-methylimidazolium tetrafluoroborate solution at room temperature (Fig. 14b). The reversible electrostatic interactions and hydrogen bonding between [BMIM]BF4 and PVA, as well as the intramolecular/intermolecular interactions of PVA, conferred excellent thermal and mechanical properties, self-healing, and shape memory effects on the hydrogels. The introduction of their ionic solutions significantly shortens the gelation time and can improve the thermal and mechanical properties as well as the self-healing efficiency of the hydrogels. In addition, the shape memory behavior of PVA/IL hydrogels is also regulated by the addition of Fe3+ to fix the temporary shape and the reduction of Fe3+ to Fe2+ in the Vc solution. This multifunctional hydrogel with memory function and self-healing and good biocompatibility has great potential for medical renewable materials, tissue engineering, sensors and actuators, and soft wearable electronic devices.
3.2.2 Self-powered function
Most current flexible sensors depend on a power source and do not work without it, making it a great challenge to develop a self-powered flexible sensor. Libin Han et al. [173] solved this problem by using a protocell structure, which synthesized 4-(methacryloyl oxy) ethyl-1- phenylene acid(MBA) and 1,4-(5-hexenyloxy) benzene (RM26) as supramolecular crosslinkers, mixed with PVA hydrogels, and used ionic solution (Fig. 15a). A series of conductive hydrogels PVA-PMR-NaCls were prepared by immersion method. The best PVA-PMR-NaCl had a toughness of 15.92MJ⋅m−3; elongation at break of 600%; tensile strength of 5.61 Mpa; Young’s modulus of 2.16 MPa; and a high specification factor (GF) of 6.24 at strains above 200% and the original hydrogel structure was assembled with zinc and copper foils by attaching wires to the original hydrogel structure to form a primary cell structure: An open-circuit voltage of 0.809 V was achieved with zinc foil as the negative electrode and copper foil as the positive electrode, again capturing tensile, bending, and compressive motions in response to current variations.

Reproduced with permission from Ref. [173]. Copyright 2022 Elsevier. b Description of the preparation of TCGP hydrogels; configuration of the generator; description of the process of electricity generation by moisture; mechanism of electricity generation by TCGP hydrogels using moisture. Reproduced with permission from Ref. [174]. Copyright 2020 RSC
a Schematic of the preparation of PVA-PMR hydrogel; schematic of the self-powered device consisting of PVA-PMR5-NaCl, zinc foil, and copper foil; monitoring of the voltage variation of the hydrogel sensor.
PVA is rich in hydroxyl groups, which can dissociate under humid conditions to release large amounts of protons, a property that makes PVA an ideal material for the preparation of proton exchange hydrogels with high proton conductivity. Peng He et al. [174] prepared a conductive hydrogel TA-CNT-Gly-PVA (TCGP) hydrogel by incorporating tannic acid-carbon nanotubes (TA-CNT) into a matrix containing water-glycerol-dispersed polyvinyl alcohol (PVA) (Fig. 15b), which can not only be used as a strain sensor for detecting various human motions, but H-MEG can also generate an output voltage from the water when the water flows continuously on the generator. With a maximum value of up to 80 mV, it can be used as an effective moisture generator. These advantages highlight the great potential of TCGP hydrogels for wearable flexible strain sensors, bioelectrodes, and next-generation green energy generators.
3.2.3 Functional responsive hydrogel
pH-responsive smart hydrogels have unique physical and chemical properties and are widely used in various fields such as bioengineering, drug release, smart coatings, and soft robotics. Shulan Jiang [175] et al. prepared pH-sensitive bilayer hydrogels by UV polymerizing the P(AAm-AAc-3-AAPBA) layer as the active actuating layer and the PAAm layer as the auxiliary actuating layer, inspired by the smart drosera system, and immersed the prepared bilayer hydrogels in acidic PVA solution to investigate the reversibility and bonding properties of the bilayer structure (Fig. 16a), and finally obtained the hydrogels with good. The bilayer hydrogels were finally obtained with good bending properties, good bonding ability, ability to make complex hydrogel patterns, and pH responsiveness. The prepared bilayer hydrogel structures are programmable and multifunctional and can be used to produce bionic flowers and bionic manipulators, which have great potential for intelligent sensing design and fabrication.

Copyright 2022 MDPI. b Preparation process of lignin reinforced hydrogels (LRP) and its applications. Photos showing the shape recovery process of LRP hydrogel in acidic/basic solutions. Mechanism of moist-electric generators based on LRP hydrogel. Reproduced with permission from Ref. [176]. Copyright 2021 Elsevier
a Schematic diagram of the fabrication of pH-responsive bilayer hydrogel structures; images of drosera and the bending mechanism of bilayer structured hydrogels; bilayer hydrogel actuators placed in solutions of pH 10–13 solution for 20 min; bending angle versus time for flower-like hydrogel contraction in acidic solution; flower-like hydrogel blooming process in alkaline solution; hand-shaped hydrogel mimicking a hand fist; reproduced with permission from Ref. [175].
Yang Zhang et al. [176] used lignin to enhance the strength of PVA hydrogels and impart pH-responsive properties (Fig. 16b), and the prepared LRP hydrogels exhibited good ionic conductivity, mechanical properties, and strain sensitivity, while the LRP hydrogels were able to output 226.6 mV from the water stream, which was a significant increase in the maximum output voltage as compared to the CNT-PVA matrix for making its generator use.
Temperature-responsive PVA-based conductive hydrogels are able to respond to temperature while strain sensing detects temperature changes. Xiangdong Wang et al. [177] proposed that HEC embedded in PVA hydrogel can reach conductivity of 5.77 S/m when immersed in NaCl solution and strength of 2.86 Mpa when the strain is 400.30%; the hydrogel was immersed in sodium chloride glycerol aqueous solution to obtain PVA-HEC organic hydrogel with high strength and high conductivity at − 30 °C ~ 65 °C, and temperature-sensitive particles were added to PHON to prepare fibers with reversible color temperature-sensitive particles are a flexible electronic material with an organic hydrogel that can directly monitor the temperature to become temperature-sensitive conductive materials.
3.2.4 Superconducting functional hydrogel
The current PVA-based conductive hydrogel in the field of wearable is sufficient to meet the application needs, and some scholars have further explored the basis of the next, to obtain a superconducting hydrogel with the function of invisibility without being detected on the basis of wearable. For example, Jiahong Tian et al. [178] successfully fabricated Epsilon-near-zero (ENZ) superconducting polyvinyl alcohol/multi-walled carbon nanotubes (PVA/MWCNTs) hydrogels by an environment-friendly method, and the experimental process and products were proved to be green, environmentally friendly, and biocompatible by cytotoxicity experiments, and the microstructure, crystalline structure, chemical composition, and dielectric properties of the hydrogels were investigated. In thermo-gravimetric analysis, it was found that when two different water states, i.e., free water molecules and bound water molecules, coexisted in these PVA/MWCNT hydrogels accounting for 37.5wt% and 15.9wt%, respectively; when the MWCNT content reached 12wt% and 15wt%, a continuous network of conducting MWCNTs was formed in the hydrogels and the ENZ phenomenon was observed at 760kHz and ENZ phenomena were observed around 580 kHz, attributed to the interband transition. The ENZ properties at radio frequencies due to the flexibility and non-toxicity of PVA/MWCNT hydrogels make them well suited for wearable invisible cloaks, flexible electronics, skin sensors, and other wearable devices.
3.3 Supercapacitor
With the development of electronic devices such as flexible displays, and portable and wearable devices, energy storage systems that are equally flexible in energy are needed. Currently, the most researched flexible energy storage systems include flexible alkaline batteries, polymer batteries, and flexible supercapacitors [179]. In flexible supercapacitors, the toughness of energy storage devices and stable energy output during dynamic deformation are two challenges to be solved in their practical applications; however, hydrogel material is used in supercapacitors with simple structure, good flexibility, and no bonding interface between electrode material and collector, which reduces the risk of contact surface damage and dislodgement. Among them, PVA hydrogel has excellent mechanical properties, supportability, and self-recovery ability, and its assembled supercapacitor can maintain stable energy output in dynamic deformation, and also has abundant hydroxyl groups, so the supercapacitor developed based on it has great potential for wearable devices.
For example, Jiwei Chen et al. [180] used a one-step solvent exchange strategy to trigger the self-assembly of PVA chains, forming a homogeneous cross-linked network enabling the PVA hydrogel electrolyte to exhibit excellent stiffness and toughness with a tensile strength of 16.54 MPa, elongation at break of 1203%, and toughness of 111.21 MJ·m−3, and assembled PANI as the electrode at 1.0 mA·cm−2 with 156.50 mF·cm−2 flexible supercapacitor. Based on the good stiffness and toughness exhibited by the PVA hydrogel, the supercapacitor shows high resistance to mechanical stimuli such as bending and pressure, and maintains stable energy output well under dynamic deformation, and the developed supercapacitor has great potential for application in wearable devices.
While Guizhen Guo et al. [181] further improved the above approach by using the synergistic effect of PVA and SA to form PVA/GO/SA/PANI conductive hydrogel without cross-linker, and PGOSAP hydrogel was used as the electrode, this is because the crystalline area of the composite hydrogel is smaller than that of the single material, thus providing more channels for electrolyte transfer. Then, the pure PVA hydrogel soaked with H2SO4 was used as the electrolyte, and the two were compounded to form the supercapacitor. The results showed no displacement and delamination between electrolyte and electrode after 3500 cycles. This sheet-integrated flexible supercapacitor with excellent mechanical properties is expected to provide a broad prospect for the preparation of deformable flexible supercapacitors.
The integration of all-hydrogel supercapacitors on the basis of their preparation is also a challenge for flexible supercapacitors. Yongliang Zou et al. [55] achieved an integrated highly flexible self-healing supercapacitor with good electrochemical properties providing an effective and low-cost strategy. The PCC hydrogel obtained by double physical cross-linking of 4-carboxyphenylboronic acid (CPBA) and calcium ions with polyvinyl alcohol-based hydrogel PCH hydrogel as electrolyte and in situ polymerization of PCH hydrogel by adding aniline to it as electrode has superior conductivity of 136 S·m−1 and large specific capacity of 351 F·g−1 (Fig. 17a), as well as good mechanical properties and self-healing capability. The all-hydrogel supercapacitor has a tensile strength of 2.21 MPa, an elongation at break of 633%, a capacitance of 78.5 F·g−1, and an energy density of 7.8 Wh·kg−1. Due to the high toughness and flexibility of the supercapacitor, it can maintain a stable energy output even after being subjected to various deformations (e.g., stretching, compression, bending, and even stapling). In addition, the severed supercapacitor can heal itself within 4 h at 70 °C. The healed supercapacitor can recover good electrochemical performance with a healing rate of 49.8%.

Copyright 2021 Elsevier. b Schematic diagram of the preparation of PANI@ Mxene/PVA hydrogel composite; schematic demonstration of a flexible supercapacitor based on PANI@ Mxene/PVA. Reproduced with permission from Ref. [182]. Copyright 2022 Elsevier. c Schematic illustration of interactions within PACP/PVA hydrogel; photographs of PACP/PVA-0.8 hydrogel with various shapes. Reproduced with permission from Ref. [183]. Copyright 2021 Springer
a Preparation route of PPHn electrode and PCH electrolyte; fabrication of integrated hydrogel supercapacitor containing PPH0.03 electrodes and PCH electrolyte through a layer-by-layer gelation strategy; reproduced with permission from Ref. [55].
Starting from the structure is likewise one of the methods to improve the performance of flexible supercapacitors. Shuqing Cao et al. [182] introduced a new two-dimensional material Mxene into the preparation of supercapacitors, and prepared a new Mxene/PVA three-dimensional interconnected structure porous sponge by sol–gel and freeze-drying methods (Fig. 17b), and then used the Mxene/PVA porous sponge as a template for in situ PAN polymerization, and the prepared PANI@Mxene/PVA hydrogel composite was used as a flexible supercapacitor electrode. The maximum surface-specific capacitance of this supercapacitor at 2 a·m−2 is 103.8 mF·cm−2, the maximum energy density is 9.2 μWh/cm−2, and the optimum power density is 800 μW·cm−2. The capacitance of this supercapacitor almost does not change under different bending angles. In addition, 99% capacitance retention was achieved after 10,000 cycles of supercapacitor charging/discharging. Throughout the process, the presence of PVA can further bind to the Mxene surface to expand the layer spacing distance, and the unique three-dimensional structure constructed increases the electrochemically active electrochemical sites, providing an effective channel for ion diffusion electron transport and contributing to the excellent electrochemical performance.
Some scholars have also designed the materials of electrodes to improve the performance of supercapacitors by using a combination of two conductive polymers to prepare electrode materials for supercapacitors to improve the electrochemical performance; e.g., Xue-Yu Tao et al. [183] used in situ polymerization of aniline and pyrrole in an aqueous PVA solution to prepare polyaniline-polypyrrole (PACP)/PVA conductive hydrogels as electrodes for supercapacitors and PVA/H2SO4 hydrogels as electrolytes for all-hydrogel flexible supercapacitors (Fig. 17c). The hydrogel electrodes exhibited high electrochemical capacitance (633.5 F g at 0.5 A g−1 and 1267 mF cm at 1 mA cm−2) and excellent cycling stability (86.4% capacitance retention after 10,000 cycles) and 81.7% initial capacitance retention after repeated bending 500 times, demonstrating their remarkable flexibility. The supercapacitor with a high surface capacitance of 317 mF cm−2 at 1 mA cm−2 and energy density of 22 Wh kg−1 at 125 W kg−1 was fabricated to provide a new direction for the preparation of flexible hydrogel electrode materials for smart wearable devices and to provide ideas for the development of next-generation wearable portable electronics.
3.4 Self-powered flexible sensors
Based on the current booming development of electronic devices, the energy storage problem becomes a major problem that needs to be solved urgently. Since Zhonglin Wang proposed the theory of friction nanogenerators, micro, and nanosystems provide long-lasting, maintenance-free, self-driven energy—nano energy has been widely paid attention to by scholars. The main mechanism of nano-energy is piezoelectric nano-generation [184, 185] and frictional electric nanopower [186]. The self-generated sensors prepared by these two autogeneration mechanisms are used in electronic skin, wearable devices [187, 188], et al. PVA conductive hydrogels have attracted a lot of attention in the field of energy conversion materials due to their tunable electrical and mechanical properties, and have a promising application in friction nanogenerators, broadening their application areas.
Hydrogel-based friction nanogenerator devices generally use contact separation [156]; single electrode two modes, its working principle is generally contact parts energized, the human body and friction layer (silicone rubber, PDMS, VHB tape, polytetrafluoroethylene (PTFE) [114, 189], etc.) when contact, the friction layer, and human skin are distributed with equal amounts of negative and positive charge; the potential difference is infinitely close to 0; when the human body and friction layer separate, the negative charge will move to promote charge balance, the resulting in the accumulation of electrons; the wire will be connected to the body’s movement in change into a detectable electrical signal.
In nanogenerators, the ability to capture motion into electrical signals such as voltage and current for sensing purposes and the transparent visualization of the structure is also a goal pursued by scholars. Kaushik Parida et al. [189] prepared the IS hydrogel by chemical cross-linking of PVA hydrogel using (Na2B4O7 ·10H2O), and then it was prepared as an electrode compounded with Ecoflex as a negative friction layer (Fig. 18a), and Cu metal sheet as another electrode prepared from human skin as a positive friction layer (IS-TENG) with 92% light transmission; its energy harvesting performance is 12 times higher than that of silver-based electron current collector, and it can withstand up to IS-TENG that is a highly deformable and transparent power source that can be quickly and automatically repaired at room temperature, and thus can be used as a power source for digital watches, touch sensors, artificial intelligence, and bio-integrated electronics.

Reproduced with permission from Ref. [190]. Copyright 2017 Wiley. b Design and schematic diagram of the operating mechanism of CPH-TENG in single electrode mode; CPH-TENG (CPH width 1.0 cm, length 1.5 cm) lit by a finger and 15 LEDs (white and blue); CPH-TENG with contact separation motion (CPH width 1.0 cm, 1.5 cm long) with open circuit voltage (VOC), short circuit charge (QSC), and short circuit current (ISC); reproduced with permission from Ref. [191]. Copyright 2020 RSC
a Schematic diagram and working mechanism of IS-TENG; digital photograph of self-healing IS-TENG (scale bar 1cm); energy harvesting performance of IS-TENG at 4 Hz when tapping nerve with finger, voltage output (Voc); current density (Is); charge transfer density (σT); digital photograph of IS-TENG powered by digital watch digital photo; digital photo of IS-TENG with human palm powering 40 LEDs; digital photo of IS-TENG under 700% lateral strain; g digital photo of IS-TENG driving 40 LEDs attached to a bifurcated fabric (scale bar 2cm).
Yang Wang et al. [191] designed a stretchable, transparent, ion-conductive cellulose/PVA (CPH) hydrogel (Fig. 18b): 80.42% light transmission at 550 nm; 747% final elongation; excellent recovery rate performance and stability between − 12.42 and 62.63 °C. Copper sheets are also utilized as electrodes. Nylon and CPH hydrogel encapsulated by VHB tape was used as friction layers, and CPH-TENG was assembled in a single-electrode working mode for energy harvesting. The test data showed that CPH-TENG has good tensile function over a wide temperature range and is stable to multiple stretching, long-term storage, or a large number of contact separation motion cycles, and the low-pressure detection limit of CPH-TENG is 0.0147 kPa, which broadens the application of PVA-based conductive hydrogel materials in the field of flexible wearable electronic devices.
PDMS [192] has been used more in PVA-based triboelectric sensors because of its simple curing method, transparency, biocompatibility, and stretchability, for example, Shasha Wang and Yin Zhang [193]. The LiCl/PVA hydrogel is used as the conductive electrode and the PDMS layer is used as the friction layer and can encapsulate the hydrogel to reduce the water loss (Fig. 19a). LP-TENG demonstrated excellent linear voltage output performance during a simple stretching process to demonstrate its potential as a wearable self-powered sensor. The open-circuit voltage and short-circuit current of the LP-TENG can reach 216 V and 1.44 µA, respectively, under simple motion, indicating that the LP-TENG can successfully capture low-frequency vibration energy from human motion and has great promise for flexible sensing devices and motion monitoring applications.

Reproduced with permission from Ref. [193]. Copyright 2022 APM. b Optical photograph of fabricated SPE-TENG. A schematic of the SPE-TENG working mechanism in SE mode. Schematic of layer structure and equivalent circuit of SPE-TENG. Reproduced with permission from Ref. [194]. Copyright 2021 Elsevier
a Schematic representation of the LiCl/PVA hydrogel as synthesized, highlighting the main and secondary cross-link networks. A schematic diagram illustrating the operation of the LP-TENG.
