Key summary points
To estimate the prevalence of frailty and prefrailty among older adults with chronic pain and review the longitudinal association between frailty status and chronic pain.
AbstractSection FindsFrailty and prefrailty are common in persons with chronic pain. Chronic pain is a risk factor for developing frailty among older persons.
AbstractSection MessageNon-frail older persons with chronic pain are more likely to experience physical frailty after an average follow-up of 5.8 years.
Abstract
Purpose
Frailty and chronic pain are prevalent among older adults. However, no study has systematically reviewed the association between frailty and chronic pain in older adults. Therefore, we aimed to estimate the prevalence of frailty and prefrailty among older adults with chronic pain and review the longitudinal association between frailty status and chronic pain.
Methods
Embase, Medline, Pubmed, and Cochrane library were searched from inception to March 2020. The methodological quality of the studies was assessed using the Newcastle Ottawa Scale. Random effect models and Mantel–Haenszel weighting were adopted to synthesize the estimates.
Results
Among the initial 846 articles retrieved, 24 were included in the review (12 cross-sectional, and 12 longitudinal). The pooled prevalence in persons with chronic pain was 18% (95% CI 14–23%; I2 = 98.7%) for frailty and 43% (95% CI 36–51%; I2 = 98.2%) for prefrailty. The pooled prevalence of chronic pain was 50% (95% CI 45–55%; I2 = 88.3%) for individuals with frailty and 37% (95% CI 31–42%; I2 = 97.1%) for individuals with prefrailty. Persons with chronic pain were 1.85 (95% CI 1.49–2.28; I2 = 93.2%) times more likely to develop frailty after an average follow-up of 5.8 years compared to those without.
Conclusion
Frailty and prefrailty are common in persons with chronic pain. Chronic pain among non-frail older persons significantly predicts the incidence of frailty after an average follow-up of 5.8 years. Future studies should explore the efficacy of different pain management strategies in reducing physical frailty and clarify the association of other types of frailty (cognitive, social and psychological) with chronic pain.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Frailty is a common clinical syndrome characterized by an underlying state of decline in reserve and function due to multisystem dysfunction [1, 2]. Frailty mainly manifests as the vulnerability to internal and external stress and the subsequent inability to restore to previous functional state. This means that even a small disturbance can render the older persons at risk of multiple adverse health outcomes, such as faster functional decline, prolonged hospitalizations, disability, higher health care-related expenses, and higher mortality rates [2,3,4,5]. Currently, there is no existing consensus regarding a standard definition of frailty. However, the frailty phenotype and deficits accumulation model has been extensively validated and widely used to conceptualize frailty. Fried et al. [5] characterized frailty as a purely physical condition of multisystem physiological dysregulation consisting of the presence of three or more of the following five components: weight loss, exhaustion, weakness, slow walking speed, and low physical activity. However, Rockwood et al. [6] defined frailty as predominantly an accumulation of deficits in various areas (symptoms, signs, functional impairment, and laboratory abnormalities). Beyond these two common definitions of frailty, several variations on the diagnostic criteria for frailty have also been developed [7]. Considering the dynamic nature of frailty and its development over time, many definitions also point out an identifiable intermediate stage between frail and non-frail known as prefrailty [8].
Studies have shown that the prevalence of pain in older adults increases with age [9]. Many older adults may have to spend most of their older age tackling the consequences of multiple chronic conditions. Among them, chronic pain is one of the most prevalent and burdensome in later life and it frequently leads to deleterious outcomes, including serious disability from reduced mobility, fall, depression, anxiety, sleep interference, isolation and sarcopenia [9,10,11]. Growing evidence suggests pain-related health consequences is linked to frailty onset and progression [12,13,14,15,16,17,18,19,20,21,22,23]. Chronic pain can be addressed through appropriate approaches, which represents one of the modifiable factors in improving frailty situation or reversing frailty status [9, 11, 24, 25]. The association between pain and frailty was not straightforward: some studies demonstrated that pain could act as a risk factor in increased frailty incidences [12,13,14,15,16,17,18,19,20,21,22,23], while other studies showed that pain is a consequence of frailty [24, 26, 27]. Since pain is a treatable condition, elucidating the association between pain and frailty can pave the way for the prevention, deceleration of progression, or even reversing the course of frailty among older people.
Therefore, the aims of our study were: (1) to perform a systematic review and meta-analysis of all studies regarding the prevalence of chronic pain and frailty and; (2) to perform a systematic review and meta-analysis of prospective studies regarding the longitudinal association between chronic pain and frailty among older persons.
Methods
The systematic review was reported following the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines (Table S1) [28].
Data sources and search strategy
We first conducted a systematic literature search in Embase, Medline, Pubmed and Cochrane library via Ovid SP for observational studies from inception through the end of March 2020 without language restrictions. The keywords were chosen by examining other reviews on similar topics. The detailed search strategies are reported in Table S2. Moreover, references from the selected studies and other relevant reviews were also manually checked to determine their fit for potential candidates as selected studies.
Selection criteria
The titles and abstracts of all the selected articles in the initial search were screened independently by two reviewers (T. Lin and Y. Zhao). If either reviewer thought further evaluation was needed after the abstract screening, a full-text review was carried out against our selection criteria. Any discrepancy regarding the screening and selection of studies or the following extraction of data was resolved through consensus with a third independent reviewer (J. Yue).
Selection criteria were presented as follows: (1) cross-sectional and longitudinal studies that reported information on any of the above-mentioned aims were included; (2) studies reported the association between frailty and chronic pain in individuals aged 60 or older were included. Studies were excluded if: (1) individuals suffering from oncological, acute, or postoperative pain; (2) frailty was defined only with an indicator measurement (timed up-and-go test or gait speed); (3) they were case reports, letters, comments or editorials. When multiple studies used the same cohort, the study on the largest number of participants was selected.
Many cohort studies investigated the longitudinal relationship between frailty and chronic pain. And if the studies also reported frailty/pre-frailty prevalence in chronic pain or chronic pain prevalence in those with frailty/pre-frailty, they were also reported as cross-sectional and their relevant data were extracted.
Data extraction
The following items were extracted independently by two reviewers (T. Lin and X. Xia) from the eligible studies. A third reviewer (N. Ge) reviewed the data extraction, and any disagreement was resolved through consensus. The items included in the data extraction were as follows: the basic information of articles (the first author name, publication year, and cohort name), study design, location, cohort size, female proportion, mean age, follow-up period, the prevalence of frailty/pre-frailty in chronic pain and pain prevalence in frailty/prefrailty older adults, effect measures of interest, pain assessment method and frailty definition. Effect measures adjusted for confounders, such as age and gender, would be included with priority. When an article provided several adjusted models, the model that adjusted for the largest number of confounders was extracted. If the estimates of ORs concerning the association between frailty and chronic pain was not reported in the original study, the relevant data included in the article were used to calculate an unadjusted effect measure.
Quality assessment
The methodological quality of the studies was evaluated independently by the two authors (T. Lin and Y. Zhao) using the Newcastle–Ottawa Scale (NOS) [29]. For observational studies, this validated assessment tool utilizes nine multiple-choice items covering three main domains: the selection of the cohort, comparability of the groups, and quality of the outcomes. The scale scores ranged from 0 to 9 points. Score ≥7 was classified as high quality, 5–7 point as moderate quality, and 4 or less indicated low quality.
Statistical analysis
All statistical analyses were performed using the metan and metainf packages in the STATA/SE (version15.1, Stata Corp, College Station, TX, USA). Two-tailed P value < 0.05 was considered statistically significant. Considering the observational design of the included studies, and the methodological differences that might have contributed to a significant share of the variance within the measures of interest, we obtained the pooled estimates through random effect models and Mantel–Haenszel weighting. Heterogeneity across the studies was assessed using the I2 statistics (significant if I2 ≥ 50%) [30]. Subgroup analysis based on different population was conducted to explore the stratified prevalence of frailty and chronic pain. Sensitivity analysis was performed by omitting each study to check the impact of individual study on the overall results. To limit the impact of extremes or outliers, we also performed sensitivity analyses which excluded studies with small sample sizes (≤ 500 participants, considering that studies with larger sample sizes are more likely to represent the general population. Publication bias was assessed using a funnel plot and Egger’s test (linear regression method) [31].
Results
Literature search
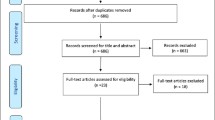
Figure 1 shows the PRISMA flow diagram presenting the literature search as well as the number and reason for study exclusion. The initial search identified 844 records, and the manual review of the references yielded 2 eligible studies. 712 studies were retained after duplications were removed. After screening the titles and abstracts, 638 studies failed our selection criteria, leaving 74 studies for full-text review. Among them, 38 studies were excluded for the following reasons: (1). they did not investigate the aims of the review; (2). they did not provide an explicit definition of frailty or evaluated with a single measure; (3). the pain definition was unreported; (4). they were conference abstracts and there were no complete data. Ultimately, 36 articles were selected for the review and 24 studies were eligible to be assessed for methodological quality and perform the meta-analyses.
Study characteristics
The characteristics of the 24 included studies [12,13,14,15,16,17,18,19,20,21,22,23,24, 26, 32,33,34,35,36,37,38,39,40,41] were summarized in Table 1. The studies involved 39,370 older adults from 13 countries with six studies from North America, two from South America, eight from Europe, three from China, two from Australia and Japan. 22 studies involved community-dwelling older adults, one study [35] enrolled in nursing home individuals, and one study [40] included older adults with HIV. Frailty was defined based on the original or modified version of the Fried phenotype in 17 studies [13,14,15,16, 19, 21,22,23, 26, 32, 34,35,36,37,38, 40, 41], three studies [17, 18, 23] used the Frailty Index (the number of deficits used ranged from 33 to 51), and one study [12] used both methods, whereas another 4 studies used FRAIL scale (n = 2) [23, 39], SOF frailty index (n = 1) [20] and Kihon Checklist (n = 1) [33]. Pain was assessed using different methods among the included studies. Most of them applied a simple question for pain evaluation: “Have you experience chronic pain lasting for several months?” Only nine studies defined pain intensity on a scale, and another seven studies defined specific locations of pain (knee, hip or hand pain). The NOS scores were presented in Table S3.
Cross-sectional association between chronic pain and frailty
Table 1 summarized the cross-sectional prevalence data from all 24 studies [12,13,14,15,16,17,18,19,20,21,22,23,24, 26, 32,33,34,35,36,37,38,39,40,41] on the association between chronic pain and frailty. One study [34] provided specific results concerning the relationship between analgesic use and frailty in community-dwelling older people. For this study, only data on the duration of pain lasting at least 3 months were extracted for our purpose. Another study [40] evaluated frailty and its association with health-related quality of life in older people with HIV. For this study, only data on chronic bodily pain and frailty were extracted. The pooled prevalence in individuals with chronic pain was 18% (95% CI 14–23%; I2 = 98.7%) for frailty (derived from 23 studies; Fig. 2) and 43% (95% CI 36–51%; I2 = 98.2%) for prefrailty (derived from 16 studies; Fig. S1). The pooled prevalence of chronic pain was 50% (derived from 17 studies; Fig. 3) among individuals with frailty (95% CI 45–55%; I2 = 88.3%) and 37% (derived from 13 studies; Fig. S2) among those with prefrailty (95% CI 31–42%; I2 = 97.1%). As high heterogeneity was observed across the above meta-analyses, we limited the analyses to studies with at least 500 participants and the estimates and heterogeneity only changed minimally (data not shown). Additionally, a sensitivity analysis suggested that no individual study significantly affected the pooled prevalence of frailty in pain. We also conducted subgroup analyses based on different population settings to investigate the stratified prevalence of frailty/prefrailty in older persons with chronic pain and the prevalence of chronic pain among older adults with frailty/prefrailty. Results revealed that older persons from a nursing home or suffering from HIV were more susceptible to frailty or a combination of chronic pain. Results also revealed a lack of statistical significance existing between different sub-groups (Supplementary Fig. S3, 4, 5, and 6).
