Key summary points
Standardizing the use of ultrasound in the assessment of muscle and sarcopenia.
AbstractSection FindingsApproach of muscle assessment has been updated according to the most recent literature and anatomical landmarks for 39 different muscles are provided.
AbstractSection MessageUsing these recommendations, ultrasonographical muscle assessment can be standardized worldwide.
Abstract
Purpose
In 2018, the SARCUS working group published a first article on the standardization of the use of ultrasound to assess muscle. Recommendations were made for patient positioning, system settings and components to be measured. Also, shortcomings in knowledge were mentioned. An important issue that still required standardization was the definition of anatomical landmarks for many muscles.
Methods
A systematic search was performed in Medline, SCOPUS and Web of Sciences looking for all articles describing the use of ultrasound in the assessment of muscle not described in the first recommendations, published from 01/01/2018 until 31/01/2020. All relevant terms used for older people, ultrasound and muscles were used.
Results
For 39 muscles, different approaches for ultrasound assessment were found that likely impact the values measured. Standardized anatomical landmarks and measuring points were proposed for all muscles/muscle groups. Besides the five already known muscle parameters (muscle thickness, cross-section area, pennation angle, fascicle length and echo-intensity), four new parameters are discussed (muscle volume, stiffness, contraction potential and microcirculation). The former SARCUS article recommendations are updated with this new information that includes new muscle groups.
Conclusions
The emerging field of ultrasound assessment of muscle mass only highlights the need for a standardization of measurement technique. In this article, guidelines are updated and broadened to provide standardization instructions for a large number of muscles.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Being one of the ultimate geriatric syndromes, the generalised skeletal muscle disorder, sarcopenia hardly needs an introduction. The progressive decline of muscle function, mass and strength affects all aspects of life, not only causing physical problems, but also psychological, social and financial ones [1]. Despite the advances in aetiology, definition and screening guidelines that have been made in the past decade, some important issues remain.
Until recently, it was only recommended to measure muscle quantity. However, it became clear in the past decade that measuring muscle quality was indispensable [2, 3]. Therefore, in the step of measuring muscle mass, looking into either quantity or quality is advised [1]. Although there is still discussion regarding the exact definition of muscle quality, for this review, it is to be interpreted as the relative presence of different components of muscle mass (e.g. muscle, vascular, fibrous and adipose tissue). Unfortunately, it is not clear yet which ‘quality’ parameters should be taken into account, also meaning that there are no cut-off point that can be used. Although dual-energy X-ray absorptiometry (DXA) and bio-impedancemetry (BIA) do have cut-off values for muscle quantity, these methods do not provide indexes for muscle quality. In comparison, computed tomography (CT) and magnetic resonance imaging (MRI) can measure both muscle quantity and quality, but have no cut-off points and are not feasible to use in clinical practice [4].
To go beyond this limitation, another technique may be re-used and revaluated. Ultrasound (US) has proven to be an accurate, reliable technique with high repeatability to measure muscle mass in different populations [5,6,7,8]. It is an affordable, non-invasive method that is portable and available bedside. Ultrasound is strongly correlated with MRI- [9,10,11], CT- [12] and DXA- [13,14,15,16] based muscle measurements. However, a standardization of methods and measures is needed to allow for extensive and comparative studies.
The first step of standardization was taken with the first SARCUS (SARCopenia through UltraSound, see Fig. 1) article on standardization of ultrasonographic muscle assessment [17], providing consensus propositions for anatomical landmarks. Also, an instructional video for these measurements was made public [18]. In the article, five main parameters were documented: muscle thickness (MT), pennation angle (PA), fascicle length (Lf), echo-intensity (EI) and cross-sectional area (CSA). The aim of the current article was to give an update of the consensus propositions, as well as to provide anatomical landmarks and standardized measuring points for additional muscles. Although a lot of research has been focused on large muscle groups (quadriceps for instance), smaller muscles can be potentially as interesting due to their specific function (e.g. swallowing muscles). These standardization efforts also enable research groups with specific interests to include muscle parameters. As in the first article, the propositions provided should be considered as updated knowledge that may evolve with practice. The current propositions of measurement points and anatomical landmarks are based upon thorough study of relevant anatomical structures, cross-referencing to articles regarding MRI or ultrasound studies and multiple ultrasound sessions on human volunteers, to try and produce the most optimal ultrasound protocol. The ultimate objective of this article was to present an updated systematic review of literature and provide standardization for specific limbs and muscles.
Methods
Registration
The protocol for this updated systematic review has been registered at PROSPERO (registration number CRD42019126106).
Search strategy
The search strategy was kept the same as in the first SARCUS standardization article [17]. The same PICO model was used, consisting of the three main components: older people as the population [19], ultrasound as the intervention and muscle as the outcome. The search was performed in Medline, SCOPUS and Web of Science, from the 1st of January 2018 up until the 31th of January 2020. This allowed an overlap with the original article, in which the final date was set upon the 20th of January 2018. English, French, Dutch and German articles were screened on applicability. Manuscripts regarding assessment of muscle(s) using ultrasound were considered for review. Additional studies were hand-searched from reference lists from included studies, keeping in mind the reference period. Some article types were excluded: cadaver studies, studies in animals, (systematic) reviews, case reports, editorials and letters to the editor.
The search strategy for Medline, SCOPUS and Web of Science was the same as in the 2018 SARCUS article. For easy reference, the full search strategy is included in the supplementary material as Table 1S.
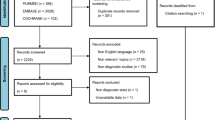
Figure 2 shows the overview of the article selection process, following the PRISMA guidelines [20]. After abstracts were gathered, duplicates were deleted. All abstracts were screened twice, once by one reviewer (SP) and once by one of 13 other SARCUS team members from different backgrounds—geriatricians, nutritional experts and specialists in body composition or physical therapy. All reviewers worked independently and were blinded from the inclusion decisions of the others using the Rayyan web-based software [21]. Manuscripts were screened for eligibility on title and abstract. Consensus of a third researcher was asked in the case of disagreement. Full-texts of the selected articles were searched for inclusion/exclusion criteria and if relevant for data extraction. Exclusion criteria for manuscripts were: content not being within the scope of this manuscript, not having an original measurement protocol (i.e. referencing to another article) and absence of description of measurement technique, patient position or anatomical landmarks. In case of referencing a protocol, the original article was included if within inclusion criteria.
