Abstract
Socio-economics development of any nation depends on Industrialization. With increase in number of industries needs, water is an essential requirement and added extra burden in terms of fresh water and wastewater. Among all the industries, sugar-processing industry is one of them. To fulfill the demand, appropriate technology will require otherwise with limited resources low quality of water can be used for operation. The aim of this research work is to treat the wastewater generated from sugarcane-processing industry using chemical coagulation and electrocoagulation. The aluminium salt suitability of both treatments was carried out for iron and metal electrode. A result shows 82% chemical oxygen demand and 84% color removal with iron electrode was attended at 156 A−2 current density, optimum pH 6 and 120 min of treatment time. Finally, settling, filtrations, X-ray diffraction and scanning electron micrograph study also conform that iron electrode is more suitable to treat sugar industry wastewater. To treat 1 m3 of wastewater, 2.0 USD will be required including all expenses.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sugar is the second major food crop in world (Oerke et al. 2012). Nearly, 24 million hectare of area was cultivated with production of 185 MT and 174 MT consumptions in year of 2017–2018 (Bordonal et al. 2018). Processing of 1 ton sugarcane requires 1500–2000 L of fresh water and release 1000 L of wastewater as effluent (Tiwari and Sahu 2017). Sugarcane-processing industry effluent contains high percentage of organic and inorganic pollutant that has major environment impact on surrounding (Gunkel et al. 2007). Generally, conventional methods, i.e., combination of physicochemical and biological were employed to treat the discharge from sugar industry (Hampannavar and Shivayogimath 2010). This treatment method is less efficient, due to their method of operation, maintenances and setup. Various post-treatment techniques were also reported such as membrane separation, advance oxidation, electrocoagulation and adsorption to treat the pollutant from sugar industry effluent. However, due to lack of technical feasibility, wastewater was not treated up to acceptable limit. The disposal of effluent in economical way is major issue for sugar-processing industry (Farhadian et al. 2007).
In the literature, lesser number of published works is available in which primary (coagulation and filtration) and tertiary (advance oxidation and electrocoagulation) treatment was discussed and compared (Oller et al. 2011). Nevertheless, these technologies can be considered as competing technologies and, therefore, the comparisons of treatment efficiencies are important. Chemical coagulation is taking into account of regular treatment and employed for complete range of pollutant on water and wastewater in economical way. The process occurs in three steps namely neutralization of charge, agglomeration and settle down of the suspended particles (Ifill and Etsell 2013). In electrocoagulation treatment, coagulant species are produced by electrolytic oxidation of electrode material (Safferman 2010). The process is suitable to remove different range of pollutant with high concentration in simple manner (Mollah et al. 2001). A very few number of researcher were informed the details of electrocoagulation and chemical coagulation process, to treat the ultrafine quartz suspensions (Kılıc et al. 2009), removal of chromium (VI) (Golder et al. 2007), virus (Zhu et al. 2005) and surface water pollutant (Bagga et al. 2008). The performance of both treatment methods depends on the alkalinity effluent, type of metal or salt uses, dosage or current applied, solution temperature, mixing of sample and retention time (Cañizares et al. 2009). For electrocoagulation process, efficiency can be also improved using electrolyte (salt like sodium hydroxide) and gap between the two electrodes, but in chemical coagulation process some coagulant aid can be used (Moussa et al. 2017). Overall, both treatment processes have more common thing and their only difference is the way of operation. Chemical coagulation process can be considered as the most primitive method and electrocoagulation can be the most modern (Donneys-Victoria et al. 2018).
The main aim of research work is to distinguish the chemical coagulation and electrocoagulation for sugar industry wastewater. Operational condition, effect of initial alkalinity of wastewater and mass loading in terms of current density and dosing were consider as dominate factor for pollutant removal. The physicochemical characteristic of sludge obtained has been studied in terms of settling, filtration, X-ray and scanning electron microscope.
Materials and methods
Material
The sugar industry wastewater was collected from Bhoramdev Sugar Industry Ltd., Kavardha (C.G.), India. The physicochemical composition of effluent is mentioned in Table 1 and stored at 4 °C until experiment. Analytical laboratory grade chemicals such as sodium hydroxide, hydrochloric acid and chemical coagulant which were supplied by Merk Limited, Mumbai, India were used for analysis. The aluminum and iron sheets were used as the electrode and were purchased from local commercial area.
Experimental methods
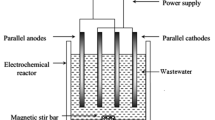
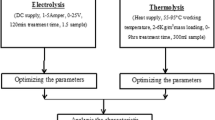
A batch experiment was conducted to treat the sugar effluent by electrocoagulation. The simplest electrochemical reactor (capacity 1.2 L) was made up of transparent sheet and fitted with two pairs of anode and cathode. The electrodes were connected with power supply system in parallel manner and maintained a gap of 20 mm. The power supply system was fitted with ammeter and voltmeter to measure the current and voltage. When a potential is applied from an external power source, the anode material undergoes oxidation, while the cathode will be subjected to reduction or reductive deposition of elemental metals. The electrochemical reactions with metal M as anode may be summarized as follows (Holt et al. 1999):
At the anode:
At the cathode:
The electrode plates were cleaned manually by abrasion with sandpaper and they were treated with 15% HCL for cleaning followed by washing with distilled water prior to their use. A known volume of wastewater was taken in the reactor and the experiment was run for 120 min. After treatment, a small amount of treated water (0.5 mL) was collected in each decided interval of experimental time for COD and color determination.
Coagulation method was carried out in standard jar test apparatus with capacity of 0.50-L glass beaker (TeKippe and Ham 1970). The initial pH of the effluent was noted and adjusted by adding aqueous NaOH (1 M) or HCl (1 M) solution. Calculated amount of the chemical coagulant was added to the effluent and flash-mixed for 5 min by a magnetic stirrer. After that, allow slowly mixing for 30 min. The effluent sample was then taken in a glass cylinder and kept quiescent for 6 h. The supernatant liquor was centrifuged and analyzed for its COD value. These steps were repeated at different dosages of the coagulant. The filtration characteristics of the solid residue formed in the treated effluent were studied using an ordinary zero haze grades. A filter paper was (pore size 7–11 µm) supplied by S.D. Fine Chemicals Ltd. The filter paper was supported on a ceramic Buchner funnel and gravity filtration was carried out.
Analytical procedure
The COD of the samples was determined by the standard dichromate reflux method (Rump 1999). The chloride concentration was determined by the standard titrimetric Volhard method (Vogel 2013). Sulfates and the phosphates were determined using standard methods (Holt et al. 1999). The concentrations of the metal ions in the filtrate and the residue were determined using an atomic absorption spectrometer (GBC, Model Awanta, Australia). The protein content was determined by the Bradford (1976) method (Kruger 2002). The color of the sample was measured in terms of the absorbance at λ = 420 nm using a UV–Vis spectrophotometer (Model Lambda 35) from Perkin-Elmer Instruments, Switzerland. After the electrolysis experiment, the microstructure of the by-products, i.e., the sludge, was examined by field emission scanning electron microscopy (FE-SEM, HITACHI, S-4700).
Results and discussion
Effect of pH on COD and color removal
The initial (adjusted) pH (0) of the wastewater has considerable effect on treatment performance. An electrocoagulation experiment was conducted at different range of pH (2–10); 20 mm electrode distance and 120 min treatment time for both iron and aluminum electrodes. The influence of initial pH on chemical oxygen demand (COD) and color removal of sugar industry wastewater (SIWW) is shown in Fig. 1a, b. Initially, when iron electrodes were used, maximum 64% COD and 70% color reduction were achieved at pH 6 (at CD 78 A/m2) and aluminum electrodes were used, maximum 60% COD and 71% color reduction were observed at pH 7 (at CD 89 A/m2). To compare the behavior of both metal electrodes, similar metal ion ferric chloride (FeCl3) and aluminum (AlCl3) were used as chemical salts at constant dosing of 30 millimoles (mm). When ferric chloride (FeCl3) was used to treat the wastewater, it shows 64% COD and 78% color reduction at pH 7 and aluminum chloride shows maximum 71.2% COD and 81% color reduction at pH 8. It can be seen that maximum removal occurred at low acidic, neutral and low alkaline pH of wastewater solution, this may be due to dominant coagulant species formation. At high alkaline range, aluminum hydroxide [Al(OH)4−] and ferrous hydroxide [Fe(OH)4−] ions are formed and these negative ions are poor to coagulate the pollutant (Duan and Gregory 2003; Ryan et al. 2008). The result also indicates that iron metal ions are more efficient as compared to aluminum ion to treat the sugar industry wastewater. This attributes to the formation of greenish color rust flock of iron electrodes, which gives better to agglomeration and settling than the gel floc formed with aluminum. The concept of pollutant particles destabilization during electrochemical treatment using iron electrodes was similar to that of chemical coagulation using ferric chloride (FeCl3) or ferrous sulfate (FeSO4) as a coagulant (Chafi et al. 2011). During the coagulation and electrocoagulation process as hydrolytic reactions, metal cations formed mononuclear and polynuclear hydroxides in the solution, which depend on the pH of the solution (Morgan and Lahav 2007).
Current density and mass loading on COD and color removal
The mass loading in terms of dosing for classical coagulation or current density for electrocoagulation directly influence the removal efficiency. Coagulant species generated by electrolysis process can usually calculate according to “Faraday’s Law” (FL), when current and treatment times are known. According to law, coagulant species produced by electrolysis on anode sides is directly proportional to the electric charge added per volume (coulombs per liter) of electrolyte (Sahu et al. 2014). To optimize, the minimum mass loading on chemical oxygen demand (COD) and color reduction of sugar industry wastewater with FeCl3 and AlCl3 at optimum pH vary from 20 to 60 mm and the effect of current density 39, 78, 117, 156, and 195 A/m2 for iron electrode, and 44.5, 89, 133.5, 178, and 222.5 A/m2 for aluminum electrode on COD and color reduction are presented in Fig. 2a, b. It was observed that COD and color reduction increase with increase in CD, maximum 82% COD and 84% color reduction shown by iron electrode at 156 A/m2 and 79% COD and 80% color reduction shown by aluminum electrode at 178 A/m2. Further increase in CD decrease the efficiency and this is because at high current, the extent of anodic dissolution of ion increases, resulting in a greater amount of precipitate for the removal of pollutants. Moreover, bubble generation rate increases and the bubble size decreases with increasing current density (Sahu and Chaudhari 2015). These effects are beneficial for high pollutant removal, but it wastes electrical energy in heating up the water (Ricordel and Djelal 2014). During the EC process, temperature also increases with increase in the reaction time and current density. It found maximum 77% COD and 85% color reduction using ferric chloride at 40 mm and same mass-loading aluminum chloride shows maximum 75% COD and 81% color reduction. In chemical coagulation, reduction percentage increases or decreases with mass loading. The predominant removal mechanisms at low mass loading of salts are adsorption and charge neutralization. However, at high mass loading of coagulant, it is sweep floc coagulation by enmeshment in the hydroxide precipitate (Fernandez et al. 2014).
Solid–liquid study
The properties of produced sludge are important because sludge treatment and disposal are one of the major cost factors in water and wastewater treatment, especially when sludge has been produced by chemicals and salts.
Settling
Gravity settling process was carried out, to determine the setting rate of electrocoagulant- and chemical-treated sugar industry wastewater without addition of coagulant aid. The solid–liquid separation with respect to time was examined for iron electrodes treated at CD 156 A/m2; pH 6; electrode distance 20 mm; treatment time 120 min and with chemical coagulant ferric chloride treated at optimum dosing 40 mm and pH 7. The solid–liquid interface is shown in Fig. 3 for 210 min of retention time. It can be observed that at the beginning, the settling rate was very slow due to Brownian Motion (BM) of the both coagulated particles (Talbot and Talbot 2018). With respect to slow process, the height of suspended particles decreases and clear water increases, which can be easily identified. Almost at the 90 min of settling time, 90:10 solid–liquid interface for electrocoagulation-treated and 75:25 solid–liquid for chemical-treated wastewater were observed clearly. The settling speed of particles depends on the rate of agglomerations (flock formation by the process) and pH of solution (wastewater).
Filtration
In literature, pressure filtration and vacuum filtration were reported for electrocoagulation generated and chemical coagulation-generated sludge (Mahesh et al. 2016). Normal gravitational filtration process was employed to obtain the experimental data for electrocoagulation and chemical coagulation-treated wastewater. The concept of force balance for the gravity filtration using a filter paper on a Buchner funnel has been fallowed, according to MaCabe et al. (2001). After filtration process, the volume of filtrate obtained with respect to time was recorded and plotted (Δt/ΔV), as shown in Fig. 4. The points lie on straight line, which indicates amount of filtrates depend on the time. The values of Rm and α can be calculated from the slope and the intercept, respectively. Values of cake resistance are found in the order 3.59 × 1011 (for iron electrode) and 7.09 × 1011 (iron metal salt). The cake resistance depends on size of particles and its porosity which is large 6.05 × 1012 for iron electrode than 7.78 × 1012 iron salts.
X-ray
The sludge (dried) with electrocoagulation and chemical coagulation treated were analyzed by X-ray diffraction, which is shown in Fig. 5a, b. It was observed that peaks are indicated the presence of Fe and little percentage of Magnetite (Fe2O4), Goethite (FeO(OH)) and Wuestite (FeO) with EC-treated sludge. When the electrocoagulation process is going on, the bubbles of hydrogen and oxygen generated move upwards in the liquid phase which carry the pollutants with them (slug/scum) (Hakizimana et al. 2017). The settling muds after treatment that are collected and dried are studied for XRD. During the EC process with iron electrode, destabilizing of suspended, emulsified, or dissolved contaminants in an aqueous medium and settle down of the pollutant in the form of sludge is observed. Chemical-treated sludge with iron salt sludge (dried) indicates that Fe exists in powder form and specifies the presence of rebeokite.
SEM
The scanning electron morphology of the floating sludge (dried) and bottom sludge is shown in Fig. 6a, b. Floating sludge shows uniformly lumpy mass, which indicates high porosity, inhomogeneous surface and particles varying in shape. Settling sludge of treated wastewater indicates the heavy lumpy masses, which is in micrometers size (Karichappan et al. 2014). The sludge particles were hard aggregates of very small particles, all under micrometer. The electrolysis eliminates the various pollutants in the wastewater by oxidation, meanwhile the iron compound particles are formed including pollutants. When the iron compound particles are agglomerated with each other, the organics may also be included into the aggregates.
Cost estimation
The cost estimation is a necessary demand for any wastewater treatment process. An electrochemical process is recommended for wastewater treatment but it has been linked with volume or nature pollutants generated. The efficiency of electrocoagulation process mainly attributed to the electricity required and electrode consumption during the treatment. The cost of energy for sugar industry (SI) treatment and the cost of iron electrode material consumed were obtained from the experimental results (without the use of any additive) and the sum was taken as the operating cost.
-
1.
Energy consumption
To treat the 1 m3 of SIWW, energy consumption approximately = 16.71 kW h/m3 Kg.
At an energy price in the Indian market = INR 5.75/kWh.
Cost of energy per m3 of SIWW treated = INR 5.75/kWh × 16.71kWh/m3 Kg = INR 96.08.
-
2.
Electrode consumption
Iron required per m3 of SIWW = 0.800 g/m3.
Cost of iron sheets on bulk purchase (cleaning, cutting and placing) = INR 50/Kg.
Cost of iron per m3 of SIWW treated = 0.8 kg × INR 50/Kg = INR 40.0/m3.
Cost of energy + cost of electrode = 96.08 + 40.0 = INR 136.08 (USD$ = 1.94 @ 70 INR/US$) per m3 of SIWW treated.
Conclusion
Electrocoagulation process was found to be a better substitute as compared to chemical coagulation for various pollutants removal from sugar industry wastewaters. It can be remedies for high quality of fresh water from colored wastewater having high concentrations of organic matter. Treated water could be used as fresh feed for industrial operation. The experimental result shows that maximum 82% COD and 84% color removal was obtained at optimum pH 6, electrode distance 20 mm, current density 156 A m−2 with iron electrode. Aluminum shows 79% COD and 80% color removal at optimum pH 7, electrode distance 20 mm, and current density 178 A m−2. Chemical treatment with ferric chloride shows maximum 75% COD and 85% color removal at 40 mm mass loading as compared to 72% COD and 81% color removal at 40 mm mass loading with aluminum chloride. The settling study shows nearly 90:10 liquid–solid interfaces with iron electrode and 75:25 liquid–solid interfaces with ferric salt in 90 min of settling time. Filtration result agrees with the settling experiment result and cake resistance was found to be 3.59 × 1011 (for iron electrode) and 7.09 × 1011 (iron metal salt). X-ray diffraction peaks show the presence of Magnetite, Goethite and Wuestite on floating and settling sludge of iron-treated wastewater. The scanning electron micrograph justified the filtration study and indicates high porous structure in settled sludge and presents an ordered structure with heterogeneous distribution of particles on both floating and settling sludge. To treat 1 m3 of wastewater, nearly 1.94 USD will required, which is economical as compared to another methods. At last, it can be concluded that iron electrode is found to be appropriate with electrocoagulation treatment process for treating the sugar industry wastewater. The comparatively study of chemical coagulation and electrocoagulation is suggested that these methods seem to be equivalent to pollutant destabilization mechanisms, metal consumption and removal efficiency. The outcome of the researcher work is that electrochemical treatment is more efficient than chemical coagulation to remove the sugar industry pollutant.
References
Bagga, A., Chellam, S., & Clifford, D. A. (2008). Evaluation of iron chemical coagulation and electrocoagulation pretreatment for surface water microfiltration. Journal of Membrane Science, 309(1–2), 82–93.
Cañizares, P., Jiménez, C., Martínez, F., Rodrigo, M. A., & Sáez, C. (2009). The pH as a key parameter in the choice between coagulation and electrocoagulation for the treatment of wastewaters. Journal of Hazardous Materials 163(1), 158–164.
Chafi, M., Gourich, B., Essadki, A. H., Vial, C., & Fabregat, A. (2011). Comparison of electrocoagulation using iron and aluminium electrodes with chemical coagulation for the removal of a highly soluble acid dye. Desalination, 281, 285–292.
de Bordonal, R. O., Carvalho, J. L. N., Lal, R., de Figueiredo, E. B., de Oliveira, B. G., & La Scala, N. (2018). Sustainability of sugarcane production in Brazil a review. Agronomy for Sustainable Development, 38(2), 13.
Donneys-Victoria, D., Marriaga-Cabrales, N., Camargo-Amado, R. J., Machuca-Martínez, F., Peralta-Hernández, J. M., & Martínez-Huitle, C. A. (2018). Treatment of landfill leachate by a combined process: Iron electrodissolution, iron oxidation by H2O2 and chemical flocculation. Sustainable Environment Research, 28(1), 12–19.
Duan, J., & Gregory, J. (2003). Coagulation by hydrolysing metal salts. Advances in Colloid and Interface Science, 100, 475–502.
Farhadian, M., Borghei, M., & Umrania, V. V. (2007). Treatment of beet sugar wastewater by UAFB bioprocess. Bioresource Technology, 98(16), 3080–3083.
Fernandez, D., Maurer, P., Martine, M., Coey, J. M. D., & Möbius, M. E. (2014). Bubble formation at a gas-evolving microelectrode. Langmuir, 30(43), 13065–13074.
Golder, A. K., Chanda, A. K., Samanta, A. N., & Ray, S. (2007). Removal of Cr (VI) from aqueous solution: electrocoagulation vs chemical coagulation. Separation Science and Technology, 42(10), 2177–2193.
Gunkel, G., Kosmol, J., Sobral, M., Rohn, H., Montenegro, S., & Aureliano, J. (2007). Sugar cane industry as a source of water pollution—case study on the situation in Ipojuca River, Pernambuco, Brazil. Water, Air, and Soil Pollution, 180(1–4), 261–269.
Hakizimana, J. N., Najid, N., Gourich, B., Vial, C., Stiriba, Y., & Naja, J. (2017). Hybrid electrocoagulation/electroflotation/electrodisinfection process as a pretreatment for seawater desalination. Chemical Engineering Science, 170, 530–541.
Hampannavar, U. S., & Shivayogimath, C. B. (2010). Anaerobic treatment of sugar industry wastewater by upflow anaerobic sludge blanket reactor at ambient temperature. International journal of environmental sciences, 1(4), 631.
Holt, P., Barton, G., & Mitchell, C. (1999). Electrocoagulation as a wastewater treatment. The Third Annual Australian Environmental Engineering Research Event, 1000, 41–46.
Ifill, R. O., & Etsell, T. H. (2013). Enhanced settling of fine silica by direct AC electrocoagulation. Mineral Processing and Extractive Metallurgy, 122(3), 137–145.
Karichappan, T., Venkatachalam, S., & Jeganathan, P. M. (2014). Optimization of electrocoagulation process to treat grey wastewater in batch mode using response surface methodology. Journal of Environmental Health Science and Engineering, 12(1), 29.
Kılıc, M. G., Hoşten, C., & Demirci, S. (2009). A parametric comparative study of electrocoagulation and coagulation using ultrafine quartz suspensions. Journal of Hazardous Materials, 171(1–3), 247–252.
Kruger, N.J., 2002. The Bradford method for protein quantitation. In The protein protocols handbook (pp. 15–21). Humana Press, New York.
MaCabe, W. L., Smith, J. C., & Harriot, P. (2001). Unit operations of chemical engineering (6th ed.) New York: McGraw-Hill.
Mahesh, S., Garg, K. K., Srivastava, V. C., Mishra, I. M., Prasad, B., & Mall, I. D. (2016). Continuous electrocoagulation treatment of pulp and paper mill wastewater: operating cost and sludge study. RSC Advances, 6(20), 16223–16233.
Mollah, M. Y. A., Schennach, R., Parga, J. R., & Cocke, D. L. (2001). Electrocoagulation (EC)—science and applications. Journal of hazardous materials, 84(1), 29–41.
Morgan, B., & Lahav, O. (2007). The effect of pH on the kinetics of spontaneous Fe (II) oxidation by O2 in aqueous solution–basic principles and a simple heuristic description. Chemosphere, 68(11), 2080–2084.
Moussa, D. T., El-Naas, M. H., Nasser, M., & Al-Marri, M. J. (2017). A comprehensive review of electrocoagulation for water treatment: Potentials and challenges. Journal of Environmental Management, 186, 24–41.
Oerke, E.C., Dehne, H.W., Schönbeck, F. and Weber, A., 2012. Crop production and crop protection: estimated losses in major food and cash crops. Elsevier, Amsterdam.
Oller, I., Malato, S., & Sánchez-Pérez, J. (2011). Combination of advanced oxidation processes and biological treatments for wastewater decontamination—a review. Science of theTotal Environment, 409(20), 4141–4166.
Ricordel, C., & Djelal, H. (2014). Treatment of landfill leachate with high proportion of refractory materials by electrocoagulation: system performances and sludge settling characteristics. Journal of Environmental Chemical Engineering, 2(3), 1551–1557.
Rump, H.H., 1999. Laboratory manual for the examination of water, waste water and soil (No. Ed. 3). Wiley-VCH Verlag GmbH, Hoboken.
Ryan, D., Gadd, A., Kavanagh, J., Zhou, M., & Barton, G. (2008). A comparison of coagulant dosing options for the remediation of molasses process water. Separation and Purification Technology, 58(3), 347–352.
Safferman, S. I. (2010). Fundamentals of coagulation and flocculation: water world. Tulsa: PennWell Corporation.
Sahu, O. P., & Chaudhari, P. K. (2015). Electrochemical treatment of sugar industry wastewater: COD and color removal. Environmental Science and Pollution Research, 739, 122–129.
Sahu, O., Mazumdar, B., & Chaudhari, P. K. (2014). Treatment of wastewater by electrocoagulation: a review. Journal of Electroanalytical Chemistry, 21(4), 2397–2413.
Talbot, D. E., & Talbot, J. D. (2018). Corrosion science and technology. Boca Raton: CRC Press.
TeKippe, R. J., & Ham, R. K. (1970). Coagulation testing: a comparison of techniques—Part 1. Journal‐American Water Works Association, 62(9), 594–602.
Tiwari, A., & Sahu, O. (2017). Treatment of food-agro (sugar) industry wastewater with copper metal and salt: chemical oxidation and electro-oxidation combined study in batch mode. Water Resources and Industry, 17, 19–25.
Vogel, A. I. (2013). A text-book of quantitative inorganic analysis-theory and practice. London; New York; Toronto: Longmans, Green And Co.
Zhu, B., Clifford, D. A., & Chellam, S. (2005). Comparison of electrocoagulation and chemical coagulation pretreatment for enhanced virus removal using microfiltration membranes. Water Research, 39(13), 3098–3108.
Acknowledgements
The author acknowledges the Department of Chemical Engineering, National Institute of Technology Raipur for funding and facilities.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sahu, O. Suitability of chemical and electrocoagulation process on sugar industry wastewater treatment. Int J Energ Water Res 3, 117–125 (2019). https://doi.org/10.1007/s42108-019-00021-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42108-019-00021-z