Longitudinal association between chronic pain and frailty
Table 1 summarizes the 12 studies [12,13,14,15,16,17,18,19,20,21,22,23] (a total sample of 27,004 community-dwelling older persons) that evaluated the longitudinal association between chronic pain and the risks for frailty occurrence during the follow-up period. Ten studies [12,13,14, 16,17,18, 20,21,22,23] included in this review provided ORs with multiple confounders adjusted of frailty risks for pain. Two studies [15, 19] did not report relevant effect measures and unadjusted ORs were calculated according to study data. As shown in Fig. 4, non-frail participants who reported chronic pain were 1.85 (95% CI 1.49–2.28; I2 = 93.2%) times more likely to develop frailty after an average follow-up of 5.8 years compared to those who reported no chronic pain. This finding revealed that chronic pain at baseline is a risk factor for frailty incidence. As higher heterogeneity was observed across these studies, we performed a sensitivity analysis by omitting every single study and no statistical significance changed (Fig. S7). The asymmetric funnel plot (Fig. S8) and Egger’s test (P = 0.002) suggested the existence of publication bias or between-study heterogeneity.
One study [14] with all 1705 older men analyzed the longitudinal association between frailty status at baseline and the risk of chronic pain in the future. In multi-adjusted models, those with baseline frailty did not independently increase the risk of developing chronic (OR = 0.82; 95% CI 0.38–1.79) or intrusive pain (OR = 1.38; 95% CI 0.70–2.74). Conversely, this study established that the presence of chronic pain had an increased likelihood of developing frailty among community-dwelling older men (OR = 1.60; 95% CI 1.02–2.51), even after adjusting for potential confounders.
Discussion
This systematic review and meta-analysis revealed that 5 out of 10 frail older adults have chronic pain, while about 2 out of 11 of older persons with chronic pain are frail. 37% of older persons with pre-frailty have chronic pain and 43% of older people with chronic pain present a condition of pre-frailty. The longitudinal studies found that baseline chronic pain increased the likelihood of developing frailty among older adults. Furthermore, only one study has been conducted to determine whether frailty predicts chronic pain incidence while failed to find a significant association between these two conditions.
When older patients suffer from the comorbidity of frailty and chronic pain, the treatment strategies might change. There are two reasons: first, frailty is related to limited life expectancy [42]. The evidence suggested that the expected years of life for frail individuals at age 70 ranged from 0.4 to 5.5 years (female) and 0.1–1.8 years (male) [42]. Treatment plans for those older persons with chronic pain and physical frailty require not only the formulation of tailored therapeutic approaches for chronic pain but also the emphasis of interventions with multidisciplinary physical frailty management [43,44,45,46]. Otherwise, the efficacy and process of pain management may be adversely affected by physical frailty [44, 45]. Second, the pain management strategies based on the existing evidence may not be suitable to be generalized for frail older persons with chronic pain, since older people with frailty usually do not participate in the clinical trials. This is extremely important when considering the observation that 50% of frail individuals studied also have chronic pain and 18% of persons with chronic pain are frail. To date, there have been no clinical trials of treatments for chronic pain which also considered the effects of frailty. Additionally, there are no clear guidelines that make any specific recommendations concerning treatments of chronic pain in frail older persons [9, 46].
Chronic pain and frailty share several mechanisms including sedentary behaviors, malnutrition, and sleep impairment [2, 4, 9, 10]. Frailty is positively associated with sedentary behaviors [47]. Similarly, a sedentary lifestyle is a major contributor to muscle weakness, which in turn leads to further declines in activity levels and loss of muscle mass and strength, causing the development of sarcopenia [48]. Nevertheless, the fear-avoidance behavior in physical exercises leads to the formation of a sedentary lifestyle and the misleading common sense for treatment recommendations is to rest as much as possible, which can be a frequent problem among patients with chronic pain, gradually experiencing mobility impairments and slow gait speed [49, 50]. Both weight loss resulted from sarcopenia, and gait speed reductions are essential components for frailty [5]. In addition, it is well-known that unhealthy dietary behaviors can often be observed in patients with chronic pain [51]. In turn, emotional anxiety, depression, and social loneliness caused by chronic pain may lead to anorexia and even malnutrition [52]. These data emphasize the importance of considering frailty when making therapeutic regimens for chronic pain as well as the importance of developing tailored exercise programs and nutrition interventions for frail patients with chronic pain.