Overview of the study selection process using the PRISMA 2009 flow chart [1]. NCDL = no clear description of location of measuring point; NCDM = no clear description of muscle measured; AOS = article outside of scope of manuscript; ROA = referencing to other articles for measurement technique
Data extraction
This update focused on patient positioning and the exact anatomical landmarks of the different muscles described. No information was extracted regarding systems, system settings, cohort sizes and ethnicity as in the first review article. Also, no measure of study quality was noted. All relevant data are summarized in proposals per muscle that are the result of consensus between SARCUS group members.
Results
Search outcomes
The initial search yielded 2.109 abstracts (PubMed = 729, SCOPUS = 595, Web of Science = 505). Nineteen additional records were identified using other sources. Duplicates were deleted (n = 306). Of the 1.542 abstracts screened, 155 were included. For two articles no full-text was found. During full-text assessment, 90 articles were discarded. In total, 65 articles were withheld for data extraction. For an overview of the selection process, see Fig. 2.
Patient positioning pre-investigation
One study investigated the need for rest before doing measurements, taking muscle thickness and echo-intensity values at 0, 5, 10 and 15 min after changing from a standing to a supine position [22]. Values for both measurements changed between 0 and 5 min after position change. Measurements taken at 10 and 15 min after position change remained the same as those taken at 5 min after position change.
Components of muscle
There are five main muscle parameters described, namely muscle thickness, muscle cross-section area, pennation angle, fascicle length and echo-intensity. In this review four additional potential parameters are introduced: muscle volume [23], muscle stiffness assessed through elastography [24], contraction potential of a muscle by correlating the cross-sectional area in rest to the cross-sectional area in maximal contraction [25] and microcirculation of a muscle [26]. Some parameters are indicative of muscle quantity (muscle thickness, cross-sectional area and volume), and others are to be regarded as quantitative parameters (pennation angle, fascicle length, echo-intensity, muscle stiffness, contraction potential and micro-circulation).
Muscle quantitative parameters
Muscle volume
One study [23] described a formula to estimate the muscle volume:
where MV = muscle volume, MT = muscle thickness and LL = limb length. This formula was based upon earlier calculated equations by the author.
Muscle thickness and muscle cross-sectional area
No new information was retrieved regarding these muscle components.
Muscle qualitative parameters
Fascicle length
Regarding calculation of fascicle length, two articles [23, 27] used the following formula:
where FL = fascicle length, mm = millimetre, MT = muscle thickness and PA = pennation angle.
One article [28] used an alternative formula to calculate fascicle length:
where FL = fascicle length, AA = aponeurosis angle, MT = muscle thickness and PA = pennation angle. The aponeurosis angle is defined as the angle between the line marked by the aponeurosis and a horizontal line drawn along the captured image [28].
There have been no comparative studies between the two formulas used.
Muscle stiffness
The interplay between the different components of muscle mass has its effect on the overall muscle stiffness, which is defined as the relation between possible deformation and compression of the muscle [29, 30]. These factors are determined by connective tissue such as collagen in the extracellular matrix that provides passive tension and muscle contraction that produces active tension [31, 32]. One study suggested that changes in muscle stiffness, measured through elastography, could be linked to muscle weakness [24]. In 77 participants divided over 3 age groups, the oldest age group had 16.5% lower muscle stiffness, which correlated with a lower muscle mass, slower walking time, less number of chair stands, lower handgrip strength and diminished isokinetic knee strength.
Muscle contraction
One study described the contraction potential of a muscle by correlating the cross-sectional area in rest to the cross-sectional area in maximal contraction [25]. This could be interesting for muscles where small variations in contraction may potentially have large functional repercussions, such as for the geniohyoid muscle, which is the major suprahyoid muscle responsible for the anterior displacement of the hyoid bone [33].
Muscle microcirculation
The microcirculation in skeletal muscle is the largest and most important site of capillary–tissue exchange of nutrients, oxygen and hormones (e.g. insulin), especially during exercise [34]. With increasing age however, a vicious circle emerges. Exercise leads to an increase in muscle microvascular volume, but in older people a lack of exercise is seen, which induces a decline in exercise capacity [35]. This decreased blood flow and thus lower oxygen delivery will lead in itself to a lower exercise capacity [36, 37]. Quantifying the muscle microvascular function in vivo is possible using contrast enhanced ultrasound [26]. Although an interesting technique, ultrasonographic contrast agents are not available in many countries.
Pennation angle and echo-intensity
No new information was retrieved regarding these muscle components.
Measuring points of the different muscles / muscle groups
For an overview of the muscles described, see Table 1 (overview of muscles of both upper and lower extremity, head and trunk).
Upper arm
The biceps brachii muscle was evaluated at 50% between the greater tubercle of the femoral head and the elbow crease, in a supine position, for muscle thickness [38, 39] and echo-intensity [38]: at 2/3 (proximal or distal was not described) between the acromion and the upper border of the olecranon, in a supine position, for muscle thickness [40].
The triceps brachii muscle was evaluated at 50% between the lateral edge of the scapular spine and the olecranon, in a prone position, for muscle thickness and echo-intensity [38]; at 3/5 (proximal or distal was not described) between the acromion and the lateral epicondyle of the humerus, in a non-defined position, for muscle thickness [41]; at 50% between the acromion and the olecranon, in a sitting position, for muscle thickness and echo-intensity [42]; at 60% (long head) (proximal or distal was not described) between the acromial process of the scapula and the lateral epicondyle of the humerus, in prone position, for muscle thickness, fascicle length, pennation angle and echo-intensity [23].
The coracobrachialis muscle was evaluated at 50% between the greater tubercle of the humeral head and the elbow crease, in a supine position, for muscle thickness [39].
Lower arm
Forearm musculature was evaluated at proximal 30% between the head of the radius and the styloid process of the ulna, in a standing position with the arm supinated, for muscle thickness [43].
Hand
Thenar muscles (abductor pollicis brevis, adductor pollicis brevis, flexor pollicis brevis and opponens pollicis) were evaluated with the probe placed transversely over the centre of the thenar eminence, as perpendicular as possible to the tendon of the flexor pollicis longus, at the thickest thenar muscles over the first metacarpal bone, in a supine position, with forearms in supination, elbows in full extension and dorsum of the hands in contact with the examination table, for muscle thickness [44].
Hypothenar muscles (opponens digiti minimi, flexor digiti minimi brevis and abductor digit minimi) were evaluated with the probe placed transversely over the centre of the hypothenar eminence, perpendicular to the axis of the 5th metacarpal bone, at the thickest thenar muscles over the first metacarpal bone, in a supine position, with forearms in supination, elbows in full extension and dorsum of the hands in contact with the examination table, for muscle thickness [44].
First dorsal interosseous muscle was evaluated at 50% between the origin and insertion of the muscle (identified by ultrasound), the probe perpendicular to the second metacarpal, in a sitting position, with the hand pronated, palm down with the thumb and index finger at a 70° angle, for muscle cross-sectional area and echo-intensity [45].
Upper leg
Gluteus medius was evaluated between the lateral surface of the iliac wing and the posteromedial surface of the trochanter major, in a lateral lying position [46].
Semitendinosus muscle was evaluated at 50% between the ischial tuberosity and the posterior knee fold joint, along the line of the semitendinosus muscle, in a prone position (hips neutral, knees extended), for muscle thickness [28], fascicle length [28] and pennation angle [28].
Quadriceps muscle (rectus femoris and vastus intermedius bellies) was evaluated at 30% (proximal or distal was not described) between the anterior superior iliac spine and the proximal end of the patella, in a supine position, for muscle thickness and echo-intensity [47]; at 50% between the anterior superior iliac spine and the proximal end of the patella, in a supine position, for muscle thickness of both bellies [48, 49] and echo-intensity of rectus femoris alone [48]; at 30% (proximal or distal was not described) between the anterior superior iliac spine and the proximal end of the patella, in a supine position, for muscle thickness and echo-intensity [50]; at 50% between the anterior superior iliac spine and the lateral epicondyle of the femur, in a standing position, for echo-intensity [51]; at 50% between the greater trochanter and the knee cleft (left or right was not described), in a supine position with the knees in 10°, for muscle thickness, cross-sectional area and echo-intensity and pennation angle [52]; at 15 cm proximal of the superior border of the patella, in a sitting position, for muscle thickness and echo-intensity [53].
Rectus femoris muscle was evaluated at 50% between the anterior superior iliac spine and the femoral lateral epicondyle, in a supine position, for muscle thickness and echo-intensity [38]; at 50% between the anterior superior iliac spine and the superior pole of the patella, in a sitting position, for echo-intensity [54]; at 50% between the anterior superior iliac spine and the superior pole of the patella, in a lying position with knees in 10°, for muscle thickness and muscle cross-sectional area [55]; at 60% (proximal or distal was not described) between the greater trochanter and the lateral epicondyle of the femur, 3 cm lateral to the midline, in a supine position, for muscle thickness and echo-intensity [56]; at 50% between the greater trochanter and the upper edge of the patella, in a supine position, for muscle thickness [57]; at 50% between the greater trochanter and the lateral condyle of the femur, in a supine position with the knees totally extended, for muscle thickness and echo-intensity [42].
Vastus lateralis muscle was evaluated at 50% between the greater trochanter and the femoral lateral condyle, in a sitting position, for muscle thickness [27, 58] and fascicle length [27, 58] and pennation angle [58]; at 50% between the greater trochanter and the tibial lateral condyle, in a supine position, for muscle thickness [59,60,61], cross-sectional area [62], fascicle length [61], pennation angle [61] and echo-intensity [59]; at 50% between the greater trochanter and the tibial lateral condyle, in a supine position with knees in a 60° angle, for muscle thickness, fascicle length and pennation angle [63]; at 50% between the greater trochanter and the superior pole of the patella, in a supine position, for echo-intensity [64]; at 1/3 (proximal or distal was not described) between the anterior superior iliac spine and the centre of the patella, in a supine position, for muscle thickness [65]; at distal 1/3 between grand trochanter and the femur medial condyle, in a sitting position (90° angle for hips and knees) for muscle thickness and pennation angle [66].
Biceps femoris muscle was evaluated at 50% between the ischial tuberosity and the posterior knee joint fold, along the line of the biceps femoris long head, in a prone position with the knees at 5° flexion, for muscle thickness and pennation angle [67].
Lower leg
Soleus muscle was evaluated at proximal 30% between popliteal crease and the lateral malleolus, in a standing position, for muscle thickness and echo-intensity [68].
Lateral gastrocnemius muscle was evaluated at proximal 30% between popliteal crease and the lateral malleolus, in a standing position, for muscle thickness and echo-intensity [68]; at 30% (proximal or distal was not described) between popliteal crease and the midpoint of the lateral malleolus, in a supine position, for muscle thickness, fascicle length and pennation angle [69].
Medial gastrocnemius muscle was evaluated at 30% (proximal or distal was not described) between popliteal crease and the medial malleolus, in a standing position, for fascicle length [70]; at 30% (proximal or distal was not described) between the lateral condyle of the tibia and the lateral malleolus, in a supine position, for muscle thickness [71]; at proximal 30% between the popliteal fossa and the posterior calcaneus, in a prone position, for muscle thickness and echo-intensity [38]; at proximal 30% between the head of the fibula and tip of the medial malleolus, in a sitting position, for muscle thickness [72].
Tibialis anterior muscle was evaluated at proximal 30% between the popliteal crease and tip of the lateral malleolus, in a sitting position, for muscle thickness [72].
Tibialis posterior muscle was evaluated at proximal 30% between popliteal crease and the lateral malleolus, in a standing position, for muscle thickness and echo-intensity [68].
Abductor hallucis muscle was evaluated between the medial calcaneal tuberosity and the navicular tuberosity, at the thickest part of the muscle 1–2 cm proximal to the navicular tuberosity, in a supine position with the hip externally rotated and the knee slightly flexed, for muscle thickness [73].
Flexor digitorum longus muscle was evaluated at 40% (proximal or distal was not described) and 50%, between the medial tibial condyle and the inferior margin of the medial malleous, in a supine position with the hip externally rotated and the knee slightly flexed, for muscle thickness [73].
Flexor hallucis longus was evaluated at 40% (proximal or distal was not described) and 50%, between the medial tibial condyle and the inferior margin of the medial malleous, in a supine position with the hip externally rotated and the knee slightly flexed, for muscle thickness [73].
Foot
Flexor hallucis brevis muscle was evaluated along the shaft of the 1st metatarsal and scanned in a proximal direction until the thickest portion of the muscle belly was located, distal to the base of the metatarsal, in a prone position with the feet hanging freely, for muscle thickness and cross-sectional area [73].
The major muscle belly of flexor digitorum brevis was evaluated on a line joining the medial tubercle of the calcaneus to the 3rd toe on the plantar surface of the foot, at the thickest portion of the muscle belly, before it divided into its four muscle fascicles, in a prone position with the feet hanging freely, for muscle thickness and cross-sectional area [73].
Abductor digiti minimi muscle was evaluated between the lateral calcaneal tuberosity and the tuberosity of the 5th metatarsal, at the thickest part of the muscle near the calcaneo-cuboid joint, in a prone position with the feet hanging freely, for muscle thickness and cross-sectional area [73].
Thoracic
Serratus anterior muscle was evaluated with the test arm placed uppermost, mid-position of scapular protraction and retraction, neutral horizontal abduction/adduction and 90° of glenohumeral flexion, with elbow in full extension, the transducer placed horizontally on the scapular inferior angle, was moved laterally until the midaxillary line, and was rotated to image the rib in cross-section until the shortest rib axis view was apparent on image, then was aligned parallel with the muscle fascia on image, to provide greatest contrast between hypoechoic muscle and highly echogenic rib, the image taken at the midpoint of the rib width, in side lying position, for muscle thickness [74].
Lower trapezius muscle was evaluated on the 5th thoracic vertebra level, with ipsilateral head rotation and 145° of glenohumeral abduction, with the palm of the hand in contact with a supporting arm pillow on an adjacent plinth, the transducer orientated horizontally over the T5 vertebra and moved laterally until the thickest part of the LT was identified, in prone position, for muscle thickness [74].
Diaphragm was evaluated at the mid-axillary line on the right side, at the level of either the 8th or the 9th intercostal space depending upon the clearest image, in a 30° head up supine position, the probe held perpendicular to the diaphragm, for muscle thickness [75]; at the midaxillary line in the apposition zone between the lung and liver on the right and between the lung and spleen on the left, in the intercostal spaces between the ninth, tenth, and eleventh ribs, 0.5–2 cm above the costophrenic sinus, for muscle thickness [76].
Abdominal
Transverse abdominis muscle was evaluated cross the abdominal wall over the anterior axillary line, with the probe held transversally at 50% between the 12th rib and the iliac crest, in a supine position with a pillow under head and knees, for muscle thickness [46, 77, 78]; across the abdominal wall 2.5 cm anterior to the anterior axillary line, between the 12th rib and the iliac crest, in a crook lying position, for muscle thickness [79]; across the abdominal wall 10 cm lateral to the umbilicus along the midaxillary line, for muscle thickness [80, 81].
Internal oblique muscle was evaluated across the abdominal wall over the anterior axillary line, with the probe held transversally at 50% between the 12th rib and the iliac crest, in a supine position with a pillow under head and knees, for muscle thickness [77, 78]; across the abdominal wall 2.5 cm anterior to the anterior axillary line, between the 12th rib and the iliac crest, in a crook lying position, for muscle thickness [79]; across the abdominal wall 10 cm lateral to the umbilicus along the midaxillary line, for muscle thickness [80].
External oblique muscle was evaluated across the abdominal wall over the anterior axillary line, with the probe held transversally at 50% between the 12th rib and the iliac crest, in a supine position with a pillow under head and knees, for muscle thickness [77, 78]; across the abdominal wall 2.5 cm anterior to the anterior axillary line, between the 12th rib and the iliac crest, in a crook lying position, for muscle thickness [79]; across the abdominal wall 10 cm lateral to the umbilicus along the midaxillary line, for muscle thickness [80].
Rectus abdominis muscle (right side) was evaluated to the right of the linea alba, 1 cm superior to the umbilicus during deep inspiration, in a supine position, for muscle thickness [40].
Lumbar multifidus muscle was evaluated between the 4th and 5th interspinous space, in a prone position with a pillow under the abdomen, for muscle thickness [46, 78]; at the 4th vertebrae, between the tip of the zygapophyseal joint and the inferior facial edge of the superior border of the muscle, for muscle thickness and cross-sectional area [82]; between the 4th and 5th lumbar vertebral level, at level of the zygapophysial joints, in prone position, for muscle thickness [81]; between the L4 and L5 regions of the spinal cord, based on the position of the Jacoby line, in a prone position, for muscle thickness and echo-intensity [42].
Quadratus lumborum muscle was evaluated at the abdominal flank above the iliac crest, in a lateral lying position [46].
Head and neck muscles
Temporal muscle was evaluated by a transversally placed transducer at 4 cm lateral from the eyelid and 2 cm above a reference line (horizontal line linking the upper edge of the external auditory canal and the corner of the eyelid), in a lateral lying position, for muscle thickness [83].
Masseter muscle was evaluated at its most prominent area at the same angle as the occlusal plane, for muscle thickness [84]; with the probe placed perpendicular to the anterior margin of the masseter muscle and external surface of the mandibular ramus, between 2 and 2.5 cm above the lower mandibular margin, in a supine position with the molars of both arches touching without pressure (light occlusal contact position), for muscle thickness [85]; by palpation to aid in placing the transducer perpendicular to the direction of the muscle fibers, approximately 2 cm above the mandible branch, for muscle thickness [86].
Suprahyoid muscles (geniohyoid muscle, digastricus muscle and stylohyoid muscle) were evaluated in the frontal plane, halfway between the mandibular mentum and the palpable thyroid cartilage, in a sitting position looking straight ahead with the mouth closed, for cross-sectional area [87].
Genioglossus muscle was evaluated submentally in the midsagittal line, with the probe aimed cranially, in a supine position, for muscle displacement [88].
Geniohyoid muscle was evaluated at 1/3 of the horizontal line between the parotid and the mandible, in a supine position, examination bed inclined at 30°, for muscle thickness and echo-intensity [89].
Tongue muscle (no subdivision) was evaluated perpendicular to the Frankfurt horizontal plane at the first premolar area for evaluation of the middle portion, and at the second premolar and tilted 45° to the Frankfurt horizontal plane for assessment of the base of the tongue, in resting position after swallowing saliva, for muscle thickness and echo-intensity [90].
Neck extensor muscles (trapezius muscle, splenius capitis muscle, semispinalis capitis muscle, semispinalis cervicis muscle and multifidus muscle) were evaluated at the level of the C4 spinous process, in a sitting position, for cross-sectional area [91].
Neck extensor muscles (trapezius, splenius capitis, semispinalis capitis and semispinalis cervicis) were evaluated 2 cm laterally to the C6 spinous process, in a sitting position, for muscle thickness [92].
Sternocleidomastoid muscle was evaluated at the C6 spinous process level at the thickest portion of the muscle, in a sitting position, for muscle thickness [92].
Discussion
The field of US muscle assessment is clearly growing, with more research groups using this technique to give more hands-on information on the muscles described. However, a clear standardization remains absent. This is primarily because for most muscle groups, no information was previously available. Therefore, standardization propositions for 39 muscles/muscle groups encountered will be discussed below.
Patient positioning pre-investigation
Whereas previously a resting period of minimum 30 min was proposed, new data show that changing from a standing to a supine position, after 5 min a normalisation of measurements can occur [22]. Although only muscle thickness, muscle cross-sectional area and echo-intensity were evaluated, this is an important improvement for clinical practice. Letting the patient wait for 30 min to restore natural fluid redistribution is not always feasible. Evolution of change in pennation angle after position change should be studied specifically before recommendation can be made.
Although muscle thickness, muscle cross-sectional area and echo-intensity do not seem to change after 5 min of rest, not all muscle parameters are studies nor is the intensity of exercise prior to examination studies. Therefore, it is still advised to not exercise in the 30 min before investigation.
Adhering to a standard position before starting the measurements is paramount. In supine position, no attention had been given before to the position of the feet. Although no comparative studies have been published, the proposition is that feet should point upwards as to have no external rotation of the leg. The test subject’s legs can be easily supported by pillows to avoid the need for muscle contractions.
One study tested whether the cross-sectional area of the rectus femoris varied depending on the variation in hip flexion/head of bed elevation. Differences between: (a) 0° and 20°, (b) 0° and 30°, (c) 0° and 60°, and (d) 20° and 60° were investigated [93]. Since there was a significant difference, clearly using a standard measurement technique regarding positioning of the patient is paramount in assessing muscle mass. For reassessment of a study subject, the same position should be used as for the first measurements.
Components of muscle
The five main muscle parameters that were described so far remain the same: muscle thickness, muscle cross-section area, pennation angle, fascicle length and echo-intensity.
Regarding muscle thickness, one article suggested that this should be corrected by dividing muscle thickness through the weight of the test subject [91]. Another article suggested this technique of allometric scaling but by dividing muscle thickness through body mass index [94]. As body weight can certainly have an influence on muscle thickness through increase of local muscle-adipose deposits, more data are welcome to support the relevance of this statement. For instance, a positive correlation was found between medial gastrocnemius muscle thickness and calf circumference in older women, but this correlation was weak and non-significant among those with excess weight [95]. Also, no comparative data exist on using either body weight or body mass index.
Regarding muscle cross-sectional area, it should be mentioned that extended field-of-view methods are also used to measure cross-sectional area. However, these were not part of this review.
Regarding echo-intensity, one study showed very good intraclass correlation coefficients (≥ 0.900) and very small standard errors of measurement (≤ 7.26%) [64]. However, there is still no good method of standardization, as no reliable, cheap dummies are available that could repeat these values between both researchers and different ultrasound machine systems.
Regarding pennation angle, a large variance could possibly exist, not only throughout the muscle bulk, but also at the earlier proposed measuring points [96]. The hypothesis is that this is linked to a larger amount of myosteatosis and/or fibrosis. No studies so far described this variance in a clinical setting, making this phenomenon and its clinical implications poorly understood.
Regarding fascicle length, two formulae are currently used in the literature, although one is only used by one article. The latter not only uses pennation angle and muscle thickness, but also the aponeurosis angle (see 4.2.2.). As there are no comparative studies between the two formulae, and also no studies linking the formulae to actual anatomical measurements, more information is needed before recommendations can be done regarding the best formula.
In some muscles/muscle groups, it is not always feasible to measure all ultrasonographic parameters. For the diaphragm for instance, measuring a cross-sectional area is not possible. This is a limitation that cannot be solved. Since it is not yet clear whether some components are more important than others, more studies are needed on this subject.
Besides the five main parameters described earlier, four new parameters are introduced: muscle volume, stiffness, contraction potential and microcirculation.
Regarding muscle volume, being able to measure muscle volume without the use of 3-dimensional ultrasound scanning techniques would prove very interesting. The formula provided only uses muscle thickness and limb length. However, the equation in this study was based upon another study in which only two muscle groups—elbow extensors and knee extensors—were evaluated through ultrasound and MRI [97]. In this study, muscle thickness only contributed to muscle volume for 41.9% (for knee extensors) and 70.4% (for elbow flexors). Also, these are muscle groups, not individual muscles. More studies are needed to correlate total muscle volume to muscle thickness of individual muscles using the proposed equation.
Regarding muscle stiffness, one must realise that during the ageing process, the distribution of the different components of normal muscle mass changes. An increased amount of fibrosis and adipose tissue are the two main features, although other factors, such as advanced glycation end products (AGE’s) can also play a role [98]. This alters the biomechanical properties of the muscle—reflected in muscle stiffness—which can be measured through elastography. Measuring stiffness could potentially give more information about the possible functionality of the muscle (force, strength, relaxation strength, i.e. range of motion) than measuring the different components separately. However, no clear information about the exact changes throughout the muscle bulk during the ageing process is available yet. Also, different types of elastography exist, with no comparison between different systems available. The last limitation for its current use in clinical practice is the cost, as this software option is rather expensive and not included in standard ultrasound systems. Nevertheless, this technique has a lot of future potential.
Regarding muscle contraction potential, the clinical implication of this technique is very unclear. Maximal voluntary contraction of a muscle can be influenced by many factors such as illness, compliance of patients, supportive techniques, pain and fatigue. Cheng et al. [25] measured the contraction potential of swallowing muscle, in an effort to provide solid numbers in a field of research where quantification is very difficult. This technique could of course be used also in larger muscles/muscle groups. Of course, the more often a muscle is contracted, the more voluminous it will get because of the increased blood flow. Strict standardization will be necessary here to ensure the smallest possible bias. If this succeeds, perhaps another more functional assessment will be available.
Regarding muscle microcirculation, one can be brief. Although yet again another potentially very interesting parameter, at this point it is too early to be used in clinical practice. Contrast agents are not yet allowed to be used in each country. Also, no clear information regarding the spreading pattern throughout different types of muscles is available yet. Promising, but not yet feasible.
Measuring points
Some muscles can easily be delineated through the use of specific anatomical landmarks. Others will still require an ultrasonographic visualisation before exact measuring points can be identified. For all the muscles/muscle groups described in this articles, anatomical landmarks and measuring points are proposed in three tables: upper extremity muscles (Table 2), lower extremity muscles (Table 3) and head and trunk muscles (Table 4). For most of these muscles—except for those where the borders of the muscle are to be visualised—example pictures will be provided in the supplemental material.
Remarks regarding measured data
Ultrasound is a technique that has a high inter- and intra-rater reliability. Still, due to various reasons, small variations can always occur. That is the reason why it is still advised to use the mean value of three measurements for all items measured.
Test–retest reliability and validity of muscle size estimation by ultrasound for both curved and linear array transducers seem to be adequate [99]
Some ultrasound systems cannot perform (all) measurements on-screen during the examination. Sometimes, one might want to take image stills and perform the measurements later. For the measurements after the ‘live’ examination, ImageJ is proposed as an easy-to-use, free alternative to more expensive software options.
Conclusion
The emerging field of ultrasonographic assessment of muscle mass only highlights the need for a standardization of measurement technique. Through this article, new insights regarding the use of ultrasound in muscle assessment are addressed and incorporated in measurement propositions for a largely expanded set of muscles/muscle groups. Because of the variety of muscles described, the foundations are laid out for a broad consensus for both muscle research in general and sarcopenia assessment in particular. As already noted, the propositions made in this article are to be viewed as starting points. Future studies will need to help guide the evolution of these modest guidelines to become an evidence-based worldwide consensus.
References
Cruz-Jentoft AJ et al (2019) Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 48(1):16–31
McGregor RA, Cameron-Smith D, Poppitt SD (2014) It is not just muscle mass: a review of muscle quality, composition and metabolism during ageing as determinants of muscle function and mobility in later life. Longev Healthspan 3(1):9
Reinders I et al (2016) Muscle quality and myosteatosis: novel associations with mortality risk: the age, gene/environment susceptibility (AGES)-Reykjavik study. Am J Epidemiol 183(1):53–60
Beaudart C et al (2016) Sarcopenia in daily practice: assessment and management. BMC Geriatr 16(1):170
Nijholt W et al (2017) The reliability and validity of ultrasound to quantify muscles in older adults: a systematic review. J Cachexia Sarcopenia Muscle 8(5):702–712
Mourtzakis M, Wischmeyer P (2014) Bedside ultrasound measurement of skeletal muscle. Curr Opin Clin Nutr Metab Care 17(5):389–395
Tillquist M et al (2014) Bedside ultrasound is a practical and reliable measurement tool for assessing quadriceps muscle layer thickness. JPEN J Parenter Enteral Nutr 38(7):886–890
English C, Fisher L, Thoirs K (2012) Reliability of real-time ultrasound for measuring skeletal muscle size in human limbs in vivo: a systematic review. Clin Rehabil 26(10):934–944
Sanada K et al (2006) Prediction and validation of total and regional skeletal muscle mass by ultrasound in Japanese adults. Eur J Appl Physiol 96(1):24–31
Tandon P et al (2016) A model to identify sarcopenia in patients with cirrhosis. Clin Gastroenterol Hepatol 14(10):1473–1480
Reeves ND, Maganaris CN, Narici MV (2004) Ultrasonographic assessment of human skeletal muscle size. Eur J Appl Physiol 91:116–118
Thomaes T et al (2012) Reliability and validity of the ultrasound technique to measure the rectus femoris muscle diameter in older CAD-patients. BMC Med Imaging 12:7
Abe T et al (2016) Ultrasound-derived forearm muscle thickness is a powerful predictor for estimating DXA-derived appendicular lean mass in Japanese older adults. Ultrasound Med Biol 42(9):2341–2344
Takai Y et al (2014) Applicability of ultrasound muscle thickness measurements for predicting fat-free mass in elderly population. J Nutr Health Aging 18(6):579–585
Takai Y et al (2013) Validity of ultrasound muscle thickness measurements for predicting leg skeletal muscle mass in healthy Japanese middle-aged and older individuals. J Physiol Anthopol 32(1):12
Abe T et al (2015) Validity of ultrasound prediction equations for total and regional muscularity in middle-aged and older men and women. Ultrasound Med Biol 41(2):557–564
Perkisas S, Baudry S, Bauer J et al (2018) Application of ultrasound for muscle assessment in sarcopenia: towards standardized measurements. Eur Geriatr Med 9(6):739–757
The SARCUS project. 2019, February, 9. SARCUS instructional video. Retrieved from https://www.youtube.com/watch?v=y4gSmxwvujE.