The simple combination of PVA conductive hydrogel and PDMS does not fully realize the potential, so scholars have designed the structure of PVA-based friction nanogenerators with PDMS as the friction layer, and Guang Li et al. [194] constructed a sandwich structure using ion-conducting hydrogel and PDMS, conductive layer to form a frictional electro-generator (TENG) with high electrical output (Fig. 19b). Mainly by combining phosphoric acid (H3PO4) with biocompatible PVA, the obtained SPE hydrogel can achieve 1058% elongation and 1.39 S·m−1 ionic conductivity due to the phosphoric acid having a plasticizer and being an ionic conductive carrier in the hydrogel. The SPE electrodes obtained are highly transparent (95% light transmission) and stretchable (1058% elongation), showing great potential as a portable power source for wearable electronic devices. SPE-TENG can maintain stable output performance over thousands of contact-disconnection cycles, showing good stability with a high output of 992 V, 44.8 μA, and 26Wm−2, and is stable under SPE-TENG that can be used as a sustainable energy source for self-powered electronics, and it is easy to manufacture, reliable, and dehydration-resistant characteristics offer a wide range of practical applications.
Jing Xin et al. [195] designed A unique PDMS pocket structure, as a way to effectively prevent hydrogel dehydration and maintain stable output during continuous operation—the output voltage reduction rate of H-TENG was within 15% after 4 weeks of exposure to the atmosphere. By synthesizing a double network hydrogel composed of double cross-linked PVA and SA and controlling the elasticity of the hydrogel by varying the concentration of SA, the significant effect of the viscoelastic properties of the hydrogel on the performance of H-TENG was verified for the first time. Finally, the best H-TENG was obtained by adjusting the conductivity and viscoelasticity of the PVA/SA hydrogel (Fig. 20a). The best H-TENG has high transparency (over 90%) and stretchability (over 250%) with peak output voltage and current of 203.4 V and 17.6µA, respectively, and can easily light up 240 green and blue LEDs simultaneously, demonstrating the ability to power small electronic devices. The H-TENG also demonstrates the ability to rapidly charge capacitors with capacitance up to 1000 μF via a bridge rectifier, and that this energy can be used to power small electronic devices, such as digital timers and pedometers.

Copyright 2020 ACS. b Schematic illustration of the as-synthesized MXene/PVA hydrogel showing the primary and secondary cross-link networks, operating principles, and output performance of the MH-TENG. Illustration of handwriting on the surface of MH-TENG. Reproduced with permission from Ref. [196]. Copyright 2021 AFM
a Description of hydrogel-based TENG fabrication process; description of hydrogel-based TENG fabrication process; output voltage of H-TENG for different materials; output voltage when H-TENG is stretched to 100%, 150%, and 200% strain; reproduced with permission from Ref. [195].
In addition to the design of the structure between the friction layer and the conductive hydrogel (Fig. 20b), some scholars focused their improvement approach on the hydrogel structure in order to further improve the performance of friction nanogenerators. Xiongxin Luo et al. [196] introduced an MXene/PVA hydrogel TENG (MH-TENG); the doping of MXene nanosheets promoted the cross-linking of PVA hydrogel and improved the stretching ability of the composite hydrogel. MXene nanosheets also formed microchannels on the surface, which could not only enhance the ion transport to improve the electrical conductivity of the hydrogel but also generate additional frictional electrical through the SVP mechanism output. In single-electrode mode, MH-TENG also measures open-circuit voltage up to 230 V. When MH-TENG is stretched to within 200% of its original length, a monotonic increasing relationship between stretching strength and short-circuit voltage can be shown. Using the excellent stretchability and high sensitivity of the MH-TENG, its application in wearable motion monitoring, high-precision writing stroke recognition, and low-frequency mechanical energy acquisition is demonstrated.
After the structural improvement, the recovery and practicality of frictional electric nano power generation are also paid attention to by scholars at the same time; for example, Wei Xu et al. [197] demonstrated a hydrogel-based frictional electric nanogenerator (H-TENG) with high flexibility, recyclability, and environmental friendliness, which can generate a maximum output power of 2 mW at a load resistance of 10 MΩ. This H-TENG can obtain mechanical energy from various human motions, including bending, twisting, and stretching movements; this H-TENG can also be used as a self-powered sensor to detect human motion. Compared with other studies, the polyvinyl alcohol hydrogel developed by Wei Xu et al. has recyclable properties and can be used to make renewable TENGs, and the recyclable H-TENG can be obtained with 92% of the original open-circuit voltage, which will be one of the ways to develop flexible energy and self-powered motion sensors.
Table 4 compares the electrical properties of PVA-based conductive hydrogels with various network architectures used in friction nanogenerators.
4 Conclusion and outlook
This paper provides an in-depth analysis of the composite ideas and current application progress of PVA-based conductive composite hydrogels. The multifunctional PVA-based conductive hydrogels made from composites have made great progress in applications such as monitoring human motion and electronic skin. Following a brief explanation of the operating principle of PVA-based conductive hydrogels, the applications of various compositions of PVA-based conductive hydrogels in human dynamic monitoring, smart wear, and other fields are briefly introduced. Strain sensing based on its capacity to self-heal, transducer, and other applications are then briefly discussed. The self-healing, transparency, anti-freezing, pH response, humidity response, shape memory, and other properties of the PVA-based conductive hydrogel are next introduced, followed by information about its use in the fields of flexible supercapacitors and self-generated flexible sensors.
PVA-based conductive hydrogel, a new material, combines the characteristics of a gel with a nanomaterial. Despite significant advancements in the current synthesis technique and property optimization, actual production applications are still some time off. One issue that needs to be resolved for PVA-based hydrogels is the mutual balance between mechanical properties and electrical conductivity. Conductive particles or ions often build a conductive network at the expense of the hydrogel’s mechanical properties, but reducing the relative mass of the conductive material will decrease the hydrogel’s electrical conductivity and sensitivity, and the need to not affect the excellent function of the original hydrogel matrix as the premise, to ensure that it still has biocompatible, environmentally friendly, and at the same time carefully selected conductive materials.
Hydrogel materials also face the challenge of extreme environments, although the research in low-temperature environments has been relatively mature, in the water-filled environment because of the osmotic pressure; hydrogel materials cannot avoid water absorption and swelling, while in the high-temperature environment in the hydrogel, water will evaporate into the environment; the above two extreme conditions will undoubtedly make its mechanical properties rapidly decline and sensor parts will fail; thus, the actual above two extremes will undoubtedly cause a rapid decline in the mechanical properties of the sensor parts, which will fail, thus affecting the actual production. The current laboratory solution is to use stretchable and biocompatible elastomer (PDMS, TPU, VHB tape, Ecoflex, etc.) materials to encapsulate it, but it will affect its sensitivity and mechanical properties to a certain extent, so overcoming extreme environmental conditions will also be one of the challenges faced by hydrogel materials to become practical.
As new industries like electronic skin, bionic robots, sensing fabrics, and human–computer interaction continue to develop, we believe that PVA-based conductive hydrogel will play an essential part in facilitating the creation of experimental solutions in the fields mentioned above. In the near future, the new PVA-based conductive hydrogel’s mechanical properties will be in many aspects (moisture, mech. future duties, and problems will undoubtedly prove challenging), but there will also be an abundance of fantastic possibilities and new items to take advantage of. This exhaustive review, in our view, may illuminate and aid researchers.
Data availability statement
Data will be available upon request.
References
Zhang J, Tao D (2021) Empowering things with intelligence: a survey of the progress, challenges, and opportunities in artificial intelligence of things. IEEE Internet Things J 8(10):7789–7817. https://doi.org/10.1109/jiot.2020.3039359
Jakab K, Csipor J, Ulbert I et al (2022) EEG sensor system development consisting of solid polyvinyl alcohol-glycerol-NaCl contact gel and 3D-printed, silver-coated polylactic acid electrode for potential brain-computer interface use. Mater Today Chem 26. https://doi.org/10.1016/j.mtchem.2022.101085
Guo Z, Liu Z, Liu W et al (2021) Multifunctional flexible polyvinyl alcohol nanocomposite hydrogel for stress and strain sensor. J Nanopart Res 23(10). https://doi.org/10.1007/s11051-021-05333-y
Karimzadeh Z, Mahmoudpour M, Rahimpour E et al (2022) Nanomaterial based PVA nanocomposite hydrogels for biomedical sensing: advances toward designing the ideal flexible/wearable nanoprobes. Adv Colloid Interface Sci 305. https://doi.org/10.1016/j.cis.2022.102705
Wen N, Jiang B, Wang X et al (2020) Overview of polyvinyl alcohol nanocomposite hydrogels for electro-skin, actuator, supercapacitor and fuel cell. Chem Rec 20(8):773–792. https://doi.org/10.1002/tcr.202000001
Li T, Wei H, Zhang Y et al (2023) Sodium alginate reinforced polyacrylamide/xanthan gum double network ionic hydrogels for stress sensing and self-powered wearable device applications 309:120678
Zhao X, Zheng M, Gao X et al (2021) The application of MOFs-based materials for antibacterials adsorption 440:213970
Lu H, Wang Y, Wang Y et al (2015) Adjusting phase transition of titania-based nanotubes via hydrothermal and post treatment 5(109):89777–89782
Wang B, Zhang H-R, Huang C et al (2017) Study on non-isothermal crystallization behavior of isotactic polypropylene/bacterial cellulose composites 7(67):42113–42122
Gao C, Liao J, Lu J et al (2021) The effect of nanoparticles on gas permeability with polyimide membranes and network hybrid membranes: a review 41(1):1–20
Wang B, Lin F-H, Zhao Y-Y et al (2019) Isotactic polybutene-1/bamboo powder composites with excellent properties at initial stage of molding. 11(12): 1981
Wang B, Mao S, Lin F et al (2021) Interfacial compatibility on the crystal transformation of isotactic poly (1-butene)/herb residue composite. 13(10): 1654
Mao S-D, Zhang M, Lin F-H et al (2022) Attapulgite structure reset to accelerate the crystal transformation of isotactic polybutene 14(18):3820
Wang B, Nie K, Xue X-R et al (2018) Preparation of maleic anhydride grafted polybutene and its application in isotactic polybutene-1/microcrystalline cellulose composites. 10(4): 393
Wang B, Lin F-H, Li X-Y et al (2018) Isothermal crystallization and rheology properties of isotactic polypropylene/bacterial cellulose composite 10(11):1284
Wang B, Lin F-H, Li X-Y et al (2019) Transcrystallization of isotactic polypropylene/bacterial cellulose hamburger composite 11(3):508
Zhao X, Zhao Y, Zheng M et al (2019) Efficient separation of vitamins mixture in aqueous solution using a stable zirconium-based metal-organic framework 555:714–721
Zhao X, Gao X, Zhang Y-N et al (2023) Construction of dual sulfur sites in metal–organic framework for enhanced mercury (II) removal. 631: 191–201
Zhao X, Wang Y, Li Y et al (2019) Synergy effect of pore structure and amount of carboxyl site for effective removal of Pb2+ in metal–organic frameworks. 64(6): 2728–2735
Hou C, Yang W, Kimura H et al (2023) Boosted lithium storage performance by local build-in electric field derived by oxygen vacancies in 3D holey N-doped carbon structure decorated with molybdenum dioxide. 142: 185–195
Lei D, Liu N, Su T et al (2022) Roles of MXene in pressure sensing: preparation, composite structure design, and mechanism. Adv Mater. https://doi.org/10.1002/adma.202110608
Shen Y, Yang W, Hu F et al (2023) Ultrasensitive wearable strain sensor for promising application in cardiac rehabilitation. Adv Compos Hybrid Mater 6(1). https://doi.org/10.1007/s42114-022-00610-3
Lin Z, Li X, Zhang H et al (2023) Research progress of MXenes and layered double hydroxides for supercapacitors.
Yang S, Shi C, Qu K et al (2023) Electrostatic self-assembly cellulose nanofibers/MXene/nickel chains for highly stable and efficient seawater evaporation and purification. 1–12
Norizan MN, Moklis MH, Demon SZN et al (2020) Carbon nanotubes: functionalisation and their application in chemical sensors. RSC Adv 10(71):43704–43732. https://doi.org/10.1039/d0ra09438b
He Y, Zhou M, Mahmoud MHH et al (2022) Multifunctional wearable strain/pressure sensor based on conductive carbon nanotubes/silk nonwoven fabric with high durability and low detection limit. Advanced Composites And Hybrid Materials 5(3):1939–1950. https://doi.org/10.1007/s42114-022-00525-z
Jiang X, Chen Y, Meng X et al (2022) The impact of electrode with carbon materials on safety performance of lithium-ion batteries: a review 191:448–470
Hernaez M (2020) Applications of graphene-based materials in sensors. Sensors 20(11). https://doi.org/10.3390/s20113196
Yuan G, Wan T, BaQais A et al (2023) Boron and fluorine Co-doped laser-induced graphene towards high-performance micro-supercapacitors 212:118101
Zhang J, Wang Y, Wang Y et al (2017) Catalytic activity for oxygen reduction reaction on CoN2 embedded graphene: a density functional theory study. 164(12): F1122
Chen S, Wang H-Z, Zhao R-Q et al (2020) Liquid metal composites Matter 2(6):1446–1480. https://doi.org/10.1016/j.matt.2020.03.016
Noah NM (2020) Design and synthesis of nanostructured materials for sensor applications. J Nanomater 2020. https://doi.org/10.1155/2020/8855321
Jiang D, Wang Y, Li B et al (2019) Flexible sandwich structural strain sensor based on silver nanowires decorated with self-healing substrate. Macromol Mater Eng 304(7). https://doi.org/10.1002/mame.201900074
Jia Z, Li Z, Ma S et al (2021) Constructing conductive titanium carbide nanosheet (MXene) network on polyurethane/polyacrylonitrile fibre framework for flexible strain sensor. Journal Of Colloid And Interface Science 584:1–10. https://doi.org/10.1016/j.jcis.2020.09.035
Srinivasan R, Sankar AR (2022) Intrinsically conducting polymers in flexible and stretchable resistive strain sensors: a review. J Mater Sci. https://doi.org/10.1007/s10853-022-07479-z
Lan D, Wang Y, Wang Y et al (2023) Impact mechanisms of aggregation state regulation strategies on the microwave absorption properties of flexible polyaniline 651:494–503
Ruan J, Chang Z, Rong H et al (2023) High-conductivity nickel shells encapsulated wood-derived porous carbon for improved electromagnetic interference shielding. 118208
Gao Z-Q, Li H-J, Gu J-Z et al (2016) Metal-organic and supramolecular networks driven by 5-chloronicotinic acid: Hydrothermal self-assembly synthesis, structural diversity, luminescent and magnetic properties. 241: 121–130
Hao XL, Ma YY, Zang HY et al (2015) A polyoxometalate‐encapsulating cationic metal–organic framework as a heterogeneous catalyst for desulfurization. 21(9): 3778–3784
Hao X-L, Jia S-F, Ma Y-Y et al (2016) Two new Keggin-type polyoxometalate-based entangled coordination networks constructed from metal-organic chains with dangling arms 72:132–137
Wang R, Wang Y, Mao S et al (2021) Different morphology MoS 2 over the gC 3 N 4 as a boosted photo-catalyst for pollutant removal under visible-light. 31: 32–42
Zhao X, Wang T, Du G et al (2019) Effective removal of humic acid from aqueous solution in an Al-based metal–organic framework. 64(8): 3624–3631
Zhao X, Wei Y, Zhao H et al (2018) Functionalized metal-organic frameworks for effective removal of rocephin in aqueous solutions 514:234–239
Zheng M, Zhao X, Wang K et al (2019) Highly efficient removal of Cr (VI) on a stable metal–organic framework based on enhanced H-bond interaction. 58(51): 23330–23337
He Y, Zhao L, Zhang J et al (2020) A breathable, sensitive and wearable piezoresistive sensor based on hierarchical micro-porous PU@CNT films for long-term health monitoring. Compos Sci Technol 200. https://doi.org/10.1016/j.compscitech.2020.108419
Yu X, Wu Z, Weng L et al (2023) Flexible strain sensor enabled by carbon nanotubes-decorated electrospun TPU membrane for human motion monitoring. Adv Mater Interfaces. https://doi.org/10.1002/admi.202202292
Yang Z, Wu Z, Jiang D et al (2021) Ultra-sensitive flexible sandwich structural strain sensors based on a silver nanowire supported PDMS/PVDF electrospun membrane substrate. Journal Of Materials Chemistry C 9(8):2752–2762. https://doi.org/10.1039/d0tc04659k
Soe HM, Abd Manaf A, Matsuda A et al (2020) Development and fabrication of highly flexible, stretchable, and sensitive strain sensor for long durability based on silver nanoparticles-polydimethylsiloxane composite. J Mater Sci-Mater Electron 31(14):11897–11910. https://doi.org/10.1007/s10854-020-03744-6
Jiang NI, Hu DW, Xu YQ et al (2021) Ionic liquid enabled flexible transparent polydimethylsiloxane sensors for both strain and temperature sensing. Adv Compos Hybrid Mater 4(3):574–583. https://doi.org/10.1007/s42114-021-00262-9
Georgopoulou A, Kummerloewe C, Clemens F (2020) Effect of the elastomer matrix on thermoplastic elastomer-based strain sensor fiber composites. Sensors 20(8). https://doi.org/10.3390/s20082399
Khalid MAU, Chang SH (2022) Flexible strain sensors for wearable applications fabricated using novel functional nanocomposites: a review. Compos Struct 284. https://doi.org/10.1016/j.compstruct.2021.115214
Wen N, Zhang L, Jiang D et al (2020) Emerging flexible sensors based on nanomaterials: recent status and applications. J Mater Chem A 8(48):25499–25527. https://doi.org/10.1039/d0ta09556g
Hou XT, Sun JX, Lian MY et al (2023) Emerging synthetic methods and applications of MOF-based gels in supercapacitors, water treatment, catalysis, adsorption, and energy storage. Macromol Mater Eng 308(2). https://doi.org/10.1002/mame.202200469
Wang H, Zhou R, Li D et al (2021) High-performance foam-shaped strain sensor based on carbon nanotubes and Ti3C2Tx MXene for the monitoring of human activities. ACS Nano 15(6):9690–9700. https://doi.org/10.1021/acsnano.1c00259
Zou Y, Chen C, Sun Y et al (2021) Flexible, all-hydrogel supercapacitor with self-healing ability. Chem Eng J 418:128616. https://doi.org/10.1016/j.cej.2021.128616
Kanoun O, Bouhamed A, Ramalingame R et al (2021) Review on conductive polymer/CNTs nanocomposites based flexible and stretchable strain and pressure sensors. Sensors 21(2). https://doi.org/10.3390/s21020341
Zhou B, Liu Z, Li C et al (2021) A highly stretchable and sensitive strain sensor based on dopamine modified electrospun SEBS fibers and MWCNTs with carboxylation. Adv Electron Mater 7(8). https://doi.org/10.1002/aelm.202100233
Fan M, Wu L, Hu Y et al (2021) A highly stretchable natural rubber/buckypaper/natural rubber (NR/N-BP/NR) sandwich strain sensor with ultrahigh sensitivity. Adv Compos Hybrid Mater 4(4):1039–1047. https://doi.org/10.1007/s42114-021-00298-x
Wu YF, Wu JB, Lin Y et al (2023) Melamine sponge skeleton loaded organic conductors for mechanical sensors with high sensitivity and high resolution. Adv Compos Hybrid Mater 6(1). https://doi.org/10.1007/s42114-022-00581-5
Kundu R, Payal P (2020) Antimicrobial hydrogels: promising soft biomaterials Chemistryselect 5(46):14800–14810. https://doi.org/10.1002/slct.202003666
Cha GD, Lee WH, Lim C et al (2020) Materials engineering, processing, and device application of hydrogel nanocomposites. Nanoscale 12(19):10456–10473. https://doi.org/10.1039/d0nr01456g
Xu J, Tsai Y-L, Hsu S-H. (2020) Design strategies of conductive hydrogel for biomedical applications. Molecules 25(22). https://doi.org/10.3390/molecules25225296
Chyzy A, Plonska-Brzezinska ME (2020) Hydrogel properties and their impact on regenerative medicine and tissue engineering. Molecules 25(24). https://doi.org/10.3390/molecules25245795
Xu JQ, Zhang MY, Du WZ et al (2022) Chitosan-based high-strength supramolecular hydrogels for 3D bioprinting. Int J Biol Macromol 219:545–557. https://doi.org/10.1016/j.ijbiomac.2022.07.206
Eivazzadeh-Keihan R, Noruzi EB, Aliabadi HAM et al (2022) Recent advances on biomedical applications of pectin-containing biomaterials. Int J Biol Macromol 217:1–18. https://doi.org/10.1016/j.ijbiomac.2022.07.016
Shokrani H, Shokrani A, Seidi F et al (2022) Biomedical engineering of polysaccharide-based tissue adhesives: recent advances and future direction. Carbohydr Polym 295. https://doi.org/10.1016/j.carbpol.2022.119787
Bakadia BM, Zhong A, Li X et al (2022) Biodegradable and injectable poly(vinyl alcohol) microspheres in silk sericin-based hydrogel for the controlled release of antimicrobials: application to deep full-thickness burn wound healing. Adv Compos Hybrid Mater 5(4):2847–2872. https://doi.org/10.1007/s42114-022-00467-6
Chang XH, Chen LR, Chen JW et al (2021) Advances in transparent and stretchable strain sensors. Adv Compos Hybrid Mater 4(3):435–450. https://doi.org/10.1007/s42114-021-00292-3
Shen J, Dai Y, Xia F et al (2022) Role of divalent metal ions in the function and application of hydrogels. Prog Polym Sci 135. https://doi.org/10.1016/j.progpolymsci.2022.101622
Zhang H, Shi LWE, Zhou J (2022) Recent developments of polysaccharide-based double-network hydrogels. J Polym Sci. https://doi.org/10.1002/pol.20220510
Ma Y, Liu K, Lao L et al (2022) A stretchable, self-healing, okra polysaccharide-based hydrogel for fast-response and ultra-sensitive strain sensors. Int J Biol Macromol 205:491–499. https://doi.org/10.1016/j.ijbiomac.2022.02.065
Tang L, Wu S, Qu J et al (2020) A review of conductive hydrogel used in flexible strain sensor. 13(18):3947. https://doi.org/10.3390/ma13183947
Nasution H, Harahap H, Dalimunthe NF et al (2022) Hydrogel and effects of crosslinking agent on cellulose-based hydrogels: a review. Gels 8(9). https://doi.org/10.3390/gels8090568
Taaca KLM, Prieto EI, Vasquez MR Jr. (2022) Current trends in biomedical hydrogels: from traditional crosslinking to plasma-assisted synthesis. Polymers 14(13). https://doi.org/10.3390/polym14132560
Takeno H, Suto N (2022) Robust and highly stretchable chitosan nanofiber/alumina-coated silica/carboxylated poly (vinyl alcohol)/borax composite hydrogels constructed by multiple crosslinking. Gels 8(1). https://doi.org/10.3390/gels8010006
Wang YS, Zheng YD, He W et al (2017) Reprint of: Preparation of a novel sodium alginate/polyvinyl formal composite with a double crosslinking interpenetrating network for multifunctional biomedical application. Compo B Eng 121:9–22. https://doi.org/10.1016/j.compositesb.2017.06.023
Pouranvari S, Ebrahimi F, Javadi G et al (2016) Chemical cross-linking of chitosan/polyvinyl alcohol electrospun nanofibers. Mater Tehnol 50(5):663–666. https://doi.org/10.17222/mit.2015.083
Karimi E, Barekati SS, Raisi A et al (2019) High-flux electrospun polyvinyl alcohol microfiltration nanofiber membranes for treatment of oil water emulsion. Desalin Water Treat 147:20–30. https://doi.org/10.5004/dwt.2019.23576
Raza MA, Jeong J-O, Park SH (2021) State-of-the-art irradiation technology for polymeric hydrogel fabrication and application in drug release system. Front Mater 8. https://doi.org/10.3389/fmats.2021.769436
Hu MF, Gao Y, Jiang YJ et al (2021) High-performance strain sensors based on bilayer carbon black/PDMS hybrids. Adv Compos Hybrid Mater 4(3):514–520. https://doi.org/10.1007/s42114-021-00226-z
Atif M, Afzaal I, Naseer H et al (2020) Review-surface modification of carbon nanotubes: a tool to control electrochemical performance. Ecs J Solid State Sci Technol 9(4). https://doi.org/10.1149/2162-8777/ab8929
Gupta N, Gupta SM, Sharma SK (2019) Carbon nanotubes: synthesis, properties and engineering applications. Carbon Letters 29(5):419–447. https://doi.org/10.1007/s42823-019-00068-2
Nag A, Alahi MEE, Mukhopadhyay SC et al (2021) Multi-walled carbon nanotubes-based sensors for strain sensing applications. Sensors 21(4). https://doi.org/10.3390/s21041261
Yu W, Sisi L, Haiyan Y et al (2020) Progress in the functional modification of graphene/graphene oxide: a review. RSC Adv 10(26):15328–15345. https://doi.org/10.1039/d0ra01068e
Tan D, Jiang C, Li Q et al (2020) Silver nanowire networks with preparations and applications: a review. J Mater Sci Mater Electron 31(18):15669–15696. https://doi.org/10.1007/s10854-020-04131-x
Liu J, Chen E, Wu Y et al (2022) Silver nanosheets doped polyvinyl alcohol hydrogel piezoresistive bifunctional sensor with a wide range and high resolution for human motion detection. Adv Compos Hybrid Mater 5(2):1196–1205. https://doi.org/10.1007/s42114-022-00472-9
Hua Z, Yu T, Liu D et al (2021) Recent advances in gold nanoparticles-based biosensors for food safety detection. Biosens Bioelectron 179. https://doi.org/10.1016/j.bios.2021.113076
De Volder MFL, Tawfick SH, Baughman RH et al (2013) Carbon nanotubes: present and future commercial applications. Science 339(6119):535–539. https://doi.org/10.1126/science.1222453
Ma P-C, Siddiqui NA, Marom G et al (2010) Dispersion and functionalization of carbon nanotubes for polymer-based nanocomposites: a review. Compos A Appl Sci Manuf 41(10):1345–1367. https://doi.org/10.1016/j.compositesa.2010.07.003
Zhang Y, Ren E, Li A et al (2021) A porous self-healing hydrogel with an island-bridge structure for strain and pressure sensors. J Mater Chem B 9(3):719–730. https://doi.org/10.1039/d0tb01926g
Gu J, Huang J, Chen G et al (2020) Multifunctional poly(vinyl alcohol) nanocomposite organohydrogel for flexible strain and temperature sensor. ACS Appl Mater Interfaces 12(36):40815–40827. https://doi.org/10.1021/acsami.0c12176
Abolpour Moshizi S, Moradi H, Wu S et al (2021) Biomimetic ultraflexible piezoresistive flow sensor based on graphene nanosheets and PVA hydrogel. Adv Mater Technol 7(1). https://doi.org/10.1002/admt.202100783
Ahmadi H, Moradi H, Pastras CJ et al (2021) Development of ultrasensitive biomimetic auditory hair cells based on piezoresistive hydrogel nanocomposites. ACS Appl Mater Interfaces 13(37):44904–44915. https://doi.org/10.1021/acsami.1c12515
Patel DK, Ganguly K, Dutta SD et al (2022) Multifunctional hydrogels of polyvinyl alcohol/polydopamine functionalized with carbon nanomaterials as flexible sensors. Mater Today Commun 32:103906. https://doi.org/10.1016/j.mtcomm.2022.103906
Wu L, Li L, Pan L et al (2020) MWCNTs reinforced conductive, self-healing polyvinyl alcohol/carboxymethyl chitosan/oxidized sodium alginate hydrogel as the strain sensor. J Appl Polym Sci 138(6):49800. https://doi.org/10.1002/app.49800
Shohreh Azadi SP, Moshizi SA, Asadnia M, Jiangtao Xu, Park I, Wang CH, Shuying Wu (2020) Biocompatible and highly stretchable PVA/AgNWs hydrogel strain sensors for human motion detection. Adv Mater Technol. https://doi.org/10.1002/admt.202000426
Murakami K, Tochinai R, Tachibana D et al (2022) Direct wiring of liquid metal on an ultrasoft substrate using a polyvinyl alcohol lift-off method. ACS Appl Mater Interfaces 14(5):7241–7251. https://doi.org/10.1021/acsami.1c20628
Liao M, Liao H, Ye J et al (2019) Polyvinyl alcohol-stabilized liquid metal hydrogel for wearable transient epidermal sensors. ACS Appl Mater Interfaces 11(50):47358–47364. https://doi.org/10.1021/acsami.9b16675
Zhang L, Wang J, Wang S et al (2022) Neuron-inspired multifunctional conductive hydrogels for flexible wearable sensors. J Mater Chem C 10(11):4327–4335. https://doi.org/10.1039/d1tc05864a
Zhang J, Zhao X, Wang Z et al (2022) Antibacterial, antifreezing, stretchable, and self-healing organohydrogel electrode based triboelectric nanogenerator for self-powered biomechanical sensing. Adv Mater Interfaces 9(15):2200290. https://doi.org/10.1002/admi.202200290
Chaudhary V, Ashraf N, Khalid M et al (2022) Emergence of MXene-polymer hybrid nanocomposites as high-performance next-generation chemiresistors for efficient air quality monitoring. Adv Funct Mat 32(33). https://doi.org/10.1002/adfm.202112913
Zhang D, Yin R, Zheng Y et al (2022) Multifunctional MXene/CNTs based flexible electronic textile with excellent strain sensing, electromagnetic interference shielding and Joule heating performances. Chem Eng J 438. https://doi.org/10.1016/j.cej.2022.135587
Zhang J, Wan L, Gao Y et al (2019) Highly stretchable and self-healable MXene/polyvinyl alcohol hydrogel electrode for wearable capacitive electronic skin. Adv Electron Mater 5(7):1900285. https://doi.org/10.1002/aelm.201900285
Zhao Y, Gao W, Dai K et al (2021) Bioinspired multifunctional photonic-electronic smart skin for ultrasensitive health monitoring, for visual and self-powered sensing. Adv Mater 33(45):e2102332. https://doi.org/10.1002/adma.202102332
Ren XDTSD (2022) Preparation of wearable strain sensor based on PVA/MWCNTs hydrogel composite. Mater Today Commun. https://doi.org/10.1016/j.mtcomm.2022.103278
Zheng W, Li Y, Xu L et al (2020) Highly stretchable, healable, sensitive double-network conductive hydrogel for wearable sensor. Polymer 211:123095. https://doi.org/10.1016/j.polymer.2020.123095
Zhu H, Xu J, Sun X et al (2022) Wearable, fast-healing, and self-adhesive multifunctional photoactive hydrogel for strain and temperature sensing. J Mater Chem A 10(43):23366–23374. https://doi.org/10.1039/d2ta06072h
Cheng B, Chang S, Li H et al (2020) Highly stretchable and compressible carbon nanofiber–polymer hydrogel strain sensor for human motion detection. Macromol Mater Eng 305(3):1900813. https://doi.org/10.1002/mame.201900813
Zhang H, Ren P, Yang F et al (2020) Biomimetic epidermal sensors assembled from polydopamine-modified reduced graphene oxide/polyvinyl alcohol hydrogels for the real-time monitoring of human motions. J Mater Chem B 8(46):10549–10558. https://doi.org/10.1039/d0tb02100h
Chen H, Huang J, Liu J et al (2021) High toughness multifunctional organic hydrogels for flexible strain and temperature sensor. J Mater Chem A 9(40):23243–23255. https://doi.org/10.1039/d1ta07127k
Wei J, Wang R, Pan F et al (2022) Polyvinyl alcohol/graphene oxide conductive hydrogels via the synergy of freezing and salting out for strain sensors. Sensors (Basel) 22(8). https://doi.org/10.3390/s22083015
Kong DS, El-Bahy ZM, Algadi H et al (2022) Highly sensitive strain sensors with wide operation range from strong MXene-composited polyvinyl alcohol/sodium carboxymethylcellulose double network hydrogel. Advanced Composites And Hybrid Materials 5(3):1976–1987. https://doi.org/10.1007/s42114-022-00531-1
Li K, Yang X, Dong X et al (2023) Easy regulation of chitosan-based hydrogel microstructure with citric acid as an efficient buffer. Carbohydr Polym 300:120258. https://doi.org/10.1016/j.carbpol.2022.120258
Li G-Y, Li J, Li Z-J et al (2022) Hierarchical PVDF-HFP/ZnO composite nanofiber-based highly sensitive piezoelectric sensor for wireless workout monitoring. Adv Compos Hybrid Mater 5(2):766–775. https://doi.org/10.1007/s42114-021-00331-z
Dong X, Tong S, Dai K et al (2022) Preparation of PVA/PAM/Ag strain sensor via compound gelation. J Appl Polym Sci 139(14). https://doi.org/10.1002/app.51883
Miao L, Wang X, Li S et al (2022) An ultra-stretchable polyvinyl alcohol hydrogel based on tannic acid modified aramid nanofibers for use as a strain sensor. Polymers (Basel) 14(17). https://doi.org/10.3390/polym14173532
Li L, Ji X, Chen K (2022) Conductive, self-healing, and antibacterial Ag/MXene-PVA hydrogel as wearable skin-like sensors. J Biomater Appl 8853282221131137. https://doi.org/10.1177/08853282221131137
Hou B, Ma C, Li S et al (2022) Modifying the conductive properties of poly(3,4-ethylenedioxythiophene) thin films in green solvents. Front Chem 10:1005266. https://doi.org/10.3389/fchem.2022.1005266
Peng Y, Yan B, Li Y et al (2019) Antifreeze and moisturizing high conductivity PEDOT/PVA hydrogels for wearable motion sensor. J Mater Sci 55(3):1280–1291. https://doi.org/10.1007/s10853-019-04101-7
Su Z, Jin Y, Wang H et al (2022) PEDOT:PSS and its composites for flexible supercapacitors. ACS Applied Energy Materials 5(10):11915–11932. https://doi.org/10.1021/acsaem.2c01524
Dodda JM, Azar MG, Bělský P et al (2022) Biocompatible hydrogels based on chitosan, cellulose/starch, PVA and PEDOT:PSS with high flexibility and high mechanical strength. Cellulose 29(12):6697–6717. https://doi.org/10.1007/s10570-022-04686-4
Shi W, Wang Z, Song H et al (2022) High-sensitivity and extreme environment-resistant sensors based on PEDOT:PSS@PVA hydrogel fibers for physiological monitoring. ACS Appl Mater Interfaces 14(30):35114–35125. https://doi.org/10.1021/acsami.2c09556
Mir A, Kumar A, Riaz U (2022) A short review on the synthesis and advance applications of polyaniline hydrogels. RSC Adv 12(30):19122–19132. https://doi.org/10.1039/d2ra02674k
Hu C, Zhang Y, Wang X et al (2018) Stable, strain-sensitive conductive hydrogel with antifreezing capability, remoldability, and reusability. ACS Appl Mater Interfaces 10(50):44000–44010. https://doi.org/10.1021/acsami.8b15287
Riaz U, Singh N, Rashnas Srambikal F et al (2022) A review on synthesis and applications of polyaniline and polypyrrole hydrogels. Polym Bull 80(2):1085–1116. https://doi.org/10.1007/s00289-022-04120-6
Cheng KC, Zou L, Chang BB et al (2022) Mechanically robust and conductive poly(acrylamide) nanocomposite hydrogel by the synergistic effect of vinyl hybrid silica nanoparticle and polypyrrole for human motion sensing. Adv Compos Hybrid Mater 5(4):2834–2846. https://doi.org/10.1007/s42114-022-00465-8
Wei HG, Li A, Kong DS et al (2021) Polypyrrole/reduced graphene aerogel film for wearable piezoresisitic sensors with high sensing performances. Adv Compos Hybrid Mater 4(1):86–95. https://doi.org/10.1007/s42114-020-00201-0
Abodurexiti A, Maimaitiyiming X (2022) Self-healing, anti-freezing hydrogels and its application in diversified skin-like electronic sensors. IEEE Sens J 22(13):12588–12594. https://doi.org/10.1109/jsen.2022.3157709
Bai H, Chen D, Zhu H et al (2022) Photo-crosslinking ionic conductive PVA-SbQ/FeCl3 hydrogel sensors. Colloids Surf, A 648:129205. https://doi.org/10.1016/j.colsurfa.2022.129205
Gong J-Y, Sun F-C, Pan Y-C et al (2022) Stretchable and tough PAANa/PEDOT:PSS/PVA conductive hydrogels for flexible strain sensors. Mater Today Commun 33:104324. https://doi.org/10.1016/j.mtcomm.2022.104324
Zhao W, Zhang D, Yang Y et al (2021) A fast self-healing multifunctional polyvinyl alcohol nano-organic composite hydrogel as a building block for highly sensitive strain/pressure sensors. J Mater Chem A 9(38):22082–22094. https://doi.org/10.1039/d1ta05586k
Zhao L, Zhang H, Guo Z et al (2022) Natural glycyrrhizic acid-tailored homogeneous conductive polyaniline hydrogel as a flexible strain sensor. ACS Appl Mater Interfaces 14(45):51394–51403. https://doi.org/10.1021/acsami.2c16129
Song M, Yu H, Zhu J et al (2020) Constructing stimuli-free self-healing, robust and ultrasensitive biocompatible hydrogel sensors with conductive cellulose nanocrystals. Chem Eng J 398:125547. https://doi.org/10.1016/j.cej.2020.125547
Ma Y, Gao Y, Liu L et al (2020) Skin-contactable and antifreezing strain sensors based on bilayer hydrogels. Chem Mater 32(20):8938–8946. https://doi.org/10.1021/acs.chemmater.0c02919
Sun X, Zhong W, Zhang Z et al (2022) Stretchable, self-healable and anti-freezing conductive hydrogel based on double network for strain sensors and arrays. J Mater Sci 57(26):12511–12521. https://doi.org/10.1007/s10853-022-07379-2
Chen X, He M, Zhang X et al (2020) Metal-free and stretchable conductive hydrogels for high transparent conductive film and flexible strain sensor with high sensitivity. Macromol Chem Phys 221(10):2000054. https://doi.org/10.1002/macp.202000054
Wang Y, Zhu Y, Xue Y et al (2020) Sequential in-situ route to synthesize novel composite hydrogels with excellent mechanical, conductive, and magnetic responsive properties. Mater Des 193:108759. https://doi.org/10.1016/j.matdes.2020.108759
Li Y, Wang Y, Liu X et al (2021) Facilely prepared conductive hydrogels based on polypyrrole nanotubes. Chem Pap 75(10):5113–5120. https://doi.org/10.1007/s11696-021-01559-1
Zhang L, Jiang D, Dong T et al (2020) Overview of ionogels in flexible electronics. Chem Rec 20(9):948–967. https://doi.org/10.1002/tcr.202000041
Di X, Ma Q, Xu Y et al (2021) High-performance ionic conductive poly(vinyl alcohol) hydrogels for flexible strain sensors based on a universal soaking strategy. Mater Chem Front 5(1):315–323. https://doi.org/10.1039/d0qm00625d
Gao Y, Peng J, Zhou M et al (2020) A multi-model, large range and anti-freezing sensor based on a multi-crosslinked poly(vinyl alcohol) hydrogel for human-motion monitoring. J Mater Chem B 8(48):11010–11020. https://doi.org/10.1039/d0tb02250k
Gong JP (2010) Why are double network hydrogels so tough? Soft Matter 6(12):2583. https://doi.org/10.1039/b924290b
Du H, Liu W, Zhang M et al (2019) Cellulose nanocrystals and cellulose nanofibrils based hydrogels for biomedical applications. Carbohydr Polym 209:130–144. https://doi.org/10.1016/j.carbpol.2019.01.020
Zhang D, Zhang M, Wang J et al (2022) Impedance response behavior and mechanism study of axon-like ionic conductive cellulose-based hydrogel strain sensor. Adv Compos Hybrid Mater 5(3):1812–1820. https://doi.org/10.1007/s42114-022-00437-y
Huiqiang Wang ZL, Zuo M, Zeng X, Tang X, Yong Sun Lu, Lin. (2022) Stretchable, freezing-tolerant conductive hydrogel for wearable electronics reinforced by cellulose nanocrystals toward multiple hydrogen bonding. Carbohydr Polymers. https://doi.org/10.1016/j.carbpol.2021.119018
Arkaban H, Barani M, Akbarizadeh MR et al (2022) Polyacrylic acid nanoplatforms: antimicrobial, tissue engineering, and cancer theranostic applications. Polymers (Basel) 14(6). https://doi.org/10.3390/polym14061259
Yongzhi Liang XS, Lv Q, Shen Y, Liang H (2020) Fully physically cross-linked hydrogel as highly stretchable, tough, self-healing and sensitive strain sensors. Polymers (Basel). https://doi.org/10.1016/j.polymer.2020.123039
Wang MX, Chen YM, Gao Y et al (2018) Rapid self-recoverable hydrogels with high toughness and excellent conductivity. ACS Appl Mater Interfaces 10(31):26610–26617. https://doi.org/10.1021/acsami.8b06567
Liu R, Chen J, Luo Z et al (2022) Stretchable, self-adhesive, conductive, anti-freezing sodium polyacrylate-based composite hydrogels for wearable flexible strain sensors. React Funct Polym 172:105197. https://doi.org/10.1016/j.reactfunctpolym.2022.105197
Qiao H, Qi P, Zhang X et al (2019) Multiple weak H-bonds lead to highly sensitive, stretchable, self-adhesive, and self-healing ionic sensors. ACS Appl Mater Interfaces 11(8):7755–7763. https://doi.org/10.1021/acsami.8b20380
Zhan Y, Xing Y, Ji Q et al (2022) Strain-sensitive alginate/polyvinyl alcohol composite hydrogels with Janus hierarchy and conductivity mediated by tannic acid. Int J Biol Macromol 212:202–210. https://doi.org/10.1016/j.ijbiomac.2022.05.071
Yang Zhou CW, Yang Y, Yang H, Wang S, Dai Z, Ji K, Jiang H, Xiaodong Chen, and Yi Long (2018) Highly stretchable, elastic, and ionic conductive hydrogel for artificial soft electronics. Adv Electro Mater. https://doi.org/10.1002/adfm.201806220
Jing X, Li H, Mi H-Y et al (2019) Highly transparent, stretchable, and rapid self-healing polyvinyl alcohol/cellulose nanofibril hydrogel sensors for sensitive pressure sensing and human motion detection. Sens Actuators B Chem 295:159–167. https://doi.org/10.1016/j.snb.2019.05.082
Ji N, Luo J, Zhang W et al (2022) A novel polyvinyl alcohol‐based hydrogel with ultra‐fast self‐healing ability and excellent stretchability based on multi dynamic covalent bond cross‐linking. Macromol Mater Eng 2200525. https://doi.org/10.1002/mame.202200525
Liu R, Chen K, Liu H et al (2022) High performance conductive hydrogel for strain sensing applications and digital image mapping. ACS Appl Mater Interfaces. https://doi.org/10.1021/acsami.2c15669
Lian M, Sun J, Jiang D et al (2022) Triboelectric nanogenerator self-heating floor - possibility to achieve intelligence in the architecture. J Mater Chem A 10(45):24353–24361. https://doi.org/10.1039/d2ta06942c
Chen G, Huang J, Gu J et al (2020) Highly tough supramolecular double network hydrogel electrolytes for an artificial flexible and low-temperature tolerant sensor. J Mater Chem A 8(14):6776–6784. https://doi.org/10.1039/d0ta00002g
Shao L, Li Y, Ma Z et al (2020) Highly sensitive strain sensor based on a stretchable and conductive poly(vinyl alcohol)/phytic acid/NH(2)-POSS hydrogel with a 3D microporous structure. ACS Appl Mater Interfaces 12(23):26496–26508. https://doi.org/10.1021/acsami.0c07717
Wen J, Tang J, Ning H et al (2021) Multifunctional ionic skin with sensing, UV-filtering, water-retaining, and anti-freezing capabilities. Adv Func Mater 31(21):2011176. https://doi.org/10.1002/adfm.202011176
Sun P, Li Q (2022) Tension‐responsive graphene oxide conductive hydrogel with robust mechanical properties and high sensitivity for human motion monitoring. Macromol Mater Eng 2200529. https://doi.org/10.1002/mame.202200529
Yu J, Dang C, Liu H et al (2020) Highly strong and transparent ionic conductive hydrogel as multifunctional sensors. Macromol Mater Eng 305(12):2000475. https://doi.org/10.1002/mame.202000475
Wen N, Song T, Ji Z, et al (2021) Recent advancements in self-healing materials: mechanicals, performances and features. React Funct Polym 168. https://doi.org/10.1016/j.reactfunctpolym.2021.105041
Wu H, Chen J, Kim J et al (2022) Facile preparation of transparent poly (γ-glutamic acid) modified poly (vinyl alcohol) hydrogels with high tensile strength and toughness. J Appl Polym Sci 139(21):52204. https://doi.org/10.1002/app.52204
Chen Z, Luo J, Hu Y et al (2022) Fabrication of lignin reinforced hybrid hydrogels with antimicrobial and self-adhesion for strain sensors. Int J Biol Macromol 222:487–496. https://doi.org/10.1016/j.ijbiomac.2022.09.197
Ke T, Zhao L, Fan X et al (2023) Rapid self-healing, self-adhesive, anti-freezing, moisturizing, antibacterial and multi-stimuli-responsive PVA/starch/tea polyphenol-based composite conductive organohydrogel as flexible strain sensor. J Mater Sci Technol 135:199–212. https://doi.org/10.1016/j.jmst.2022.06.032
Sun P, Qiu M, Li G et al (2022) Artificial jelly channel inspired by the shark for sensing specific ions and environmental perturbation. Mater Today Chem 26:101047. https://doi.org/10.1016/j.mtchem.2022.101047
Pan L, Han L, Liu H et al (2022) Flexible sensor based on Hair-like microstructured ionic hydrogel with high sensitivity for pulse wave detection. Chem Eng J 450:137929. https://doi.org/10.1016/j.cej.2022.137929
Yagmurcukardes M, Qin Y, Ozen S et al (2020) Quantum properties and applications of 2D Janus crystals and their superlattices. Appl Phys Rev 7(1). https://doi.org/10.1063/1.5135306
Wang Y, Liu S, Wang Q et al (2022) Nanolignin filled conductive hydrogel with improved mechanical, anti-freezing, UV-shielding and transparent properties for strain sensing application. Int J Biol Macromol 205:442–451. https://doi.org/10.1016/j.ijbiomac.2022.02.088
Yan Y, He C, Zhang L et al (2023) Freeze-resistant, rapidly polymerizable, ionic conductive hydrogel induced by Deep Eutectic Solvent (DES) after lignocellulose pretreatment for flexible sensors. Int J Biol Macromol 224:143–155. https://doi.org/10.1016/j.ijbiomac.2022.10.111
Xiao G, Wang Y, Zhang H et al (2021) Cellulose nanocrystal mediated fast self-healing and shape memory conductive hydrogel for wearable strain sensors. Int J Biol Macromol 170:272–283. https://doi.org/10.1016/j.ijbiomac.2020.12.156
Du H, Wang J, Xu N et al (2022) Transparent, self-healable, shape memory poly(vinyl alcohol)/ionic liquid difunctional hydrogels assembled spontaneously from polymer solution. J Mol Liq 366:120226. https://doi.org/10.1016/j.molliq.2022.120226
Han L, Zhou Q, Chen D et al (2022) Flexible sensitive hydrogel sensor with self-powered capability. Colloids Surf A 639:128381. https://doi.org/10.1016/j.colsurfa.2022.128381
He P, Wu J, Pan X et al (2020) Anti-freezing and moisturizing conductive hydrogels for strain sensing and moist-electric generation applications. J Mater Chem A 8(6):3109–3118. https://doi.org/10.1039/c9ta12940e
Jiang S, Xia L (2022) Bioinspired high-performance bilayer, pH-responsive hydrogel with superior adhesive property. Polymers (Basel) 14(20). https://doi.org/10.3390/polym14204425
Zhang Y, MohebbiPour A, Mao J et al (2021) Lignin reinforced hydrogels with multi-functional sensing and moist-electric generating applications. Int J Biol Macromol 193(Pt A):941–947. https://doi.org/10.1016/j.ijbiomac.2021.10.159
Wang X, Wang X, Pi M et al (2022) High-strength, highly conductive and woven organic hydrogel fibers for flexible electronics. Chem Eng J 428:131172. https://doi.org/10.1016/j.cej.2021.131172
Tian J, Fan R, Zhang Z et al (2022) Flexible and biocompatible poly (vinyl alcohol)/multi-walled carbon nanotubes hydrogels with epsilon-near-zero properties. J Mater Sci Technol 131:91–99. https://doi.org/10.1016/j.jmst.2022.05.019
Yousaf M, Shi HTH, Wang Y et al (2016) Novel pliable electrodes for flexible electrochemical energy storage devices: recent progress and challenges. Adv Energy Mater. https://doi.org/10.1002/aenm.201600490
Chen J, Shi D, Yang Z et al (2022) A solvent-exchange strategy to develop stiff and tough hydrogel electrolytes for flexible and stable supercapacitor. J Power Sources 532:231326. https://doi.org/10.1016/j.jpowsour.2022.231326
Guo G (2022) Facile synthesis of new conductive hydrogels for application in flexible all-solid-state supercapacitor. Int J Electrochem Sci 220528. https://doi.org/10.20964/2022.05.18
Cao S, Zhao T, Li Y et al (2022) Fabrication of PANI@Ti3C2Tx/PVA hydrogel composite as flexible supercapacitor electrode with good electrochemical performance. Ceram Int 48(11):15721–15728. https://doi.org/10.1016/j.ceramint.2022.02.108
Tao X-Y, Wang Y, Ma W-B et al (2021) Copolymer hydrogel as self-standing electrode for high performance all-hydrogel-state supercapacitor. J Mater Sci 56(28):16028–16043. https://doi.org/10.1007/s10853-021-06304-3
Liu R, Liu H, Lyu T et al (2022) Tri‐state recyclable multifunctional hydrogel for flexible sensors. J Appl Polym Sci 139(39). https://doi.org/10.1002/app.52928
Das Mahapatra S, Mohapatra PC, Aria AI et al (2021) Piezoelectric materials for energy harvesting and sensing applications: roadmap for future smart materials. Adv Sci 8(17). https://doi.org/10.1002/advs.202100864
Wang, Z. L. (2021) From contact electrification to triboelectric nanogenerators. Rep Prog Phys 84(9). https://doi.org/10.1088/1361-6633/ac0a50
Walden R, Aazem I, Babu A et al. (2023) Textile-triboelectric nanogenerators (T-TENGs) for wearable energy harvesting devices. Chem Eng J 451. https://doi.org/10.1016/j.cej.2022.138741
Babu A, Aazem I, Walden R et al. (2023) Electrospun nanofiber based TENGs for wearable electronics and self-powered sensing. Chem Eng J 452. https://doi.org/10.1016/j.cej.2022.139060
Lian M, Sun J, Jiang D et al. (2023) Waterwheel-inspired high-performance hybrid electromagnetic-triboelectric nanogenerators based on fluid pipeline energy harvesting for power supply systems and data monitoring. Nanotechnology 34(2). https://doi.org/10.1088/1361-6528/ac97f1
Parida K, Kumar V, Jiangxin W et al. (2017) Highly transparent, stretchable, and self-healing ionic-skin triboelectric nanogenerators for energy harvesting and touch applications. Adv Mater 29(37). https://doi.org/10.1002/adma.201702181
Wang Y, Zhang L, Lu A (2020) Highly stretchable, transparent cellulose/PVA composite hydrogel for multiple sensing and triboelectric nanogenerators. Journal of Materials Chemistry A 8(28):13935–13941. https://doi.org/10.1039/d0ta02010a
Wolf MP, Salieb-Beugelaar GB, Hunziker P (2018) PDMS with designer functionalities—properties, modifications strategies, and applications. Prog Polym Sci 83:97–134. https://doi.org/10.1016/j.progpolymsci.2018.06.001
Wang S, Zhang Y (2022) A functional triboelectric nanogenerator based on the LiCl/PVA hydrogel for cheerleading training. Mater Technol 37(13):2752–2757. https://doi.org/10.1080/10667857.2022.2073117
Li G, Zhang J, Huang F et al (2021) Transparent, stretchable and high-performance triboelectric nanogenerator based on dehydration-free ionically conductive solid polymer electrode. Nano Energy 88:106289. https://doi.org/10.1016/j.nanoen.2021.106289
Jing X, Li H, Mi HY et al (2020) Enhancing the performance of a stretchable and transparent triboelectric nanogenerator by optimizing the hydrogel ionic electrode property. ACS Appl Mater Interfaces 12(20):23474–23483. https://doi.org/10.1021/acsami.0c04219
Luo X, Zhu L, Wang YC et al (2021) A flexible multifunctional triboelectric nanogenerator based on MXene/PVA hydrogel. Adv Func Mater 31(38):2104928. https://doi.org/10.1002/adfm.202104928
Xu W, Huang L-B, Wong M-C et al (2017) Environmentally friendly hydrogel-based triboelectric nanogenerators for versatile energy harvesting and self-powered sensors. Adv Energy Mater 7(1):1601529. https://doi.org/10.1002/aenm.201601529
Dai X, Long Y, Jiang B et al (2022) Ultra-antifreeze, ultra-stretchable, transparent, and conductive hydrogel for multi-functional flexible electronics as strain sensor and triboelectric nanogenerator. Nano Res 15(6):5461–5468. https://doi.org/10.1007/s12274-022-4153-5
Liu, P., Sun, N., Mi, Y., et al. (2021) Ultra-low CNTs filled high-performance fast self-healing triboelectric nanogenerators for wearable electronics. Compos Sci Technol 208. https://doi.org/10.1016/j.compscitech.2021.108733
Long Y, Wang Z, Xu F et al (2022) Mechanically ultra-robust, elastic, conductive, and multifunctional hybrid hydrogel for a triboelectric nanogenerator and flexible/wearable sensor. Small. https://doi.org/10.1002/smll.202203956
Qu M, Shen L, Wang J et al (2022) Superhydrophobic, humidity-resistant, and flexible triboelectric nanogenerators for biomechanical energy harvesting and wearable self-powered sensing. Acs Applied Nano Materials 5(7):9840–9851. https://doi.org/10.1021/acsanm.2c02026
Zhou Z, Yuan W, Xie X (2022) A stretchable and adhesive composite hydrogel containing PEDOT:PSS for wide-range and precise motion sensing and electromagnetic interference shielding and as a triboelectric nanogenerator. Materials Chemistry Frontiers 6(22):3359–3368. https://doi.org/10.1039/d2qm00690a
Funding
This work was supported by the Heilongjiang Province Postdoctoral Funded Project (LBH-Q21019), Heilongjiang Province Natural Science Foundation (LH2020E087). This study is supported via funding from Prince Sattam bin Abdulaziz University project number (PSAU/2023/1444).
Author information
Authors and Affiliations
Contributions
Zijian Wu: conceived and designed the review. Qi Xu: prepared figures and visualization, wrote the main text. Wei Zhao and Mingpeng He: formal analysis and editing. Ning Guo: data collection and curation. Ling Weng, Zeinhom M. El-Bahy, and Mohamed M. Ibrahim: review and editing. Man Vir Singh: data collection and curation. Zhiping Lin: data collection and curation. Junna Ren, Manal F. Abou Taleb, and Mohamed M. Ibrahim: review and editing.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xu, Q., Wu, Z., Zhao, W. et al. Strategies in the preparation of conductive polyvinyl alcohol hydrogels for applications in flexible strain sensors, flexible supercapacitors, and triboelectric nanogenerator sensors: an overview. Adv Compos Hybrid Mater 6, 203 (2023). https://doi.org/10.1007/s42114-023-00783-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42114-023-00783-5