The meta-analysis of longitudinal studies showed a nearly two-fold increase in the likelihood of developing frailty in older adults affected by chronic pain compared with persons without after an average follow-up of 5.8 years. This observation is consistent with the results of a previous systematic review [53]. A possible explanation for our findings could be that chronic diseases, including chronic pain, are generally considered to be the determinants of frailty, and the adverse outcomes, such as disability and malnutrition, induced by chronic pain can result in frailty incidence [5]. It is estimated that 66% of older adults have at least two chronic conditions [54]. Thus, effective and tailored prevention strategies for comorbidity are crucial to reduce the overall disease burden.
Nonetheless, only one longitudinal study assessed the impact of frailty on the development of chronic pain, and no statistical significance was found. Lack of evidence does not mean a lack of significant association between these two conditions. Since there was only one cohort study included, we were unable to conduct a meta-analysis. This finding might be explained by the fact that frail individuals are associated with limited life expectancy and persons with frailty could be more likely to be lost in follow-up [42]. As a result, it is difficult to draw any firm conclusions about the causal association between baseline frailty and future chronic pain.
This study had several unique characteristics. It was the first systematic review and meta-analysis on the prevalence of frailty and prefrailty among older patients with chronic pain. Also, we conducted a comprehensive literature search and rigorous literature selection as well as methodological evaluation, providing a reliable review of the evidence concerning the association between frailty and chronic pain.
However, our study has some limitations. First, substantial heterogeneity was detected among the included studies, which could be explained by the lack of standard diagnostic methods for frailty and chronic pain and by the demographic discrepancies across studies. However, in the meta-analyses of observational studies, heterogeneity is often inevitable, and it does not necessarily invalidate the research results [55]. Second, only one longitudinal analyzed the association between frailty and the development of chronic pain, which limited the opportunity to conclude whether a causal association existed between frailty and chronic pain. Third, there are limited original studies examining the impact of chronic pain on cognitive, social, or psychological frailty. Therefore, more studies are needed to better understand the association between pain and types of frailty other than physical frailty.
Conclusion
We have found that non-frail older persons with chronic pain were more likely to experience physical frailty after an average follow-up of 5.8 years. We also found that frailty and prefrailty were common in older persons with chronic pain. Taken together, our findings suggest that early assessment and effective interventions of chronic pain may help reduce physical frailty and improve the quality of life. Future studies should explore the efficacy of different pain management strategies in reducing physical frailty and clarify the association of other types of frailty (cognitive, social and psychological) with chronic pain.
References
Collard RM, Boter H, Schoevers RA et al (2012) Prevalence of frailty in community-dwelling older persons: a systematic review. J Am Geriatr Soc 60(8):1487–1492. https://doi.org/10.1111/j.1532-5415.2012.04054.x
Clegg A, Young J, Iliffe S et al (2013) Frailty in elderly people. Lancets 381(9868):752–762. https://doi.org/10.1016/s0140-6736(12)62167-9
Fried LP, Ferrucci L, Darer J et al (2004) Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J Gerontol A Biol Sci Med Sci. 59(3):255–263. https://doi.org/10.1093/gerona/59.3.m255
Morley JE, Vellas B, Van Kan GA et al (2013) Frailty consensus: a call to action. J Am Med Dir Assoc 14(6):392–397. https://doi.org/10.1016/j.jamda.2013.03.022
Fried LP, Tangen CM, Walston J et al (2001) Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 56(3):M146–156. https://doi.org/10.1093/gerona/56.3.m146
Rockwood K, Andrew M, Mitnitski A (2007) A comparison of two approaches to measuring frailty in elderly people. J Gerontol A Biol Sci Med Sci 62(7):738–743. https://doi.org/10.1093/gerona/62.7.738
Malmstrom TK, Miller DK, Morley JE (2014) A comparison of four frailty models. J Am Geriatr Soc 62(4):721–726. https://doi.org/10.1111/jgs.12735
Lang PO, Michel JP, Zekry D (2009) Frailty syndrome: a transitional state in a dynamic process. Gerontology 55(5):539–549. https://doi.org/10.1159/000211949
Abdulla A, Adams N, Bone M et al (2013) Guidance on the management of pain in older people. Age Ageing 42(Suppl 1):i1–57. https://doi.org/10.1093/ageing/afs200
Frederick E (2016) Relieving pain in America: a blueprint for transforming prevention, care, education, and research. Mil Med 181(5):397–399. https://doi.org/10.7205/milmed-d-16-00012
Guerriero F (2017) Guidance on opioids prescribing for the management of persistent non-cancer pain in older adults. World J Clin Cases 5(3):73–81. https://doi.org/10.12998/wjcc.v5.i3.73
Rodriguez-Sanchez I, Garcia-Esquinas E, Mesas AE et al (2019) Frequency, intensity and localization of pain as risk factors for frailty in older adults. Age Ageing 48(1):74–80. https://doi.org/10.1093/ageing/afy163
Bindawas SM, Vennu V, Stubbs B (2018) Longitudinal relationship between knee pain status and incident frailty: data from the osteoarthritis initiative. Pain Med 19(11):2146–2153. https://doi.org/10.1093/pm/pnx296
Megale RZ, Ferreira ML, Ferreira PH et al (2018) Association between pain and the frailty phenotype in older men: longitudinal results from the Concord Health and Ageing in Men Project (CHAMP). Age Ageing 47(3):381–387. https://doi.org/10.1093/ageing/afy012
Dapp U, Minder CE, Anders J et al (2014) Long-term prediction of changes in health status, frailty, nursing care and mortality in community-dwelling senior citizens-results from the Longitudinal Urban Cohort Ageing Study (LUCAS). BMC Geriatr 14:141. https://doi.org/10.1186/1471-2318-14-141
Veronese N, Maggi S, Trevisan C et al (2017) Pain increases the risk of developing frailty in older adults with osteoarthritis. Pain Med 18(3):414–427. https://doi.org/10.1093/pm/pnw163
Wade KF, Lee DM, Mcbeth J et al (2016) Chronic widespread pain is associated with worsening frailty in European men. Age Ageing 45(2):268–274. https://doi.org/10.1093/ageing/afv170
Wade KF, Marshall A, Vanhoutte B et al (2017) Does pain predict frailty in older men and women? Findings from the English longitudinal study of ageing (ELSA). J Gerontol A Biol Sci Med Sci 72(3):403–409. https://doi.org/10.1093/gerona/glw226
Ferrer A, Formiga F, Cunillera O et al (2015) Predicting factors of health-related quality of life in octogenarians: a 3-year follow-up longitudinal study. Qual Life Res 24(11):2701–2711. https://doi.org/10.1007/s11136-015-1004-9
Misra D, Felson DT, Silliman RA et al (2015) Knee osteoarthritis and frailty: findings from the multicenter osteoarthritis study and osteoarthritis initiative. J Gerontol A Biol Sci Med Sci 70(3):339–344. https://doi.org/10.1093/gerona/glu102
Wise BL, Parimi N, Zhang Y et al (2014) Frailty and hip osteoarthritis in men in the MrOS cohort. J Gerontol A Biol Sci Med Sci 69(5):602–608. https://doi.org/10.1093/gerona/glt126
Sodhi JK, Karmarkar A, Raji M et al (2020) Pain as a predictor of frailty over time among older Mexican Americans. Pain 161(1):109–113. https://doi.org/10.1097/j.pain.0000000000001711
Yang F, Wang S, Qin H et al (2019) Frailty progress and related factors in the elderly living in community: a prospective study. Zhonghua Liu Xing Bing Xue Za Zhi 40(2):186–190. https://doi.org/10.3760/cma.j.issn.0254-6450.2019.02.012
Shega JW, Dale W, Andrew M et al (2012) Persistent pain and frailty: a case for homeostenosis. J Am Geriatr Soc 60(1):113–117. https://doi.org/10.1111/j.1532-5415.2011.03769.x
Gill TM, Gahbauer EA, Allore HG et al (2006) Transitions between frailty states among community-living older persons. Arch Intern Med 166(4):418–423. https://doi.org/10.1001/archinte.166.4.418
Lohman MC, Whiteman KL, Greenberg RL et al (2017) Incorporating persistent pain in phenotypic frailty measurement and prediction of adverse health outcomes. J Gerontol A Biol Sci Med Sci 72(2):216–222. https://doi.org/10.1093/gerona/glw212
Karp JF, Shega JW, Morone NE et al (2008) Advances in understanding the mechanisms and management of persistent pain in older adults. Br J Anaesth 101(1):111–120. https://doi.org/10.1093/bja/aen090
Moher D, Liberati A, Tetzlaff J et al (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 339:b2535. https://doi.org/10.1136/bmj.b2535
Margulis AV, Pladevall M, Riera-Guardia N et al (2014) Quality assessment of observational studies in a drug-safety systematic review, comparison of two tools: the Newcastle-Ottawa Scale and the RTI item bank. Clin Epidemiol 6:359–368. https://doi.org/10.2147/clep.S66677
Higgins JP, Thompson SG, Deeks JJ et al (2003) Measuring inconsistency in meta-analyses. BMJ 327(7414):557–560. https://doi.org/10.1136/bmj.327.7414.557
Egger M, Davey Smith G, Schneider M et al (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315(7109):629–634. https://doi.org/10.1136/bmj.315.7109.629
Blyth FM, Rochat S, Cumming RG et al (2008) Pain, frailty and comorbidity on older men: the CHAMP study. Pain 140(1):224–230. https://doi.org/10.1016/j.pain.2008.08.011
Hirase T, Kataoka H, Nakano J et al (2018) Impact of frailty on chronic pain, activities of daily living and physical activity in community-dwelling older adults: a cross-sectional study. Geriatr Gerontol Int 18(7):1079–1084. https://doi.org/10.1111/ggi.13314
Koponen MP, Bell JS, Karttunen NM et al (2013) Analgesic use and frailty among community-dwelling older people: a population-based study. Drugs Aging 30(2):129–136. https://doi.org/10.1007/s40266-012-0046-8
Morais D, Terassi M, Inouye K et al (2017) Chronic pain in elderly caregivers at different levels of frailty. Rev Gaucha Enferm 37(4):e60700. https://doi.org/10.1590/1983-1447.2016.04.60700
Tian X, Wang C, Qiao X et al (2018) Association between pain and frailty among Chinese community-dwelling older adults: depression as a mediator and its interaction with pain. Pain 159(2):306–313. https://doi.org/10.1097/j.pain.0000000000001105
Tse MM, Lai C, Lui JY et al (2016) Frailty, pain and psychological variables among older adults living in Hong Kong nursing homes: can we do better to address multimorbidities? J Psychiatr Ment Health Nurs 23(5):303–311. https://doi.org/10.1111/jpm.12303
Coyle PC, Sions JM, Velasco T et al (2015) Older adults with chronic low back pain: a clinical population vulnerable to frailty? J Frailty Aging 4(4):188–190. https://doi.org/10.14283/jfa.2015.75
Nakai Y, Makizako H, Kiyama R et al (2019) Association between chronic pain and physical frailty in community-dwelling older adults. Int J Environ Res Public Health. https://doi.org/10.3390/ijerph16081330
Zeballos D, Lins L, Brites C (2019) Frailty and its association with health related quality of life in older HIV patients, in Salvador Brazil. AIDS Res Hum Retroviruses 35(11–12):1074–1081. https://doi.org/10.1089/aid.2019.0103
Hermsen LA, Leone SS, Smalbrugge M et al (2014) Frequency, severity and determinants of functional limitations in older adults with joint pain and comorbidity: results of a cross-sectional study. Arch Gerontol Geriatr 59(1):98–106. https://doi.org/10.1016/j.archger.2014.02.006
Romero-Ortuno R, Fouweather T, Jagger C (2014) Cross-national disparities in sex differences in life expectancy with and without frailty. Age Ageing 43(2):222–228. https://doi.org/10.1093/ageing/aft115
Makris UE, Abrams RC, Gurland B et al (2014) Management of persistent pain in the older patient: a clinical review. JAMA 312(8):825–836. https://doi.org/10.1001/jama.2014.9405
Vermeiren S, Vella-Azzopardi R, Beckwée D et al (2016) Frailty and the prediction of negative health outcomes: a meta-analysis. J Am Med Dir Assoc 17(12):1163.e1161–1163.e1117. https://doi.org/10.1016/j.jamda.2016.09.010
Romanowski KS, Curtis E, Palmieri TL et al (2018) Frailty is associated with mortality in patients aged 50 years and older. J Burn Care Res 39(5):703–707. https://doi.org/10.1093/jbcr/irx024
Reid MC, Eccleston C, Pillemer K (2015) Management of chronic pain in older adults. BMJ 350:h532. https://doi.org/10.1136/bmj.h532
Kehler DS, Clara I, Hiebert B et al (2019) The association between patterns of physical activity and sedentary time with frailty in relation to cardiovascular disease. Aging Med (Milton) 2(1):18–26. https://doi.org/10.1002/agm2.12059
Landi F, Marzetti E, Martone AM et al (2014) Exercise as a remedy for sarcopenia. Curr Opin Clin Nutr Metab Care 17(1):25–31. https://doi.org/10.1097/mco.0000000000000018
Geneen LJ, Moore RA, Clarke C et al (2017) Physical activity and exercise for chronic pain in adults: an overview of Cochrane reviews. Cochrane Database Syst Rev 4:Cd011279. https://doi.org/10.1002/14651858.CD011279.pub3
Kroll HR (2015) Exercise therapy for chronic pain. Phys Med Rehabil Clin N Am 26(2):263–281. https://doi.org/10.1016/j.pmr.2014.12.007
Meleger AL, Froude CK, Walker J 3rd (2014) Nutrition and eating behavior in patients with chronic pain receiving long-term opioid therapy. PM R 6(1):7–12.e11. https://doi.org/10.1016/j.pmrj.2013.08.597
Feingold D, Brill S, Goor-Aryeh I et al (2017) Depression and anxiety among chronic pain patients receiving prescription opioids and medical marijuana. J Affect Disord 218:1–7. https://doi.org/10.1016/j.jad.2017.04.026
Saraiva MD, Suzuki GS, Lin SM et al (2018) Persistent pain is a risk factor for frailty: a systematic review and meta-analysis from prospective longitudinal studies. Age Ageing 47(6):785–793. https://doi.org/10.1093/ageing/afy104
Ofori-Asenso R, Chin KL, Curtis AJ et al (2019) Recent patterns of multimorbidity among older adults in high-income countries. Popul Health Manag 22(2):127–137. https://doi.org/10.1089/pop.2018.0069
Noubiap JJ, Balti EV, Bigna JJ et al (2019) Dyslipidaemia in Africa-comment on a recent systematic review—Authors’ reply. Lancet Glob Health 7(3):e308–e309. https://doi.org/10.1016/s2214-109x(18)30517-5
Funding
This study was supported by Grants from Chinese National Science & Technology Pillar Program (2020YFC2005600); 1·3·5 project 269 for disciplines of excellence–Clinical Research Incubation Project, West China 270 Hospital, Sichuan University (19HXFH012); National Clinical Research Center for 271 Geriatrics, West China Hospital, Sichuan University (Z20191003).
Author information
Authors and Affiliations
Contributions
TL and JY designed the study, developed the study protocol, and performed all analyses, and oversaw the management of all aspects of the study. All co-author conducted the literature search and participated in screening, full-text review and data extraction. JY and NG advised on analysis and contributed to the interpretation of findings. TL contributed to the writing of the final manuscript and all co-author approved the final version for submission.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Informed consent not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lin, T., Zhao, Y., Xia, X. et al. Association between frailty and chronic pain among older adults: a systematic review and meta-analysis. Eur Geriatr Med 11, 945–959 (2020). https://doi.org/10.1007/s41999-020-00382-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41999-020-00382-3