van de Glind EM et al (2012) Search filters to identify geriatric medicine in Medline. J Am Med Inform Assoc 19(3):468–472
Moher D et al (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 62(10):1006–1012
Ouzzani M et al (2016) Rayyan-a web and mobile app for systematic reviews. Syst Rev 5(1):210
Lopez P, Pinto MD, Pinto RS (2018) Does rest time before ultrasonography imaging affect quadriceps femoris muscle thickness, cross-sectional area and echo intensity measurements? Ultrasound Med Biol 45(2):612–616
Merrigan JJ et al (2018) Differences in elbow extensor muscle characteristics between resistance-trained men and women. Eur J Appl Physiol 118(11):2359–2366
Alfuraih AM et al (2019) The effect of ageing on shear wave elastography muscle stiffness in adults. Aging Clin Exp Res 31(12):1755–1763
Cheng DTH et al (2018) Sonographic assessment of swallowing in irradiated nasopharyngeal carcinoma patients. The Laryngoscope 128(11):2552–2559
Dunford EC et al (2018) Cardiovascular aging and the microcirculation of skeletal muscle: using contrast-enhanced ultrasound. Am J Physiol Heart Circ Physiol. 315(5):H1194–H1199
Kruse NT, Hughes WE, Casey DP (2018) Mechanistic insights into the modulatory role of the mechanoreflex on central hemodynamics using passive leg movement in humans. J Appl Physiol 125(2):545–552
Alonso-Fernandez D et al (2018) Effects of the Nordic hamstring exercise on the architecture of the semitendinosus. Isokinet Exerc Sci 26(2):81–88
Ikezoe T et al (2012) Associations of muscle stiffness and thickness with muscle strength and muscle power in elderly women. Geriatr Gerontol Int 12(1):86–92
Creze M et al (2018) Shear wave sonoelastography of skeletal muscle: basic principles, biomechanical concepts, clinical applications, and future perspectives. Skeletal Radiol 47(4):457–471
Hug F et al (2015) Elastography for muscle biomechanics: toward the estimation of individual muscle force. Exerc Sport Sci Rev 43(3):125–133
Eby SF et al (2013) Validation of shear wave elastography in skeletal muscle. J Biomech 46(14):2381–2387
Pearson WG Jr, Langmore SE, Zumwalt AC (2011) Evaluating the structural properties of suprahyoid muscles and their potential for moving the hyoid. Dysphagia 26(4):345–351
Dunford EC et al (2018) Cardiovascular aging and the microcirculation of skeletal muscle: using contrast-enhanced ultrasound. Am J Physiol Heart Circ Physiol 315(5):H1194–H1199
Billot M et al (2020) Preserving mobility in older adults with physical frailty and sarcopenia: opportunities, challenges, and recommendations for physical activity interventions. Clin Interv Aging 15:1675–1690
Vincent MA et al (2006) Mixed meal and light exercise each recruit muscle capillaries in healthy humans. Am J Physiol Endocrinol Metab 290(6):E1191–E1197
Inyard AC et al (2007) Contraction stimulates nitric oxide independent microvascular recruitment and increases muscle insulin uptake. Diabetes 56(9):2194–2200
Chang KV et al (2018) Limb muscle quality and quantity in elderly adults with dynapenia but not sarcopenia: an ultrasound imaging study. Exp Gerontol 108:54–61
Hadda V et al (2018) Reliability of ultrasonographic arm muscle thickness measurement by various levels of health care providers in ICU. Clin Nutr Espen 24:78–81
Shah SP et al (2018) Comparison of bedside screening methods for frailty assessment in older adult trauma patients in the emergency department. Am J Emerg Med 37(1):12–18
Michael AE, Young P (2018) Is triceps hypertrophy associated with ulnar nerve luxation? Muscle Nerve 58(4):523–527
Yoshiko A et al (2018) Muscle quality characteristics of muscles in the thigh, upper arm and lower back in elderly men and women. Eur J Appl Physiol. 118(7):1385–1395
Abe T et al (2018) Prediction and validation of DXA-derived appendicular fat-free adipose tissue by a single ultrasound image of the forearm in japanese older adults. J Ultrasound Med 37(2):347–353
Misirlioglu TO, Taskiran OO (2018) Reliability of sonographic muscle thickness measurements of the thenar and hypothenar muscles. Muscle Nerve 57(1):E14–E17
Sterczala AJ et al (2018) Age-related differences in the motor unit action potential size in relation to recruitment threshold. Clin Physiol Funct Imaging. 38(4):610–616
Aboufazeli M et al (2018) Comparison of selective local and global muscle thicknesses in females with and without chronic low back pain. Ortopedia Traumatol Rehabil 20(3):197–204
Akazawa N et al (2018) Relationships between muscle mass, intramuscular adipose and fibrous tissues of the quadriceps, and gait independence in chronic stroke survivors: a cross-sectional study. Physiotherapy. 104(4):438–445
Fukumoto Y et al (2018) Association of physical activity with age-related changes in muscle echo intensity in older adults: a 4-year longitudinal study. J Appl Physiol 125(5):1468–1474
Nozoe M et al (2018) Quadriceps muscle thickness changes in patients with aneurysmal subarachnoid hemorrhage during the acute phase. Top Stroke Rehabil 25(3):209–213
Akazawa N et al (2019) Low body mass index negatively affects muscle mass and intramuscular fat of chronic stroke survivors. PLoS ONE 14(1):e0211145
Watanabe Y et al (2018) Association between echo intensity and attenuation of skeletal muscle in young and older adults: a comparison between ultrasonography and computed tomography. Clin Interv Aging 13:1871–1878
Silva CRD et al (2018) Quadriceps muscle architecture ultrasonography of individuals with type 2 diabetes: reliability and applicability. PLoS ONE 13(10):e0205724
Kawai H et al (2017) Morphological and qualitative characteristics of the quadriceps muscle of community-dwelling older adults based on ultrasound imaging: classification using latent class analysis. Aging Clin Exp Res 30(4):283–291
Harris-Love MO et al (2018) The comparative associations of ultrasound and computed tomography estimates of muscle quality with physical performance and metabolic parameters in older men. J Clin Med 7(10):340
Magrini MA et al (2018) Muscle size, strength, power, and echo intensity, but not specific tension, are affected by age in physically active adults. Isokinet Exerc Sci 26(2):95–103
de Lima FD et al (2018) Cancer-related fatigue and muscle quality in Hodgkin’s lymphoma survivors. Integr Cancer Ther 17(2):299–305
Hida T et al (2018) Ultrasound measurement of thigh muscle thickness for assessment of sarcopenia. Nagoya J Med Sci 80(4):519–527
Murtagh CF et al (2018) The neuromuscular determinants of unilateral jump performance in soccer players are direction-specific. Int J Sports Physiol Perform 13(5):604–611
Blue MNM et al (2018) The effects of high intensity interval training on muscle size and quality in overweight and obese adults. J Sci Med Sport. 21(2):207–212
Franchi MV et al (2018) Muscle thickness correlates to muscle cross-sectional area in the assessment of strength training-induced hypertrophy. Scand J Med Sci Sport 28(3):846–853
Bartolomei S et al (2019) Relationships between muscle architecture, deadlift performance, and maximal isometric force produced at the midthigh and midshin pull in resistance-trained individuals. J Strength Cond Res
Franchi MV et al (2018) Muscle thickness correlates to muscle cross-sectional area in the assessment of strength training-induced hypertrophy. Scand J Med Sci Sports 28(3):846–853
Bey ME et al (2019) Vastus lateralis architecture changes during pregnancy - a longitudinal study. Front Physiol 10:1163
Burton AM, Stock MS (2018) Consistency of novel ultrasound equations for estimating percent intramuscular fat. Clin Physiol Funct Imaging. 38(6):1062–1066
Gaffney KA et al (2018) Nil whey protein effect on glycemic control after intense mixed-mode training in type 2 diabetes. Med Sci Sports Exerc 50(1):11–17
Aubertin-Leheudre M et al (2019) The usefulness of muscle architecture assessed with ultrasound to identify hospitalized older adults with physical decline. Exp Gerontol 125:110678
Simunic B et al (2019) Tensiomyography detects early hallmarks of bed-rest-induced atrophy before changes in muscle architecture. J Appl Physiol 126(4):815–822
Akagi R et al (2018) Muscle size-strength relationship including ultrasonographic echo intensity and voluntary activation level of a muscle group. Arch Gerontol Geriatr 75:185–190
Allison SJ et al (2018) High and odd impact exercise training improved physical function and fall risk factors in community-dwelling older men. J Musculoskelet Neuronal Interact 18(1):100–107
Kato E, Nakamura M, Takahashi H (2018) Effect of compression garments on controlled force output after heel-rise exercise. J Strength Condition Res 32(4):1174–1179
Saito A et al (2018) Age-related changes in muscle elasticity and thickness of the lower extremities are associated with physical functions among community-dwelling older women. Geriatr Gerontol Int. 19(1):61–65
Lee HJ et al (2018) Intra- and inter-rater reliabilities for novel muscle thickness assessment during Co-contraction with dual-rehabilitative ultrasound imaging. Phys Therap Sport 32:109–114
Mickle KJ, Nester CJ (2018) Morphology of the toe flexor muscles in older adults with toe deformities. Arthritis Care Res (Hoboken). 70(6):902–907
McKenna LJ et al (2018) Measurement of muscle thickness of the serratus anterior and lower trapezius using ultrasound imaging in competitive recreational adult swimmers, with and without current shoulder pain. J Sci Med Sport 21(2):129–133
Holtzhausen S et al (2018) An investigation into the use of ultrasound as a surrogate measure of diaphragm function. Heart Lung 47(4):418–424
Cappellini I et al (2018) Recovery of muscle function after deep neuromuscular block by means of diaphragm ultrasonography and adductor of pollicis acceleromyography with comparison of neostigmine vs. sugammadex as reversal drugs: study protocol for a randomized controlled trial. Trials 19(1):135
Aboufazeli M, Afshar-Mohajer N (2018) Within-day and between-day reliability of thickness measurements of abdominal muscles using ultrasound during abdominal hollowing and bracing maneuvers. J Bodywork Mov Therap 22(1):122–128
Emami F, Yoosefinejad AK, Razeghi M (2018) Correlations between core muscle geometry, pain intensity, functional disability and postural balance in patients with nonspecific mechanical low back pain. Med Eng Phys 60:39–46
Kim DK et al (2018) A comparison of ultrasonography measurement on the abdominal muscle thickness between adolescent idiopathic scoliosis and healthy subjects. J Back Musculoskelet Rehabil 31(1):65–74
Mangum LC et al (2018) Ultrasound assessment of the transverse abdominis during functional movement. J Ultrasound Med 37(5):1225–1231
Sutherlin MA et al (2018) Changes in muscle thickness across positions on ultrasound imaging in participants with or without a history of low back pain. J Athletic Train 53(6):553–559
Cuellar WA et al (2019) Vitamin D supplements for trunk muscle morphology in older adults: secondary analysis of a randomized controlled trial. J Cachexia Sarcopenia Muscle 10(1):177–187
Hasegawa Y et al (2019) Temporal muscle thickness as a new indicator of nutritional status in older individuals. Geriatr Gerontol Int 19(2):135–140
Park KM et al (2018) The relationship between masseter muscle thickness measured by ultrasonography and facial profile in young Korean adults. Imaging Sci Dentistry. 48(3):213–221
Poveda-Roda R et al (2018) Myofascial pain: ultrasound width of the masseter muscle. J Oral Facial Pain Head 32(3):298–303
Carletti TM et al (2019) Influence of lower complete denture use on masseter muscles and masticatory function: a longitudinal study. J Oral Rehabil 46(2):127–133
Kajisa E et al (2018) The relationship between jaw-opening force and the cross-sectional area of the suprahyoid muscles in healthy elderly. J Oral Rehabil. 45(3):222–227
Kwan BCH et al (2018) Influence of respiratory mechanics and drive on genioglossus movement under ultrasound imaging. PLoS ONE 13(4):e0195884
Ogawa N et al (2017) Ultrasonography to measure swallowing muscle mass and quality in older patients with Sarcopenic dysphagia. J Am Med Dir Assoc. 19(6):516–522
Chantaramanee A et al (2019) Association between echo intensity of the tongue and its thickness and function in elderly subjects. J Oral Rehabil 46(7):634–639
Goodarzi F et al (2018) The effects of forward head posture on neck extensor muscle thickness: an ultrasonographic study. J Manipulative Physiol Ther 41(1):34–41
Hemelryck W et al (2018) Ultrasonographic assessment of neck muscular size and range of motion in rugby players. Int J Sports Phys Therap 13(1):28–38
Hacker ED, Peters T, Garkova M (2016) Ultrasound assessment of the rectus femoris cross-sectional area: subject position implications. West J Nurs Res 38(9):1221–1230
Linek P (2018) The importance of body mass normalisation for ultrasound measurements of the morphology of oblique abdominis muscles: the effect of age, gender, and sport practice. Folia Morphol (Warsz) 77(1):123–130
Macedo Fraiz G et al (2020) Relationship between muscle thickness and calf circumference in healthy older women. Arch Gerontol Geriatr 86:103942
Infantolino BW, Challis JH (2014) Short communication: pennation angle variability in human muscle. J Appl Biomech 30(5):663–667
Miyatani M et al (2004) The accuracy of volume estimates using ultrasound muscle thickness measurements in different muscle groups. Eur J Appl Physiol 91(2–3):264–272
Drenth H et al (2016) The Contribution of Advanced Glycation End product (AGE) accumulation to the decline in motor function. Eur Rev Aging Phys Act 13:3
Nijholt W et al (2020) Reliability and validity of ultrasound to estimate muscles: a comparison between different transducers and parameters. Clin Nutr ESPEN 35:146–152
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no relevant conflict of interest.
Ethical approval
Not applicable.
Informed consent
Not applicable.
Registration
The protocol for this updated systematic review has been registered at PROSPERO (registration number CRD42019126106) on the 9th of August 2019.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
SARCUS working group on behalf of the Sarcopenia Special Interest Group of the European Geriatric Medicine Society.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Perkisas, S., Bastijns, S., Baudry, S. et al. Application of ultrasound for muscle assessment in sarcopenia: 2020 SARCUS update. Eur Geriatr Med 12, 45–59 (2021). https://doi.org/10.1007/s41999-020-00433-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41999-020-00433-9