Abstract
Metals such as Fe, Mn, and Zn act as micronutrients for plants, but at excessive levels, these pose genotoxic, mutagenic, and carcinogenic risks to living beings. The present study was planned to assess the levels of Fe, Mn and Zn in intensively cultivated agricultural soils and crops (berseem, mustard, sugarcane, and sorghum) from six villages situated around the rivers Beas and Sutlej in Punjab, India during pre and post-monsoon seasons. It was observed that the levels of Zn exceeded the maximum permissible limits in some crop samples. Contamination factor calculated for metal contamination indicated high contamination of soil samples with Zn. Onion (Allium cepa) root chromosomal aberration assay revealed high genotoxic potential of soil samples which can be attributed to significant metal contents in soils. The soil-to-plant metal bioaccumulation factor was found to be highest for Zn for mustard in pre-monsoon (2.09) and sorghum in post-monsoon (1.23) seasons. Such high bioaccumulation of Zn in these crops can lead to toxic effects in animals and humans consuming these crops. Thus, steps must be taken to reduce the level of Zn and other metals in the soils of these areas.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Environmental pollutants such as metals pose severe impacts on various ecological and environmental systems. Metallic elements having relatively high density can cause toxic effects in living systems at concentrations above permissible limits (Nagajyoti et al. 2010; Galal et al. 2016).
Soil is a diverse and dynamic system, which plays a vital role for human civilization due to its requirement for agricultural activities (Bhatti et al. 2017). However, soil quality is deteriorating rapidly throughout the globe due to anthropogenic activities such as rapid urbanization, industrialization, intensive agriculture, changes in land use patterns, etc. (Pathak et al. 2015). Metal contamination of agricultural soils is an issue of severe concern due to its potential negative impacts on the agro-ecosystems (Bhatti et al. 2016a; Tian et al. 2017).
Iron (Fe), manganese (Mn), and zinc (Zn) are important metals for plants and act as essential micronutrients (Nagajyoti et al. 2010). Fe is required for various important processes, including photosynthesis, respiration, and chlorophyll biosynthesis, and is essential component of heme proteins (cytochrome, catalase, peroxidase, etc.), the Fe-sulfur cluster proteins (ferredoxin, aconitase, etc.), and other Fe-binding sites (Kobayashi and Nishizawa 2012). Mn is an important constituent of photosynthetic proteins and enzymes in plants. Its deficiency is harmful for chloroplasts due to its effects on the water-splitting system of photosystem II (PSII), which provides the necessary electrons for photosynthesis (Millaleo et al. 2010). Zn is a vital component in several metabolic processes in plants and is required for the six enzyme classes viz. oxidoreductases, transferases, hydrolases, lyases, isomerases, and ligases. It also forms complexes with DNA and RNA and affects the stability of these macromolecules (Broadley et al. 2007; Jain et al. 2010). Thus, these three metals are necessary for survival of plants and must be present in soil at suitable concentrations. The normal contents of Fe, Mn, and Zn in plants growing in uncontaminated environment are 140, 15–100, and 8–100 mg/kg of dry weight, respectively (Misra and Mani 1991; Nagajyoti et al. 2010). The ranges of Mn and Zn in agricultural crops growing in uncontaminated soils are 15–100 and 15–200 mg/kg of dry weight, respectively (Allaway 1968). Though these metals are essential for survival of plants, but when present in excessive contents, they can pose severe threats to plant growth and survival (El-Jaoual and Cox 1998; Rout and Das 2003; Suresh 2005). In addition, these metals can accumulate in important food and fodder crops and, thus, can adversely affect animal and human systems (Bhatti et al. 2016b). Therefore, regular monitoring of the agricultural soils must be carried out to determine contents of these metals. However, analysis of contents of these metals alone is not enough to determine their toxic effects in soils and crops. Further statistical and biological tests must be carried out to determine the level of contamination and toxic genotoxic effects of these metals. Therefore, factors such as Contamination Factor (CF) are calculated for assessment of the level of contamination of soils with metals (Chandrasekaran et al. 2015; Pathak et al. 2015). These factors are based upon comparative analysis of metal contents in contaminated sites with an uncontaminated soil environment (Sakram et al. 2015). Plant assays such as onion (Allium cepa) root chromosomal aberration assay are very effective in analyzing the genotoxic effects of metals in soils (Leme and Marin-Morales 2009). Onion root chromosomal aberration assay is a sensitive, reliable, cost effective, and widely used assay for genotoxicity monitoring of soils (Katnoria and Nagpal 2008; Bhat et al. 2014; Kaur et al. 2014).
Punjab is an important agrarian state of India. The soils of Punjab are highly fertile and perennial rivers (Sutlej, Beas, and Ravi) provide irrigation water for whole year. Usually, the natural concentrations of Fe (0.71–1.65%), Mn (150–414 mg/kg) and Zn (13–17 mg/kg) are at non-hazardous levels in sandy loam textured soils of Punjab as reported by Sharma et al. (1992). However, natural (floods, storms, bedrock erosion, etc.) and anthropogenic activities such as vehicular emissions, addition of organic and inorganic fertilizers, industrial activities such as smelting, steel production and sewage discharge in water systems pose risk of elevation of these metals to dangerous levels in agricultural soils of Punjab (Nagajyoti et al. 2010; Singh et al. 2013; Kumar et al. 2016). The level of these metals in soils of Punjab is also affected by change of seasons due to variation caused by precipitation and other climatic factors. Therefore, the present seasonal study (pre- and post-monsoon) was conducted to analyze the contents of Fe, Mn, and Zn in agricultural soils and four crops viz. berseem (Trifolium alexandrinum), sugarcane (Saccharum officinarum), mustard (Brassica juncea) and sorghum (Sorghum bicolor) from six villages situated on the banks of rivers Beas and Sutlej of Punjab, India. Furthermore, the level of contamination of soils with these metals was analyzed using CF and genotoxic potential of soil samples was assessed using Onion root chromosomal aberration assay. Finally, the study focused on the uptake and accumulation of these metals in the four crops under investigation.
Materials and Methods
Study Area
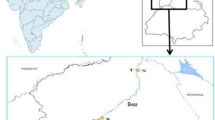
Punjab (Lat. 29º30′–32º32′N and Long. 73º55′–76º50′E) is located in the north-western part of India bordering Pakistan. Agriculture is the main occupation of people of Punjab. Punjab has a continental, semiarid to subhumid climate and two main crop seasons, Rabi (pre-monsoon) and Kharif (post-monsoon). The annual rainfall in Punjab is 435.6 mm. Six villages situated on the banks of rivers Beas and Sutlej were selected for sampling. The names of villages, their districts, adjoining rivers, geographical coordinates, village codes, and crops (sugarcane, berseem, mustard and sorghum) cultivated during pre- and post-monsoon seasons are given in Fig. 1 which shows the map of studied area. The soil of the study area is mostly sandy-loam in texture, slightly alkaline, and calcisolic in nature. The soil organic matter, soil nutrients, and metal contents (Cr, Cu, Cd, Co, and Pb) in these soils were found to be very low in the previous studies (Bhatti et al. 2016a, b, 2017).
Map of the study area showing localization of villages@ around rivers Beas and Sutlej, Punjab, India. @Geographical coordinates of villages given in figure at each village site. Village Information: JA: Village—Jalalabaad; District—Tarn Taran; Adjoining river—Beas (upstream Harike wetland); Crops—Sugarcane (pre-monsoon and post-monsoon). RA: Village—Rajewal; District—Kapurthala; Adjoining river—Beas (upstream Harike wetland); Crops—Berseem (pre-monsoon) and Sugarcane (post-monsoon). YP: Village—Yousufpur; District—Kapurthala; Adjoining river—Sutlej (upstream Harike wetland); Crops—Berseem (pre-monsoon) and Sugarcane (post-monsoon). TT: Village—Tibbi Taiba; District—Firozpur; Adjoining river—Sutlej (upstream Harike wetland); Crops—Berseem (pre-monsoon) and Sugarcane (post-monsoon). DO: Village—Doomniwala; District—Tarn Taran; Adjoining river—Sutlej (downstream Harike wetland); Crops—Berseem (pre-monsoon) and Sorghum (post-monsoon). GA: Village—Gatta Badshah; District—Firozpur; Adjoining river—Sutlej (downstream Harike wetland); Crops—Mustard (pre-monsoon) and Sorghum (post-monsoon).
Sampling and Preparation
Soil sampling was done during pre-monsoon (April–May, 2013) and post-monsoon seasons (September–October 2013). Composite soil samples in triplicates were collected from fields under cultivation of studied crops from each site. At least five subsamples of soil were pooled to form a composite sample. Soil samples were taken from depths of 0–15 cm. Composite samples of the crops under study (whole shoots including leaves and flowers) were collected in triplicates from the corresponding soil sampling fields from each site for the metal analysis. Therefore, a total number of soil samples were 12 (six of pre-monsoon and six of post-monsoon season) and the total number of plant samples was also 12 (six of pre-monsoon and six of post-monsoon season, as described in Table 1). All soil and plant samples were stored in clean polythene bags and were brought to the laboratory. The soil samples were air-dried, ground, and passed through 2 mm sieve for physicochemical and metal analysis. The crop samples were washed with deionised water, oven dried at 70 °C, and then ground-to-fine powder with pestle and mortar.
Metal Analysis
For metal (Fe, Mn, and Zn) determination, 1 g of soil was digested with 15 mL of aqua regia (HNO3: HCl in 3:1 ratio) and 1 g crop (shoot) samples with 15 mL of triacid mixture (HNO3:H2SO4:HClO4 in 5:1:1 ratio) at 80 °C until a transparent solution was obtained (Allen et al. 1986). The digested samples were filtered and diluted with de-ionized water up to 50 mL and analyzed for the metals viz. Iron (Fe), manganese (Mn), and zinc (Zn) using Flame Atomic Absorption Spectrophotometer (FAAS) (Agilent 240 FS AA model). Properly washed glassware, double distilled water, and analytical grade reagents were used throughout the study. Individual standard solutions of selected metals, i.e., Fe, Mn, and Zn, were procured from Agilent (1000 mg/L) and were used to make solutions of varying concentrations by dilution of the standards. After every ten sample readings, the standards were run to assure the working of machine with 95% accuracy (Arora et al. 2008). The limit of detection (quantification) of the instrument (FAAS) for Fe, Mn, and Zn was 7.3, 1, and 1.6 µg/L, respectively.
Metal Contamination Assessment
The level of metal contamination was assessed by calculating CF. This factor is dependent comparison of metal content at a site to a reference/background value (Marrugo-Negretea et al. 2017). Since negligible sources were available regarding the background metal data for soils of the study area, the concentrations of elements in the earth’s crust (Taylor and Mclennan 1995) were taken as the background values as done previously by Sakram et al. (2015). The CF was determined as follows:
Contamination Factor
Contamination Factor (CF) is a reflection of anthropogenic inputs of metals (Ahmed et al. 2016). CF was calculated as per (Hakanson 1980) as
where \(C_{0}^{i}\) is the concentration of metal i from the sampling site, and \(C_{n}^{i}\) is the concentration of metal in reference/background soil environment (Taylor and Mclennan 1995). The CF is classified into four categories by Hakanson (1980), i.e., CF < 1 for low; CF: 1 to < 3 for moderate; CF: 3 to < 6 for considerable; and CF: ≥ 6 for very high contamination.
Analysis of Genotoxic Potential of Soil
The genotoxicity or genotoxic potential of soil samples was analyzed using onion (Allium cepa) root chromosomal aberration assay. Fresh, healthy, and young uniform sized onion bulbs (2n = 16) of about 10–15 g weight were purchased from the local market for the assay. The primary roots and loose outer scales of the onions were removed using forceps. For the treatment, the denuded onion bulbs were grown directly in small pots containing soil samples (five replicates for each sample). Distilled water was used as negative control as described by Rank and Nielsen (1998) and Kaur et al. (2014). The pots containing bulbs were kept in B.O.D. incubator at 20 ± 1 °C for 48 h for rooting. After the treatment, the onion bulbs were thoroughly washed, roots were plucked from the best three onions among the five replicates and preserved in three different vials (for triplicate analysis) in Farmer’s Fluid (glacial acetic acid and ethanol in a ratio of 1:3) for each soil sample (Bhat et al. 2014). After fixation for 24 h, the root tips were hydrolyzed in 1 N HCl for 1 min and then stained in aceto-orcein and 1 N HCl (9:1) with intermittent heating (60 °C) for 10–15 min (Bhat et al. 2014; Kaur et al. 2014). The meristematic zone of root tips was cut and washed in 45% glacial acetic acid for excessive dye removal, squashed under a cover slip on a glass slide, and sealed with DPX (a mixture of distyrene, a plasticizer (tricresyl phosphate) and xylene). All slides were coded and examined under a light microscope for Mitotic Index (MI), i.e., percentage of dividing cells in each slide and Percent Aberrant Cells (PAC), i.e., number of aberrant cells per 100 dividing cells. For each soil sample, 450–500 (approximately 150–200 per replicate) dividing cells were scanned. Two types of aberrations, i.e., physiological (attributed to spindle inhibition) and clastogenic (attributed to direct breaking action on chromosomes) were observed during analysis which included abnormal metaphases, abnormal anaphases, stickiness, delayed anaphases, c-mitosis, chromatin bridges, and chromosomal breaks.
Metal Bioaccumulation Factor (BAF)
Metal concentrations in soil and each crop were calculated on the basis of dry weight. The metal bioaccumulation factor is a ratio of metal concentration of crop to soil (Zhuang et al. 2013) and was calculated as follows:
where Cplant and Csoil are the concentrations of metal in crop and soil, respectively, on a dry weight basis.
Statistical Analysis
The metal content and genotoxicity analysis of different soil samples from each village was done in triplicates, and the average values from the six villages are represented as mean ± SE. The normality distribution of the data was analyzed using Kolmogorov–Smirnov test. Most of the data sets were found to be normally distributed. Following the normal distribution analysis of data sets, one-way ANOVA followed by Tukey’s HSD test as post hoc was used to compare the means of metal contents in soils and crops from the six villages as done in the previous studies from the study area (Bhatti et al. 2017, 2018). The difference between the soil samples of pre-monsoon and post-monsoon seasons was analyzed by Student’s Independent t test. Tukey’s test and Student t test were also used for comparing means of genotoxicity parameters (MI and PAC) in soil samples from six villages. Chi-square test was used for comparing the MI and PAC observed in case of soils samples with negative control (distilled water). Differences at p < 0.05 were considered statistically significant for Tukey’s HSD test, Student’s t test, and Chi square test. Pearson’s correlation coefficients were calculated to analyze the correlation between metals and genotoxic potential (MI and AC) of the soil samples. Statistical analysis was done with the help of IBM SPSS version 16.0 (Chicago, IL, USA) and Minitab version 14.0 (Pennsylvania, USA) and PAST computer software programs.
Results and Discussion
Metal Contents in Soil
The analytical details of Fe, Mn, and Zn contents in soil samples from both seasons are given in Fig. 2. Fe is a major constituent of soil and iron concentrations in soils worldwide range from 20 to 550 g/kg (Bodek et al. 1988) which vary significantly, even within localized areas, due to soil type and presence of other sources. The Fe content in the soils of the present study area varied from 11.02 (GA) to 23.80 g/kg (RA) in pre-monsoon samples (Fig. 2a). The post-monsoon levels of Fe in soils ranged from 13.65 (TT) to 21.25 g/kg (RA). Thus, Fe contents in the present study were low in accordance to the global range of 20–550 g/kg (Bodek et al. 1988). Such low levels of Fe in the present study can be attributed to the sandy texture of soil reported earlier for the present study area (Bhatti et al. 2016a, 2017). Usually, Fe contents are found to be lowest in sandy soils and highest in clayey soils (McGovern 1987). The Tukey analysis showed that the Fe contents in soils in the present study varied significantly among the six villages during both seasons, thus indicating the diversity in soil properties of the study area. However, the seasonal variation (pre-monsoon to post-monsoon) of Fe contents was not significant (Student’s t test) in the six villages. Such non-significant seasonal variation can be attributed to the lithogenic origins of Fe as very few anthropogenic factors are present in the study area which could affect the Fe contents. Therefore, the Fe contents were almost similar in the six villages in both seasons. In addition, the trend of seasonal variation in the six villages was not similar as Fe contents increased in villages JA, YP, and GA, but decreased in RA, TT, and DO. The Fe contents in the present study were found to be higher than the contents reported by Chahal et al. (2014) in agricultural soils of Punjab, India, but lower than the contents reported by Pathak et al. (2015) from Haryana, India.
Heavy metal contents (g/kg) in soil samples. @Described in Fig. 1. Bars showing mean values with standard error of metal contents. Different letters above the bar (lowercase for pre-monsoon, uppercase for post-monsoon) indicate statistically significant difference (one-way ANOVA; Tukey’s test, p ≤ 0.05) among soils of six villages. *Indicates statistically significant difference between pre-monsoon and post-monsoon values of metal contents (Independent Student’s t test, p ≤ 0.5) for soil of same village. a Fe, b Mn and c Zn
Globally, among transition metals, Mn is the second most abundant metal after Fe. The average global upper crustal content of Mn is around 0.60 g/kg and bulk continental crust content is around 1.40 g/kg (Taylor and McLennan 1995). The Mn content in the pre-monsoon samples was found to be minimum for village Gatta Badshah (0.20 g/kg) and maximum for village Rajewal (0.48 g/kg) (Fig. 2b). Among post-monsoon samples, minimum Mn content was found in soil samples from village Tibbi Taiba (0.30 g/kg), while maximum was observed for village Rajewal (0.57 g/kg). As with Fe contents, the level of Mn in soil samples observed in the present study was also below the global average, which can be attributed to sandy texture and low soil organic matter reported earlier for the soils of study area (Bhatti et al. 2016a, b, 2017). The Mn contents in soil observed in the present study were also lower than the limit of 2.0 g/kg prescribed by European Union (2009). The levels of Mn observed among different villages were found to be significantly different in both the seasons (Tukey test). Significant differences were found among pre- and post-monsoon season concentrations of Mn (Student’s t test) for only two villages Rajewal and Gatta Badshah, which indicated the effect of monsoon on observed variations in the contents of metals in soil. However, in similarity to Fe, a clear trend in seasonal variation of Mn contents was not observed, since Mn contents increased in post-monsoon season in villages RA, DO, and GA, but decreased in villages JA, YP, and TT. The Mn contents observed in the present study were found to be in similar ranges to the contents reported by Kaur et al. (2014), but higher than the contents reported by Chahal et al. (2014) in agricultural soils of Punjab, India. Pathak et al. (2015) recorded higher levels of Mn in agricultural soils of Haryana, India, while Galal (2016) recorded lower values of Mn in agricultural soils of Egypt.
Zinc (Zn) is among major constituents of earth’s crust. Globally, Zn concentrations in the soils range from 0.01 to 0.30 g/kg with an average of 0.050 g per kg (Alloway 2008). Zn contents in the present study (Fig. 2c) ranged from 0.06 (GA) to 0.14 g/kg (JA) in pre-monsoon samples and from 0.15 (JA) to 0.28 g/kg (RA) for post-monsoon samples. Thus, in contrast to Fe and Mn, the Zn contents observed in the present study were found to be relatively higher in range as compared to the global average. Overall, Zn contents in soil samples of the present study were below the Indian maximum permissible limits (0.30–0.60 g/kg) given by Awashthi (2000) and maximum permissible limit (0.30 g/Kg) given by European Union (2009). The Indian permissible limits were not available for Fe and Mn. Such low level can also be attributed to the sandy texture and low organic matter of soil in these areas, which leads to leaching of the metals to lower soil layers (Bhatti et al. 2016a). Very less variation was observed in Zn contents of soil among the six villages in both the seasons, but significant variation was observed among seasons (pre- and post-monsoon) for villages Rajewal, Gatta Badshah, Tibbi Taiba, and Yousufpur. In addition, in contrast to Fe and Mn, the contents of Zn increased in post-monsoon season samples in all the villages. Such seasonal variation can be attributed to influx of Zn from various sources (parent rock material from upstream areas, industrial emissions from upstream cities, etc.) due to flooding and silting in these areas during monsoon period. Our study on Zn contents is in line with that of Chahal et al. (2014) who recorded similar range of Zn contents in agricultural soils of Punjab, whereas Kaur et al. (2014) recorded lower levels of Zn from agricultural soils of Punjab, India. Pathak et al. (2015) from Haryana, India, (Marrugo-Negretea et al. 2017) from Colombia recorded higher levels of Zn in agricultural soil in comparison with the contents reported in the present study. On the contrary da Silva et al. (2016) from Brazil, (Galal et al. 2016) from Egypt and Tian et al. (2017) from China reported lower levels of Zn in agricultural soil in comparison with the present study.
Pearson’s correlation analysis (Table 1) showed significant positive correlation among contents of Fe and Mn in soils of the present study. This positive correlation indicated similar sources of these metals which could be natural and anthropogenic. The natural sources of these metals mainly include parent rock material as these three metals are major constituents of earth’s crust (Taylor and McLennan 1995). The anthropogenic sources include irrigation water polluted by industrial effluents and sewage, etc. (Nagajyoti et al. 2010; Kaur et al. 2014; Tian et al. 2017).
Metal Contamination Assessment
Contamination of soils with the analyzed metals (Fe, Mn and Zn) was estimated by calculating Contamination Factor (Table 2). The CF compares the contamination in local environment to a global background level (Khorshid and Thiele-Bruhn 2016). The CF values for Fe and Mn were found to be < 1 in soil samples for both seasons, which indicated that soils analyzed in the present study were very less contaminated with these metals. However, the CF values for Zn were found to be > 1 for pre-monsoon samples and > 3 for post-monsoon samples, which indicated that the soil samples in present study had moderate contamination of Zn in pre-monsoon samples and considerable contamination in post-monsoon samples (Hakanson 1980). Such high CF values indicated anthropogenic impacts in elevation of Zn contents in soil (Tian et al. 2017). The anthropogenic inputs of Zn may come from various sources such as agricultural practices using chemical fertilizers, pesticides, weedicides, manures and sewage sludge, vehicular emissions, and traffic-related activities, and use of irrigation water contaminated with industrial effluents from upstream areas such as Ludhiana and Jalandhar (Sakram et al. 2015; Ahmed et al. 2016; Bhatti et al. 2016a; Khorshid and Thiele-Bruhn 2016). Thus, the CF analysis showed that the soils in the present study were contaminated with Zn in both seasons.
Assessment of Genotoxic Potential of Soils
The genotoxic potential of soil samples was determined using onion root chromosomal assay (Fig. 3). Rooting of onion bulbs in different soil samples revealed variation in Mitotic Index (MI) and induction of different types of chromosomal aberrations such as stickiness, c-mitosis, chromosomal bridges, etc. in root tip cells of onion. MI was observed to range from 8.01 (YP) to 11.78 (GA) in pre-monsoon samples and 10.68 (DO) to 13.61 (TT) in post-monsoon samples. The MI observed for negative control was 6.08. The MI values observed for most post-monsoon samples were relatively higher (statistically significant for villages RA, YP, and TT) than those observed for pre-monsoon samples. This could be attributed to higher levels of Zn in post-monsoon samples as Zn is an important nutrient for root and shoot growth (Nagajyoti et al. 2010). Pearson’s correlation matrix also indicates significant positive correlation between Zn and MI (Table 1). Chi-square test revealed that MI values observed for most of the soil samples were significantly higher (p ≤ 0.5) than the negative control. Higher value of MI in case of soil samples can be a result of increase in cell division which can be harmful as a result of disordered cell proliferation leading to formation of tumor tissues (Hoshina 2002; Leme and Marin-Morales 2009).
Genotoxic potential of different soil samples based on their effects on MI and PAC in Allium cepa root chromosomal aberration assay. @Described in Fig. 1. MI mitotic index; PAC percent aberrant cells. Bars showing mean values with standard error of MI and PAC. Different letters above the bar (lowercase for pre-monsoon, uppercase for post-monsoon) indicate statistically significant difference (one-way ANOVA; Tukey’s test, p ≤ 0.05) among soils of six villages. *Indicates statistically significant difference between pre-monsoon and post-monsoon values of MI and PAC (Independent Student’s t test, p ≤ 0.5) for soil of same village. #On bar indicates statistically significant difference between soil samples and negative control values of MI and PAC (Chi square test, p ≤ 0.5). a Mitotic index (%) and b Percent aberrant cells (%)
Exposure of onion bulbs to different soil samples also resulted in an increase in number of percent aberrant cells (PAC) as compared to negative control (5.73%). The PAC were found to range from 8.28% (DO) to 12.89% (RA) for pre-monsoon samples and 7.45% (DO) to 14.37% (GA) for post-monsoon samples. Student’s t test revealed that the variation in post-monsoon samples for PAC was significant for villages RA and GA only. The PAC observed for most villages was found to be significantly higher (Chi-square test; p ≤ 0.5) than those observed for negative control. This can be attributed to metal contamination of soil samples (Katnoria et al. 2008; Kaur et al. 2014). Pearson’s correlation matrix (Table 1) also revealed significant positive correlation between PAC and contents of Mn. Some positive correlation was also observed between PAC and contents of Fe and Zn. Hence, metal contamination of soils might have contributed to genotoxic potential of these soils.
Metal Contents in Crops
The metal contamination of soil can lead to their uptake and deposition in various plant parts such as roots, leaves, fruits, etc. (Singh et al. 2014). Such uptake and deposition of metals in food and fodder crops is severely dangerous for animal and human health as these metals can enter the animal and human systems and cause health issues (Malik et al. 2017). In the present study, Fe, Mn, and Zn contents were analyzed in food and fodder crops, i.e., sugarcane, berseem and Indian mustard in pre-monsoon season and sugarcane and sorghum in post-monsoon season (Fig. 4).
Heavy metal contents (g/kg) in crops samples. @Described in Fig. 1. Bars showing mean values with standard error of metal contents. Different letters above the bar (lowercase for pre-monsoon, uppercase for post-monsoon) indicate statistically significant difference (one-way ANOVA; Tukey’s test, p ≤ 0.05) among soils of six villages. *Indicates statistically significant difference between pre-monsoon and post-monsoon values of metal contents (Independent Student’s t test, p ≤ 0.5) in crop samples from the same village. a Fe, b Mn and c Zn
The Fe (Fig. 4a) content was found to be minimum in sugarcane from village Jalalabaad (1.07 g/kg) and maximum in berseem from village Rajewal (3.43 g/kg) in pre-monsoon season. In post-monsoon season, the Fe contents were found to be lowest in sugarcane from village Rajewal (0.69 g/kg) and highest in sorghum from village Gatta Badshah (3.71 g/kg). The Fe contents observed in the present study were found to be above the safe limit of 0.45 g/kg given by WHO/FAO (2007) for food crops. Also, Suresh (2005) observed that Fe contents above 0.30 g/kg are toxic to plants. Thus, Fe contents observed in different crops in the present study indicated that Fe was accumulated to dangerous and toxic levels in the crops of the study area. Such high levels of Fe could lead to free radical production in plant tissues that can alter cellular structure irreversibly and cause damage to DNA and proteins (Nagajyoti et al. 2010; Chauhan 2014).
The Mn (Fig. 4b) content in the present study in pre-monsoon crops ranged from 0.07 g/kg in village Doomniwala to 0.14 g/kg in village Rajewal in berseem. In the post-monsoon crops, minimum and maximum contents of Mn were found in sugarcane from village Rajewal (0.05 g/kg) and Tibbi Taiba (0.14 g/kg), respectively. The Mn contents recorded in crops in the present study were below the WHO/FAO (2007) maximum permissible limit of 0.50 g/kg. Clarkson (1988) also noted that Mn contents in the range of 0.03–0.50 g/kg were normal, but above 0.50 g/kg Mn in plants can be toxic. Thus, the Mn contents in crop plants in the present study were below the toxicity levels.
Among the crops analyzed in the present study, maximum (0.21 g/kg in village Yousufpur) and minimum (0.10 g/kg in village Tibbi Taiba) levels of Zn in pre-monsoon season were found in berseem (Fig. 4c). In post-monsoon season samples, maximum (0.22 g/kg in village Yousufpur) and minimum (0.14 g/kg in village Jalalabaad) levels of Zn were found in sugarcane. The Zn levels observed in the present study in many samples (especially in post-monsoon crops) were above the optimum range of 0.15–0.20 g/kg prescribed by Kabata-Pendias (2001). Such high levels of Zn in plants can lead to toxic effects such as chlorosis of leaves, hindrance in root and shoot developments, Mn and Cu deficiencies in plants and oxidative damages to various plant tissues (Rout and Das 2003; Nagajyoti et al. 2010). The levels of Zn observed in sugarcane and Indian mustard in the present study were also above the WHO/FAO (2007) limit of 0.06 g/kg in food crops. Thus, high Zn contents in these food crops are expected to pose severe health threat to humans consuming these crops. Excess amount of Zn uptake by humans can lead to symptoms like vomiting, diarrhea, bloody urine, icterus (yellow mucus membrane), liver failure, kidney failure, and anemia (Fosmire 1990) and can further lead to impairment of growth and reproduction (Duruibe et al. 2007).
In comparison with the present study Galal (2016) observed lower levels of Fe, Mn, and Zn in berseem grown in metal contaminated soils of Egypt. Singh et al. (2014) also observed lower levels of Zn in berseem collected from Uttar Pradesh, India. The contents of Zn observed in Indian mustard in the present study were higher than the contents observed by Khan et al. (2008) in Indian mustard grown on wastewater irrigated soils in Beijing, China. The Zn contents observed in sugarcane samples in the present study were also higher than the contents recorded by Alghobar and Suresha (2015) from India and Wang et al. (2017) from China. In addition, Mn and Zn contents observed in Sorghum in the present study were higher in comparison with the levels of these metals in sorghum recorded by Malik et al. (2017) from Pakistan. Thus, comparative analysis also indicated that the Fe, Mn, and Zn contents recorded in crops in the present study were high and can cause toxic effects in plants, animals, and humans.
Metal Bioaccumulation in Crops
The metal from soils is taken up and accumulated by plants (Galal 2016). To quantitatively analyze the level of uptake and accumulation of metals from soil, the soil to plant Bioaccumulation Factor (BAF) is very useful (Khan et al. 2008; Wang et al. 2017). The BAF values of > 1 indicate significant bioaccumulation of metals in the plants and BAF of > 1 in food and fodder crops can be a cause of concern for humans and animals (Bhatti et al. 2016a).
In the present study, (Fig. 5) BAF values > 1 were observed for Zn in all the pre-monsoon crop samples (berseem, sugarcane, and Indian mustard) and sorghum (village DO) among post-monsoon crops. Thus, BAF values of > 1 for Zn observed in the food and fodder crops indicated that soils and crops analyzed in the present study were highly contaminated with Zn and thus, can pose threat to animal and humans consuming these crops.
Heavy metal Bioaccumulation factor (BAF) in crop samples. @Described in Fig. 1
Therefore, steps must be taken to reduce the levels of these metals (Fe, Mn, and Zn) in the soils of the study area by regulating the anthropogenic inputs of these metals in soils. Phytoremediation of soil from these metals using suitable plant species can provide a green solution for the already contaminated soils. Thus, the reduction of metal contents (especially Zn) in soils of the study would positively lead to reduction in genotoxicity of soil samples and less metal uptake by food and fodder crops.
Conclusions
The contents of Fe, Mn, and Zn which are important nutrients for plants were analyzed in the agricultural soil and crop (sugarcane, berseem, mustard, and sorghum) samples collected during pre- and post-monsoon seasons from 6 villages situated on the banks of rivers Beas and Sutlej in Punjab, India. The study revealed that the contents of these metals in soil samples were below the national and international maximum permissible limits. However, the metal contamination assessment results (using CF) revealed that the soils analyzed in the present study were significantly contaminated with Zn. The genotoxicity analysis of soil samples using onion root chromosomal aberration assay revealed that the soils had significant genotoxic potential which could be attributed to the presence of metals in these soils. The metal analysis in crop samples revealed that the crop samples were highly enriched with Fe and Zn and these metals can cause toxic effects in plants. The metal bioaccumulation analysis in crops indicated very high bioaccumulation of Zn in the crops analyzed in the present study. Overall, soil and crop samples in the present study were significantly contaminated with metals (especially Zn) and steps must be taken to reduce the level of these metals in soils and crops.
References
Ahmed F, Fakhruddin ANM, Imam MDT et al (2016) Spatial distribution and source identification of heavy metal pollution in roadside surface soil: a study of Dhaka Aricha highway, Bangladesh. Ecol Process 5:2. https://doi.org/10.1186/s13717-016-0045-5
Alghobar MA, Suresha S (2015) Evaluation of nutrients and trace metals and their enrichment factors in soil and sugarcane crop irrigated with wastewater. J Geosci Environ Prot 3:46–56
Allaway WH (1968) Agronomic control over the environmental cycling of trace elements. Adv Agron 20:235–274
Allen SE, Grimshaw HM, Rowland AP (1986) Chemical analysis. In: Moore PD, Chapman SB (eds) Methods in plant ecology. Blackwell, Oxford, pp 285–344
Alloway BJ (2008) Zinc in soils and crop nutrition, 2nd edn. IZA and IFA, Brussels and Paris
Arora M, Kiran B, Rani S et al (2008) Heavy metal accumulation in vegetables irrigated with water from different sources. Food Chem 111(4):811–815
Awashthi SK (2000) Prevention of food adulteration act no. 37, of 1954, central and state rules as amended for 1999, Editions, Ashoka Law House, New Delhi
Bhat SA, Singh J, Vig AP (2014) Genotoxic assessment and optimization of pressmud with the help of exotic earthworm Eisenia fetida. Environ Sci Pollut Res 21(3):8112–8123
Bhatti SS, Sambyal V, Nagpal AK (2016a) Heavy metals bioaccumulation in Berseem (Trifolium alexandrinum) cultivated in areas under intensive agriculture, Punjab, India. Springerplus 5:173. https://doi.org/10.1186/s40064-016-1777-5
Bhatti SS, Kumar V, Singh N et al (2016b) Physico-chemical properties and heavy metal contents of soils and kharif crops of Punjab, India. Proced Environ Sci 35:801–808
Bhatti SS, Sambyal V, Singh J et al (2017) Analysis of soil characteristics of different land uses and metal bioaccumulation in wheat grown around rivers: possible human health risk assessment. Environ Dev Sustain 19:571–588
Bhatti SS, Kumar V, Sambyal V et al (2018) Comparative analysis of tissue compartmentalized heavy metal uptake by common forage crop: a field experiment. CATENA 160:185–193
Bodek I, Lyman WJ, Reehl WF (1988) In: Walton BT, Conway RA (eds) Environmental inorganic chemistry: properties, processes, and estimation methods. SETAC special publication series. Pergamon, New York
Broadley MR, White PJ, Hammond JP et al (2007) Zinc in plants. New Phytol 173:677–702
Chahal V, Chand P, Nagpal A et al (2014) Evaluation of heavy metals contamination and its genotoxicity in agricultural soil of Amritsar, Punjab, India. Int J Res Chem Environ 4(4):20–28
Chandrasekaran A, Ravisankar R, Harikrishnan N et al (2015) Multivariate statistical analysis of heavy metal concentration in soils of Yelagiri Hills, Tamilnadu, India—spectroscopical approach. Spectrochim Acta Part A Mol Biomol Spectrosc 137:589–600
Chauhan G (2014) Toxicity study of metals contamination on vegetables grown in the vicinity of cement factory. Int J Sci Res Publ 4(11):1–8
Clarkson DT (1988) The uptake and translocation of manganese by plant roots. In: Graham RD, Hannam RJ, Uren NJ (eds) Manganese in soil and plants. Kluwer, Dordrecht, pp 101–111
da Silva FBV, do Nascimento CWA, Araujo PRM et al (2016) Assessing heavy metal sources in sugarcane Brazilian soils: an approach using multivariate analysis. Environ Monit Assess 188:457. https://doi.org/10.1007/s10661-016-5409-x
Duruibe JO, Ogwuegbu MOC, Egwurugwu JN (2007) Heavy metal pollution and human biotoxic effects. Int J Phys Sci 2(5):112–118
El-Jaoual T, Cox DA (1998) Manganese toxicity in plants. J Plant Nutr 21(2):353–386
European Union. 2009. Heavy metals in wastes. European Commission on Environment. http://ec.europa.eu/environment/waste/mining/studies/pdf/heavymetalsreport.pdf
Fosmire GJ (1990) Zinc Toxicity. Am J Clin Nutr 51(2):225–227
Galal TM (2016) Impact of environmental pollution on the growth and production of Egyptian clover. Int J Plant Prod 10(3):303–315
Hakanson L (1980) An ecological risk index for aquatic pollution control. A sedimentological approach. Water Res 14:975–1001
Hoshina MM (2002) Evaluation of a possible contamination of the waters of the claro river-municipality of Rio Claro, part of the Corumbataí River Basin, with the mutagenicity tests using Allium cepa. 52f. Monograph (Bachelor’s and Teaching degrees). State University of Sao Paulo, Rio Claro, SP (in Portuguese)
Jain R, Srivastava S, Solomon S et al (2010) Impact of excess zinc on growth parameters, cell division, nutrient accumulation, photosynthetic pigments and oxidative stress of sugarcane (Saccharum spp.). Acta Physiol Plant 32:979–986
Kabata-Pendias A (2001) Trace elements in soils and plants, 3rd edn. CRC Press, Boca Raton, p 413
Katnoria JK, Arora S, Nagpal A (2008) Genotoxic potential of agricultural soils of Amritsar. Asian J Sci Res 1(2):122–129
Kaur M, Soodan RJ, Katnoria JK et al (2014) Analysis of physico-chemical parameters, genotoxicity and oxidative stress inducing potential of soils of some agricultural fields under rice cultivation. Trop Plant Res 1(3):49–61
Khan S, Cao Q, Zheng YM et al (2008) Health risks of heavy metals in contaminated soils and food crops irrigated with wastewater in Beijing, China. Environ Pollut 152:686–692
Khorshid MSH, Thiele-Bruhn S (2016) Contamination status and assessment of urban and non-urban soils in the region of Sulaimani City, Kurdistan, Iraq. Environ Earth Sci 75:1171. https://doi.org/10.1007/s12665-016-5972-z
Kobayashi T, Nishizawa NK (2012) Iron uptake, translocation, and regulation in higher plants. Annu Rev Plant Biol 63:131–152
Kumar V, Sharma A, Chawla A et al (2016) Water quality assessment of river Beas, India, using multivariate and remote sensing techniques. Environ Monit Assess 188:137. https://doi.org/10.1007/s10661-016-5141-6
Leme DM, Marin-Morales MA (2009) Allium cepa test in environmental monitoring: a review on its application. Mutat Res 682:71–81
Malik A, Jadoon S, Arooj M et al (2017) Assessment of heavy metals in fodder crops leaves being raised with Hudiara drain water (Punjab-Pakistan). Int J Advanced Eng Res Sci 4(5):93–102
Marrugo-Negretea J, Pinedo-Hernandeza J, Diez S (2017) Assessment of heavy metal pollution, spatial distribution and origin in agricultural soils along the Sinu River Basin, Colombia. Environ Res 154:380–388
McGovern EC (1987) Background concentrations of 20 elements in soils with special regard for New York state. Wildlife Pathology Unit, New York State Department of Environmental Conservation, Albany, New York
Millaleo R, Reyes-Diaz M, Ivanov AG et al (2010) Manganese as essential and toxic element for plants: transport, accumulation and resistance mechanisms. J Soil Sci Plant Nutr 10(4):476–494
Misra SG, Mani D (1991) Soil pollution. Ashish Publishing House, Punjabi Bagh
Nagajyoti PC, Lee KD, Sreekanth TVM (2010) Heavy metals, occurrence and toxicity for plants: a review. Environ Chem Lett 8:199–216
Pathak AK, Kumar R, Kumar P et al (2015) Sources apportionment and spatio-temporal changes in metal pollution in surface and sub-surface soils of a mixed type industrial area in India. J Geochem Explor 159:169–177
Rank J, Nielsen MH (1998) Genotoxicity of wastewater sludge using the Allium cepa anaphase-telophase chromosome aberrations assay. Mutat Res 418:113–119
Rout GR, Das P (2003) Effect of metal toxicity on plant growth and metabolism: I. Zinc. Sustain Agric 23:3–11. https://doi.org/10.1051/agro:2002073
Sakram G, Machender G, Dhakate R et al (2015) Assessment of trace elements in soils around Zaheerabad Town, Medak District, Andhra Pradesh, India. Environ. Earth Sci. 73:4511–4524
Sharma BD, Sidhu PS, Nayyar VK (1992) Distribution of micronutrients in arid zone soils of Punjab and their relation with soil properties. Arid Soil Res Rehabil 6:233–242
Singh CK, Rina K, Singh RP et al (2013) Geochemical characterization and heavy metal contamination of groundwater in Sutlej River Basin. Environ Earth Sci. https://doi.org/10.1007/s12665-013-2424-x
Singh SN, Goyal SK, Rai JP et al (2014) Heavy metal accumulation in plants grown in peri-urban metal contaminated areas of eastern UP. Agriways 2(1):8–11
Suresh S (2005) