Abstract
Alzheimer disease is one of the most common and fastest growing neurodegenerative diseases in the western countries. Development of different biomarkers tools are key issues for diagnosis of Alzheimer disease and its progression, in early stages. Electroencephalogram (EEG) signal analysis can be well suited for automated diagnosis of Alzheimer’s disease. This paper focuses on spectral and wavelet features for diagnosis of Alzheimer using EEG signals with effective increase in diagnostic accuracy for improvement in diagnosis in early stage. The use of spectral and wavelet based features are proposed in this paper, with effective increase in classification accuracy by use of supervised classifiers giving 94% diagnostic accuracy for early Alzheimer’s diagnosis. As compared to MRI, PET based diagnosis; the EEG based method of diagnosis of Alzheimer disease is much more cost effective. Frequency bump modeling is used to observe the effect of early stage diagnosis of Alzheimer disease using EEG. This research work of Alzheimer disease using EEG signals, explores new tool in the form of bump modeling of EEG signals; for Alzheimer disease diagnosis in early stages.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Alzheimer disease is one of the Neuro-degenerative diseases which are found to be complex in the present scenario. It is the common form of dementia and by the time it affects the brains cells [1, 2]. Alzheimer Disease (AD) is a chronic Neuro-degenerative disorder that has ranked as third most expensive disease and sixth leading cause of death in United States. The progression of AD is classified into four stages. The first stage is termed as “MCI” i.e. Mild Cognitive Impairment. It usually presents some memory impairment. It retains their abilities in other cognitive domains and functional activities. Some MCI patients (6 to 25%) develop AD. The next stage is characterized by growing cognitive deficit. The second and third stage is termed as Mild AD and Moderate AD, while last stage is termed as Severe AD; it is complete dependent on caregivers. Mild and Moderate AD are key stages, an early diagnosis of AD in these stages can be done and proves beneficial since medications works in this stage. At final stage of disease, patient cannot be cured and hence it can lead to death. Thus, diagnosis in early stage is essential. On the other hand, early diagnosis allows the patients family to take financial decisions related to disease and to plan the future needs involving the care of patient. Electroencephalogram (EEG) basically reflects the electrical activity of large number of cortical neurons which is mainly associated with the neural information processing of brain regions [3, 4]. The Table 1 shows the comparison of different techniques used for Alzheimer disease.
In present scenario, the use of EEG as a diagnostic tool continues to be challenging part in current studies. Focusing on previous studies obtained in literature, none of the existing systems are not clinically or analytically validated. Due to such reasons, the systems require significant improvements.
This paper presents analysis of EEG signals in time and frequency domain for detection of Alzheimer disease in early stage using Spectral and Complexity based features of EEG by use of suitable machine learning algorithm. EEG abnormalities in AD reflect the anatomical and functional deficits of the cerebral cortex damaged by the disease. Nonlinear dynamic analysis (NDA) of EEG reveals out the decreased complexity of EEG signals and reduction in functional connections in Alzheimer patients. Thus, NDA of EEG signals provides valuable information about the progress of the disease as compared to other conventional techniques [10]. In literature, it is observed that Spectral Based features such as EEG relative power, magnitude square coherence, phase synchrony and EEG amplitude modulation energy are widely used which plays a significant role in AD diagnosis giving accuracy of about more than 80%.
The Table 2 summarizes the research done by different researchers. In the proposed research work, the goal is to improve the diagnostic accuracy for classification between two groups.
2 Materials and methods
2.1 Participants information
Standard database for Alzheimer is not available in public domain. Obtaining such database is itself challenging thing. But to have generalized conclusion from the observation one must have sufficient number of database [4]. Database was obtained from SKN Medical College and General Hospital consisting of both mild Alzheimer disease and age-matched healthy patients termed as Normal subjects. The AD patients were diagnosed by experienced Neurologists according to NINCDS-ADRDA criteria and based on Indian version of Mini Mental State Examination and Clinical Dementia Rating (CDR). Multichannel EEG signal recordings were collected from 100 patients separated into 2 groups as shown in Table 3. M represent male and F represent female patients in database.
Patients belonging to the abnormal group, also underwent Single photon emission computed tomography (SPECT) scanning and it was followed for 14–15 months. Even then functional tests such as MMSE and CDR are not enough to set Alzheimer diagnosis. An additional criteria used was the presence of functional and cognitive decline over last 10 months based on interview with knowledgeable informants. The patients were also tested for different disorders such as diabetes, kidney disease, thyroid tests and vitamin B12 deficiency since they can also cause cognitive decline. The EEG signal recordings and the study was approved from Ethical committee of the hospital and participants.
2.2 Test setup for acquisition of EEG data
The EEG recording was done according to International 10–20 electrode placement system as recommended by American EEG society. Recorders and Medicare Systems (RMS) EEG machine was used for recording with 12 bits resolutions and sampling rate of 200 Hz. Twenty-four channel EEG signals were acquired with participants awake, relaxed and their eyes closed for 15–20 min. Impedance of the EEG machine was maintained below 10 M Ohms. EEG signals were Bandpass filtered using third order Butterworth filter between 0.5 and 30 Hz [11]. After successful recording, EEG data has been successfully inspected by Clinical technician. EEG recordings are prone to certain artifacts such as electronic smog, head movement and muscular activity etc. For each subject, one EEG segment of 20 s (termed as ‘epochs’) was extracted for analysis. These epochs are further used in the study for analysis.
2.3 Characteristics of EEG signals for Alzheimer diagnosis
EEG is one of the well-known modality for measuring the electrical activity generated by neurons of the cortex in brain regions. The EEG signals (Bioelectric signals) are recorded noninvasively through a set of electrodes placed on the scalp of human brain according to international 10–20 standards. Now a day’s EEG is not only treated as key diagnostic tool for neurologists but it is more widely used in brain computer interface (BCI) applications. The use of EEG in the early diagnosis of Alzheimer disease is supported by typical abnormalities observed in the literature [3, 12, 13] as follows:
-
(a)
Slowing The slowing effect in EEG signals of Alzheimer patients is associated with the increase of relative power (RP) of the low frequency bands (Delta, 0.5–4 Hz, and Theta, 4–8 Hz), along with reductions in power in high frequency bands (Alpha, 8–12 Hz and Beta, 12–30 Hz).
-
(b)
Reduced complexity Reduced complexity is measured by use of different non-linear features such as Information Theory and other signal processing measures [12, 13]. The non-linear measure shows the increase in regularity in EEG signals of Alzheimer patients.
-
(c)
Loss of synchrony measures The obtained Synchrony measures may be significantly affected by brain events other than changes of synchrony, and by choices (like the reference electrodes) that necessary have to be made during the analysis. Several of the Synchrony measures can be applied such as the Pearson Correlation Coefficient, Magnitude and Phase Coherence, Granger Causality, Phase Synchrony etc. Some typical measures includes Coherence, Granger Measures, state space based synchrony measures, Phase Synchrony (PS) and stochastic event synchrony measures. All these measures seek to quantify the relationships between two or more signals.
3 Analysis of the EEG data
EEG signal is a non-stationary signal and it is quite difficult to analyze it clinically. A brain rhythm exists in time as well as frequency domain. In present work, analysis of EEG signal is explored in time, frequency and time–frequency domain [14, 15]. In this section, Spectral and Complexity based features are explored in detail [16, 17]. These features provide better performance results for distinguishing the subjects between two groups.
3.1 Feature extraction and proposed features
The diagnosis of Alzheimer disease is done using different features such as.
-
1.
Spectral features (measurement of RP in each sub band of EEG signal).
-
2.
Wavelet features (calculation of mean and variance of each wavelet coefficient).
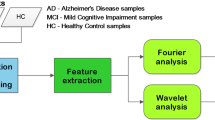
The block schematic for Alzheimer disease diagnosis is shown below in Fig. 1.
Alzheimer Disease diagnosis system consists of three stages as, (1) pre-processing of raw EEG signal, (2) feature extraction of EEG data, and (3) Classification as shown in Fig. 1. Initially, EEG signal is pre-processed to make signals suitable for feature extraction. In pre-processing, artifacts of the signal are removed. In pre-processing stage, the EEG signal is filtered using third order Butterworth band pass filter between 0.5 and 30 Hz. In feature extraction stage, spectral and complexity features have been computed and it is given as an input to classifier. MATLAB (2013b) software is used for implementing the algorithms proposed in present research. Besides this, all algorithms were executed on hardware platform of Laptop, Model: DELL INSPIRON Win7PC, Core i5 @250 GHz, RAM: 8 GB, 64-bit OS.
3.2 Spectral features
Spectrum of EEG signal is affected by neurodegenerative diseases such as MCI and AD. Recent research studies have reported that AD and MCI causes EEG signal to slow down. Slowing effect in EEG signal of AD patients is observed by computation of Relative EEG power in each EEG frequency bands. EEG signal frequency bands are helpful in obtaining important information of the patients. From the results obtained in the study, it is observed that RP is high in low frequency bands (delta and theta bands) i.e. frequency range between 0.5 and 8 Hz. This effect of increase in power of low frequency bands and decrease of power in high frequency bands of EEG signal is observed in AD patients [3, 14, 15, 24]. This irregularity of EEG signal is also quantified by using various standard measures one of those is Lempel–Ziv complexity discussed in [12, 15, 24]. EEG spectrum is helpful in understanding brain activity [26]. For extracting spectral features i.e. RP, EEG signal is classified in specific frequency bands such as: 0.5–4 Hz (delta), 4–8 Hz (theta), 8–12 Hz (Alpha) and 30–100 Hz (Gamma) using wavelet decomposition function Parham [18].
In study, “Daubechies” mother wavelet was used for decomposition of EEG signal into different five sub bands. The reasons for using Daubechies wavelet includes (1) wide smoothing characteristics of it (2) easy understanding of their nature and (3) changes in the EEG signals are easily seen [13]. EEG signals are decomposed using “db2” wavelet at 5th level of decomposition. In present work, EEG RP of four electrodes namely Frontal (F3 and F4), Central, parietal and temporal is computed for all five EEG sub bands.
The power spectral density (PSD) function helps in assessment of spectral characteristics of EEG activity of each epoch. Power in each sub band of EEG signal is computed as Fourier transform of its autocorrelation function. The normalized PSD in frequency range of 0.5–30 Hz is given as,
3.3 Feature extraction using discrete wavelet transform
EEG signal is analyzed at different temporal resolutions using Discrete Wavelet Transform (DWT) technique by its decomposition into several frequency bands by means of scaling and a wavelet function associated with low pass and high-pass filters. For example, EEG signal x(t) can be related as a discrete signal x[i], which is first passed through half-band high pass filter, h[i] and a low-pass filter, g[i]. This filtering of signal which is followed by sub-sampling generates one level of decomposition and it is expressed as,
Where \(a_{1} \left[ k \right]\) and \(d_{1} \left[ k \right]\) are level 1 detail and approximate coefficients at k translation; which are outputs of the high and low-pass filters after process of sub-sampling. This process is termed as Sub-band coding. This process is repeated for further decomposition of signal until no more sampling is possible. In present work, five level of decomposition is done which results in D1 (related to gamma spectral frequency band) through D5 (related upper delta frequency band) and through A5 (related to lower delta) [18].
Mean and variance are given by,
where xi’s are the computed coefficients of the signal at each sub-band, n is the number of coefficients at each band and N is the number of band.
Similarly, variance is calculated by using following formula,
where µ is the expected value. The above calculated values of mean and variance is taken for classification purpose. The necessity of using these features is that they depict the reduced complexity in case of EEG signals of AD patients.
4 Machine learning and classification techniques
Machine learning is technique of programming to optimize a performance criterion based on past experience. The performance of the system is analyzed by means various machine learning algorithms. For testing the performance of system, different classifiers such as Decision tree, KNN, Naive Bayes, Support Vector Machine (SVM) and Multilayer feed forward Neural Network can be been used. Waikato environment for knowledge analysis (WEKA) is also one the technique for classifying the data using JAVA platform. But, in present research work, MATLAB Pattern Recognition toolbox is used for classification. These algorithms are briefly described as follows. There are total 150 samples of data from 100 patients. 2/3rd data samples are used for training and 1/3rd data samples are left for testing to test the performance of the system. Supervised method is used for classification. SVM and KNN (K Nearest Neighbor) classifies are used for classification.
4.1 Performance analysis of the EEG data
The complete system is implemented stepwise considering each process i.e. preprocessing, feature extraction and classification. Each feature was computed and analyzed accordingly for getting better result in terms of accuracy, sensitivity and specificity.
The Fig. 2 shows depicts time–frequency computation [19] of EEG signals for the subjects belonging to three categories (Normal—Fig. 2a and AD—Fig. 2b, and MCI stage—Fig. 2c) with respect to Frontal electrode. EEG signals of Temporal (T), Frontal (F), Central (C) and Parietal (P) lobes of both normal and Alzheimer disease patients were taken into the consideration for study as these electrodes plays significant role in AD diagnosis.
The RP is calculated by means of time–frequency maps. Time–frequency maps of this EEG is quite sparse. Most energy is contained in specific regions of time frequency maps called as “bumps” [15]. The time frequency bumps observed in the EEG signal of the Alzheimer’s disease patients. In case of AD patients, the EEG signal shows the slowing effect due to the neuronal loss observed in the brain regions (cerebral cortex); but this phenomenon is not observed in case of normal subjects. The slowing effect is assume to be proportional with respect to stages of AD.
It is observed that transient oscillations in the EEG signals of MCI and Alzheimer’s disease patients occur more often at low frequencies compared to the normal subjects. This is the signal of severe Alzheimer disease; in which the signal is slower. Thus, cognitive deficits are tremendously affected in this stage. The above figure also shows the time–frequency representation using wavelet transform; along with it the RP is also calculated which is decreased in delta and theta bands in case of Alzheimer’s disease patients. These bumps are not observed in case of Normal patients since they do not exhibit slowing effect.
As observed in Fig. 2c, the frequency bumps are at middle of graph for early stage of AD, while for letter stage of AD the frequency bumps are right corner towards higher time duration are observed in Fig. 2b. There is correlation between the stage of Alzheimer and the observed frequency bumps.
5 Results
The spectral and wavelet features were computed successfully on EEG data. The Table 4 indicates the observed values for above features used which are further used for training and testing of data for classification purpose.
The Table 4 shows the Power calculated in each bands of the EEG signals. Sample I and II consist of Alzheimer disease patients whereas Sample III and IV consist of Normal subjects. The yellow colored boxes concludes that the power in low frequency bands such as Delta and Theta in sample data I and II is increased whereas it shows the decreased power in case of Alpha and Beta bands. The computed values show the power obtained is decreased in case of AD patient which shows that there is less complexity in EEG signal. In case of sample III and IV, it is observed that Power in low frequency bands is low whereas the Power in high frequency bands is high in case of Normal patients (observable mainly in frontal region of brain). In this way, results obtained in the literature are verified using above database used in the study. In the study, “db2” mother wavelet is used at level of decomposition. These mean and variance values are then further extracted for classification purpose for distinguishing between two groups i.e. Alzheimer’s disease patients and Normal subjects. The obtained values for 20 samples are listed in the following Table 5.
The Tables 5 and 6 shows the computation of mean and variance of wavelet based coefficients calculated for both Normal as well as Alzheimer’s disease patients. From the observation of two tables, it is concluded that the values of means and variance in case of Normal patients is high, suggesting that the EEG of Normal subjects is more complex as compared to Alzheimer’s patients. This is due to the growth of Neurons residing in the brain regions. Similarly, the value tends to be decreased in case of Alzheimer’s disease patients due to the neuronal loss of brain cells or death of neurons in the cortical regions. This verifies that an EEG signal of EEG patients tends to be less complex as compared to that of the Normal subjects.
The Fig. 3 shows ‘weka’ tool analysis of Normal and Alzheimer signal each of 20 samples. The blue color signals are normal patients where as red colored signals are Alzheimer patients. The signal indicates that, it’s difficult to classify the patients into Normal or Alzheimer patient’s categories as there is lots of overlapping of features.
The computed values for different features used in different electrodes are used for training and testing purpose. This means 50 patients database was used for training while 50 patients database was left for testing purpose. The reason behind this is that each feature is tested individually during classification and on the similar basis the results were obtained. Based on the features calculated and classifier used, we have calculated the accuracy of classification based on following terminology [21].
The algorithm sensitivity is the “ability of a test to correctly identify those with the disease” (true positive rate), whereas test specificity is the “ability of the test to correctly identify those without the disease” (true negative rate).
The specificity can be defined as the extent to which a diagnostic test is specific for a particular condition.
Where TP stands for True Positive (AD individuals correctly classified), TN stands for True Negative (NC individuals correctly classified), FP stands for False Positives (NC individuals misclassified), FN stands for False Negative (AD individuals misclassified).
The following Table 7 shows results obtained after testing of database after computation of features as discussed in Sect. 3.
Results in terms of classification rates as well as diagnostic accuracy is compared with each spectral and wavelet features using SVM and KNN classifiers. The result in percentage for different features and classifiers is given in Table 8.
Table 9 shows that SVM classifier gives out good classification accuracy with minimum time as compared to that of the KNN classifier. It is due to SVM classifier classifies each input data efficiently in case of high dimensional data. It is also verified that SVM classifier takes less computational time for classification of data.
6 Discussion
In this paper, Spectral and wavelet based features are explored briefly for early Alzheimer disease diagnosis. The objective of current study was to provide better results in terms of classification rates and/or diagnostic accuracy. Improvements in results in terms of classification rates are observed using the proposed features in this paper. Each individual feature was computed and classified by use of both the classifiers discussed in paper. It is also to note that EEG data analysis is mainly done for four brain regions such as Frontal, parietal, central and temporal regions as these are the regions where changes in the brain tends to occurs at initial stage in case of Alzheimer’s. EEG spectrum provides useful information clearly stating the changes occurring in the Alzheimer patients. Spectral features such as RP is useful in discriminating the two subjects for classification in terms of accuracy obtained. RP is computed in each sub band of EEG signal to highlight the difference between spectrum of EEG signal of Normal and Alzheimer affected person. From this EEG spectrum, different non-linear features are also calculated which also provided satisfactory results in terms of diagnostic accuracy and specificity. Appearance of plagues and neurofibrillary tangles in the cortex and decrease in volume of hippocampus slows down the EEG of Alzheimer disease patients. In present study, SPECT scans of Alzheimer patients were also studied with expert clinicians since only functional tests such as MMSE and CDR are not enough to valid the results for diagnosis obtained in present research. It is also to highlight that each band of EEG signal equally contributes for analysis.
7 Conclusion and future work
This work explores the use of EEG as a new tool for Alzheimer disease diagnosis in early stages. It is concluded that when combination of spectral and wavelet features is done together, classification rate as well as diagnostic accuracy obtained is more and it provides comparatively better results than individuals in terms of accuracy (up to 94%). Future work in this study involves making analysis of each frequency bands in depth to observe, whether they carry any other significant information for better diagnosis by means of various signal processing algorithms and features extraction techniques. The slowing down of EEG signal with respect to stages of AD needs to be modeled mathematically and to be verified clinically. The future work includes implementing present algorithms on hardware devices such as DSP processors ASIC (application specific integrated circuits) devices to make a standalone device for diagnosis which might be useful for doctors for correctly diagnosing the patients in early stage.
References
Mattson M (2004) Pathways towards and away from Alzheimer’s disease. Nature 430:631–639
Meek PD, McKeithan K, Shumock GT (1998) Economics considerations of Alzheimer’s disease. J Pharmacother 18:68–73
Van der Hiele K, Vein AA, Reijntjes RH, Westendorp RG, Bollen EL, van Buchem MA, van Dijk JG, Middelkoop HA (2007) EEG correlates in the spectrum of cognitive decline. J Clin Neurophysiol 118(9):1931–1939
Chiang H-S, Pao S-C (2016) An EEG-based fuzzy probability model for early diagnosis of Alzheimer’s Disease. Springer J Med Syst 40(5):1–9
Silverman D (2009) PET in the Evaluation of Alzheimer’s disease and related disorders. Springer, New York
Horvath A, Szucs A, Csukly G, Sakovics A, Stefanics G, Kamondi A (2018) EEG and ERP biomarkers of Alzheimer’s disease: a critical review. Front Biosci (Landmark Ed) 23:183–220
Crosson B, Ford A, McGregor KM, Meinzer M, Cheshkov S, Li X (2010) Functional imaging and related techniques: an introduction for rehabilitation researchers. J Rehabil Res Dev 47(2):7–31
Simpraga S, Alvarez-Jimenez R, Mansvelder HD, Van Gerven JMA, Groeneveld GJ, Poil S-S, Linkenkaer-Hansen K (2017) EEG machine learning for accurate detection of cholinergic intervention and Alzheimer’s disease. Sci Rep 7(5775):1–11
Ruiz-Gómez SJ, Gómez C, Poza J, Gutiérrez-Tobal GC, Tola-Arribas MA, Cano M, Hornero R (2018) Automated multiclass classification of spontaneous EEG activity in Alzheimer’s Disease and mild cognitive impairment. Entropy 20(1)35:1–15
Wan J, Zhang Z, Rao BD, Fang S, Yan J, Saykin AJ, Shen L (2014) Identifying the neuroanatomical basis of cognitive impairment in Alzheimer’s disease by correlation and nonlinearity-aware sparse Bayesian learning. IEEE Trans Med Imaging 33(7):1475–1487
Kulkarni N (2018) Use of complexity based features in diagnosis of mild Alzheimer disease using EEG signals. Int J Inf Technol 10(1):59–64
Czigler B, Csikos D, Hidasi Z, Anna Gaal Z, Csibri E, Kiss E, Salacz P, Molnar M (2008) Quantitative EEG in early Alzheimer’s disease patients power spectrum and complexity features. Int J Psychophysiol 68(1):75–80
Daubechies (2008) Ten lectures on wavelets. Society for Industrial and Applied Mathematics, Philadelphia
Jeong J (2004) EEG dynamics in patients with Alzheimer’s disease. J Clin Neurophysiol 15(7):1490–1505
Dauwels J, Srinivasan K, Ramasubba Reddy M, Musha T, Vialatte F-B, Latchoumane C, Jeong J, Cichocki (2011) A slowing and loss of complexity in Alzheimer’s EEG: two sides of the same coin? Int J Alzheimers Dis 539621
Kang Y, Escudero J, Shin Dae (2015) Principal dynamic mode analysis of EEG data for assisting the diagnosis of Alzheimer’s Disease. IEEE J Transl Eng Health Med 3:1–10
Staudinger T, Polikar R (2011) Analysis of complexity based EEG features for diagnosis of Alzheimer Disease. In: Proc Intl Conf IEEE-EMBC, Boston, USA, pp 2033–2036
Ghorbanian P, Devilbiss DM, Verma A, Bernstein A, Hess T, Simon AJ, Ashra H (2013) Identification of resting and active state EEG features Of Alzheimer’s disease using discrete wavelet transform. Ann Biomed Eng 41(6):1243–1257
Vialatte F, Sole-Casals J, Dauwels J, Maurice M, Cichocki A (2009) Bump time-frequency toolbox: a toolbox for time–frequency oscillatory bursts extraction in electrophysiological signals. BMC Neurosci 10.1:46:1–12
Dai Z, Yan C (2012) Discriminative analysis of early Alzheimer Disease using multi-modal imaging and multilevel characterization with multi-classifier (M3). J Neuroimage Elsevier 59(3):2187–2195
Rueda A, Gonzalez FA (2014) Extracting salient brain patterns for imaging based classification of neurodegenerative diseases. IEEE Trans Med Imaging 33(6):1262–1274
Dauwels J, Vialatte Fr, Cichocki A (2010) Diagnosis of Alzheimer’s disease from EEG signals: where are we standing? Curr Alzheimer Res 7(6):487–505
De Bock T, Das S, Mohsin M (2010) Early detection of Alzheimer’s Disease using nonlinear analysis of EEG via Tsallis entropy. IEEE Biomed Sci Eng Conf 1–4
Cassani R, Falk TH, Fraga FJ, Kanda PAM, Anghinah R (2014) The effects of automated artifact removal algorithms on electroencephalography-based Alzheimer’s disease diagnosis. Front Aging Neurosci 6:1–13
Ahmadlou M, Adeli H (2010) Wavelet-synchronization methodology: a new approach for EEG based diagnosis of ADHD. Clin EEG Neurosci 41(1):1–10
Fattouh A (2016) An emotional model based on wavelet coherence analysis of EEG recordings, BIJIT-BVICAM’s. Int J Inf Technol 8(2):1023–1028
Acknowledgements
The author would like to thank the S P Pune University, India for financially supporting this work under BCUD research Grant (15ENG000865) for researchers and the Sinhgad General Hospital, Pune for their valuable help and support. The author would like to thank all authors of the references which have been used, as well as reviewers of the paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bairagi, V. EEG signal analysis for early diagnosis of Alzheimer disease using spectral and wavelet based features. Int. j. inf. tecnol. 10, 403–412 (2018). https://doi.org/10.1007/s41870-018-0165-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41870-018-0165-5