Abstract
The purification of wastewater, including heavy metals (Cu2+, Cd2+, Pb2+, and Zn2+), was investigated using the coconut shell-based activated carbon that the different agents modified of acid, alkali, potassium permanganate, and iron salt. Several effects using the modified coconut shell-based activated carbon were observed through the experimental characterization. First, the shortened time of adsorption equilibrium to heavy metal ions, reduced addition of carbon absorbents, and improved removal purification was observed. Second, the agents of acid and potassium-modified coconut shell-based activated carbon showed improved removal rate on heavy metal wastewater containing Zn2+ with up to 95% and 96.5%, respectively. Alkali modification showed the highest removal effect on wastewater containing Cd2+ with 98%, and the iron-salt modification improved the adsorption capacity most for Pb2+ with 92%. Third, the increase of the adsorption reaction time and the amount of adsorbent showed a direct relationship to the adsorption effect. Saturated adsorption to reach an equilibrium was observed as the reaction time exceeded. Among agents, acid, alkali, and potassium specifically had a better impact on the purification than iron salt. With a 1-g dosage of adsorbent, three agents successfully removed more than 90% of the wastewater pollutants. Also, weak acidic conditions with pH = 5 showed more effect on removing heavy metal ions.
Graphical abstract
Purification of heavy metals in wastewater using the coconut shell-based activated carbons with experimental verification.

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Along with the rapid development of mining, metallurgy, and other industries, the production and discharge of heavy metal wastewater and derived pollution have become a global environmental problem. The composition of heavy metal wastewater is complicated, and its toxicity is severe. Heavy metals can interact strongly with proteins and enzymes in the human body through the food chain and accumulate in human organs. Once the concentration of heavy metal ions in the body exceeds the human body’s tolerance limit, it causes toxic reactions in the human body. The treatment of heavy metal pollution becomes imminent. In recent, removing heavy metal ions from wastewater has been a concern for health and environmental problems. For the low concentration of heavy metal ions, the traditional chemical precipitation method and ion exchange resin method are limited in the purification effect. Instead, it was disclosed that the adsorption method could effectively and solve this problem with improved purification. Adsorption method has become more interested in removing heavy metal ions due to its advantages of low cost, high efficiency, environmental protection, and recyclability of heavy metals [1, 2]. Some research reported the modification of absorbent by using physical and chemical methods and showed the adsorbent’s enhanced selectivity to heavy metal ions [3,4,5].
Coconut shell is easily obtained from agriculture and forestry and has low cost, dense structure, low content of ash content, and high strength. It has been used to remove heavy metal ions from water [6,7,8,9], organic matter [10], and separate and purify mixed exhaust gases of CO2 and CH4 [11,12,13]. In this study, four different reagents of nitric acid, sodium hydroxide, potassium permanganate, and iron salt were used to modify coconut shell-based activated carbon to investigate the purification effect of heavy metals from wastewater. The reaction time, adsorbent input amount, and pH value on the purification were experimentally investigated. Figure 1 depicts the processes in this research from materials preparation, modification, and absorption reaction.
2 Materials and methods
2.1 Material preparation of coconut shell-based activated carbon
The coconut shell-based activated carbon used in experiments was purchased from gongyi guo qing shui material co., LTD. The physical and chemical properties are shown in Table 1. About 500 g of coconut shell-based activated carbon was mixed with ionized water in a 1000-mL beaker, heated to boil for 30 min with a gentle stir, and cooled down. After taking it out, it was rinsed with deionized water 4–5 times and then filtered. The cleaned activated carbon was placed at 383 K and dried for 12 h to obtain an activated carbon sample (named AC-0).
2.2 Modification of activated carbon by different agents
The prepared activated carbon of coconut shell (AC-0) was immersed in HNO3 and 4% NaOH solution with a mass concentration of 1% and oscillated 24 h. After washing, filtration, drying, nitric acid, and sodium-hydroxide modified activated carbon were prepared (named as AC-1 and AC-2, respectively).
The prepared activated carbon of coconut shell (AC-0) was immersed in 1.5% KMnO4 solution. After 12-h oscillation under room temperature, the modified coconut shell activated carbon was washed with ultra-pure water and then dried in a 110 °C constant temperature oven for 12 h to prepare potassium permanganate modified activated carbon (named as AC-3).
About 2 g of AC-0 was oscillated for 24 h in FeSO4 solution with a concentration of 50 mL of 0.04 mol/L. After filtration, it was washed with deionized water until no color and dried in a 40 °C oven for 4 h. The iron salt modified activated carbon was prepared (named as AC-4).
2.3 Characterization of surface morphology of activated carbon
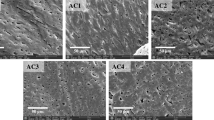
The surface morphology of coconut shell-based activated carbon before and after modification (AC-0 through AC-4) was characterized using scanning electron microscopy—Zeiss GeminiSEM 300.
3 Adsorption experiments
Each coconut shell-activated carbon samples were weighed and placed in a 250-mL conical flask, and a 50-mL beaker containing 5 mg/L of Cd2+, Cu2+, Pb2+, and Zn2+ solution was added. The beaker was put on a constant temperature oscillator with 25 ± 1 °C. After adjusting the pH value, it then oscillated at a speed of 200 rpm. The supernatant solution was filtered with a 0.45-μm filter membrane to determine the content of heavy metal ions in the filtrate. Removal rate E in percentage was calculated as follows:
where C0 is the concentration of heavy metal ions in the solution before adsorption (mg/L) and C1 represents the concentration of heavy metal ions in the solution after adsorption (mg/L).
4 Results and analysis
4.1 Surface morphology characterization of coconut shell activated carbon
As shown in Fig. 2 for the surface morphology, more prominent pores and irregular structures were observed in modified coconut shell-based activated carbon (AC-1 through AC-4) than natural carbon (AC-0). The pore structure in modified carbons is more complex and changeable. The shape of pores in both modified and natural carbons are mainly ellipses, but the observed pores are macropores with more complex pore structure.
4.2 The effect of pH on the adsorption of Cu2+, Cd2+, Pb2+, and Zn2+
To investigate the effect of different pH values of coconut carbons on the removal rate of heavy metals, pH value ranges from 2 to 9. The initial condition of the solution of Cd2+, Cu2+, Pb2+,, and Zn2+ are five mg/L concentration, 60 min of adsorption time, room temperature, pH = 5, and adsorbent additive quantity under the condition of 1 g. The results on absorption performance with removal rate are shown in Fig. 3.
The change of pH value in wastewater solution affects the removal rate of heavy metal ions in coconut shell activated carbon [14,15,16,17,18,19,20,21]. Different pH value affects the charge on the surface of activated carbon and electrostatic interaction between activated carbon and heavy metal ions and influences the heavy metal ions in aqueous solution.
As shown in graphs in Fig. 3, as the pH value in the aqueous solution increased, the removal rate of heavy metal ions gradually increased. This effective absorption comes from the slow dissociation of the functional group of H+ in coconut carbons and its more prolonged exposure to the absorption area. Around a pH value of 5, the removal rate of heavy metal ions by modified coconut carbons with nitric acid, sodium hydroxide, and potassium permanganate (AC-1 through AC-3) reached a peak of above 90%. With more pH in aqueous solution than pH 5, these modified coconut carbons’ adsorption capacity showed a decreasing trend. On the other hand, modified coconut carbons with iron salt (AC-4) showed its peak near pH = 7, and natural carbon (AC-0) showed an increase of removal rate as increased pH value.
4.3 Dosage of adsorbent of heavy metal ions
Under several initial conditions of concentrations of heavy metals (Cu2+, Cd2+, Pb2+, and Zn2+) in aqueous solution with 5 mg/L, 60 min of the adsorption time, room temperature of 25 °C, and the pH value = 5, the influence of the dosage of activated carbon on the removal effect of heavy metal ions was investigated with the range of 0.1–7 g and shown in Fig. 4.
As shown in Fig. 4, for a low dosage of activated carbon’s adsorbent (< 1 g), the adsorption rate of heavy metal ions modified by nitric acid, sodium hydroxide, and potassium hyper-menthol (AC-1 through 3) increases rapidly. And when it reaches about 1 g, these three modified carbons show more than 90% for the four heavy metals’ removal rate. For further increase of dosage, the adsorption rate seems to be saturated with a slow rise, which may be caused by the overlapped adsorption sites by the overcrowding of adsorbent particles. For the dosage of both iron-salt modified carbon (AC-4) and unmodified coconut shell activated carbon (AC-0), when it is less than 6 g, the adsorption rate of both was positively correlated with the dosage. At the dosage of 6 g of AC-0, the adsorption of both reached a balance, and the removal rate was above 73%.
In general, the increase of adsorbent dosage leads to the improved removal rate of the adsorbent to heavy metal ions. However, the rise of absorbent dosage is only one factor in absorption effects due to its saturation. Instead, the continued increase of the adsorbent amount will reduce the adsorption amount of the adsorbent per unit mass, resulting in the waste of the adsorbent.
4.4 Adsorption kinetics of heavy metals (Cu2+, Cd2+, Pb2+, and Zn2+) to different coconut shell activated carbons
Under the initial conditions of concentrations of Cu2+, Cd2+, Pb2+, and Zn2+ with 5 mg/L, the temperature of 25 °C, dosage of activated carbon of 1 g, and pH = 5, the influence of contact time (ranging from 5 to 240 min) on the adsorption effect of heavy metal ions with different types of coconut shell-based activated carbons were investigated. The relationship between reaction time and the removal rate of heavy metal ions within wastewater with different carbons is shown in Fig. 5.
The adsorption kinetic curves of coconut shell activated carbons for natural (AC-0) and modified (AC-1 through 4) show a relationship of the contact time between activated carbons and the removal capacity of heavy metal ions wastewater.
At the initial stage of adsorption, the driving force of diffusion of Cu2+, Cd2+, Pb2+, and Zn2+ in an aqueous solution was large enough to increase the absorption rate. As the adsorption increased continuously, the heavy metal ions spread into the holes of coconut shell activated carbons until those make adsorbents in the center. The removal rate was further improved, and its rate gradually decreased, and finally reached the equilibrium points with saturation.
During the first 60 min, the four heavy metal ions’ adsorption rate on the natural coconut shell activated carbon (AC-0) was faster. When the contact time exceeded 60 min, the adsorption rate of AC-0 decreased slowly. With the continuous increase of the contact time, the adsorption capacity gradually reached saturation. The adsorption and removal capacity of AC-0 on four heavy metal ions is significantly different. Its adsorption and removal effect on Pb2+ is the most, reaching 87%, while the removal rate of Cd2+ is only 44%.
The adsorption of four heavy metal ions in an aqueous solution with the modified carbons of acid (AC-1), alkali (AC-2), and potassium (AC-3) was settled to a balance point within 60 min. When the contact time was extended to 120 min, the adsorption with iron salt modified carbon (AC-4) was also saturated. After reaching adsorption equilibrium, the removal rates of the four heavy metal ions with modified carbons (AC-1, AC-2, and AC-3) got to 88%. Among them, the removal of Zn2+ by AC-1 and AC-3 was the highest with 95%, 96.5%, respectively. For removing Cd2+, AC-2 was the highest at 98%, and the adsorption effect of Pb2+ by AC-4 was the best at 92%.
Compared with the natural carbon (AC-0), the adsorption equilibrium time of modified carbons (AC-1 through AC-4) to heavy metal ions is shorter, and the removal effect is better. The modified coconut shell activated carbons provide more adsorption sites to have increased contacts between heavy metal ions and the adsorbent and result in rapid adsorption.
5 Conclusion
Compared with the natural coconut shell activated carbon (AC-0), modified carbons (AC-1 through AC-4) responded to heavy metal ions with a shorter response time and fast saturation and showed improved removal effect. Among different modifications, the adsorption capacity with iron-salt modified coconut shell activated carbon showed an inferior performance than the other three modifications with acid, alkali, and potassium permanganate. More than 90% of heavy metal ions in wastewater were absorbed and removed successfully by nitric acid (AC-1), sodium hydroxide (AC-2), and potassium high-mentholated (AC-3). The adsorption and removal effect of iron-salt modified carbon (AC-4) and natural carbon (AC-0) reach the best with 6 g of dosage. A reasonable increase in adsorbent dosage shows enhancing the adsorption and removal capacity to heavy metal ions in an aqueous solution. At the pH value of the aqueous solution with 5, the adsorption of heavy metal ions with three modified carbons (acid, alkali, and potassium modified with high menstruate) reached the optimal level. As the pH value increased to 7, iron-salt modified carbon’s adsorption effect reached the optimal level. In the weak acid condition, the binding ability of hydrogen ions to the carboxyl group is vital. With the increase of pH value in the solution, the ionization degree of hydroxyl groups increases. The possibility of heavy metal ions being adsorbed increases, so the removal rate increases rapidly. When the pH value is 5, the adsorption capacity of three kinds of modified coconut shell activated carbon (acid, alkali, and potassium permanganate) is the strongest. With the further increase of the solution’s pH value, the adsorption effect of the adsorbent for heavy metal ions showed a slight decrease, which may be caused by the gradual increase of chemical interaction between heavy metal ions and hydroxyl ions.
References
Cay S, Sayin S, Engin MS, Eymur S (2017)Preparation and characterization of calix [4] aren-immobilized magnetic microcapsule and its application in heavy metal removal. Polycycl Aromat Compd
Rastogi S, Kandasubramanian B (2020) Progressive trends in heavy metal ions and dyes adsorption using silk fibroin composites. Envi Sci Pollut Res, 1-28
Wang Z, Guo J, Ma J, Shao L (2015) Highly regenerable alkali-resistant magnetic nanoparticles inspired by mussels for rapid selective dye removal offer high-efficiency environmental remediation. J Mat Chem A 3:19960–19968. https://doi.org/10.1039/C5TA04840K
Yang X et al (2020) Polyphenol-sensitized atomic layer deposition for membrane interface hydrophilization. Adv Func Mater 30:1910062. https://doi.org/10.1002/adfm.201910062
Zhang F, Li J, Tan J, Wang B, Huang F (2013) Advance of the treatment of heavy metal wastewater by adsorption. Chem Ind Eng Prog 32, 2749-2756
Bernard E, Jimoh A, Odigure J (2013) Heavy metals removal from industrial wastewater by activated carbon prepared from coconut shell. Res J Chem Sci 2231:606X
Ks G, Belagali SL (2013) Removal of heavy metals and dyes using low cost adsorbents from aqueous medium—a review. IOSR J Environ Sci Toxicol Food Technol 4:56–68
Onyeji LI, Aboje AA (2011) Removal of heavy metals from dye effluent using activated carbon produced from coconut shell. Int. J. Engin. Sci. Technol. (IJEST) 3:8238–8246
Sekar M, Sakthi V, Rengaraj S (2004) Kinetics and equilibrium adsorption study of lead (II) onto activated carbon prepared from coconut shell. J Colloid Interface Sci 279:307–313
Song M et al (2018) Study on adsorption properties and mechanism of Pb2+ with different carbon based adsorbents. Sci Total Environ 618:1416–1422
Abdeljaoued A et al (2018) Preparation and evaluation of a coconut shell-based activated carbon for CO2/CH4 separation. Energies 11:1748
Lima SB, Borges SMS, Rangel MdC, Marchetti SG (2013) Effect of iron content on the catalytic properties of activated carbon-supported magnetite derived from biomass. J BR Chem Soc 24, 344-354
Prauchner MJ, Sapag K, Rodríguez-Reinoso F (2016) Tailoring biomass-based activated carbon for CH4 storage by combining chemical activation with H3PO4 or ZnCl2 and physical activation with CO2. Carbon 110:138–147
Mohammad-Khah A, Ansari R (2009) Activated charcoal: preparation, characterization and applications: a review article. Int J Chem Tech Res 1:859–864
Cholico-González D, Ortiz Lara N, Fernández Macedo AMA, Chavez Salas J (2020) Adsorption Behavior of Pb (II), Cd (II), and Zn (II) onto Agave Bagasse, Characterization, and Mechanism. ACS omega 5, 3302-3314
Jiang C et al (2020) Construction of a lignosulfonate-lysine hydrogel for the adsorption of heavy metal ions. J Agri Food Chem 68:3050–3060
Jin Q et al (2019) Effective removal of Cd 2+ and Pb 2+ pollutants from wastewater by dielectrophoresis-assisted adsorption. Front Environ Sci Eng 13:16
Eruola AO, Ogunyemi IO (2014) Evaluation of the adsorption capacity of the coconut shell and palm-kernel shell adsorbents powder for the sorption of cadmium (II) ions from aqueous solution. IOSR J Environ Sci Toxicol Food Technol 8, 55-63
Taoufik N, Elmchaouri A, Korili SA, Gil A (2020) Optimizing the removal of nitrate by adsorption onto activated carbon using response surface methodology based on the central composite design. J Appl Water Eng Res 8:66–77
Amuda OS, Giwa A, Bello IA (2007) Removal of heavy metal from industrial wastewater using modified activated coconut shell carbon. Biochem Eng J 36:174–181
Zhang M, Tang Z, Zhao Y, Wang X, Xu X (2020) Study on the adsorption properties of different insect feces on Cu 2 and Cd 2. E&ES 450:012124
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Deng, Z., Sun, S., Li, H. et al. Modification of coconut shell-based activated carbon and purification of wastewater. Adv Compos Hybrid Mater 4, 65–73 (2021). https://doi.org/10.1007/s42114-021-00205-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42114-021-00205-4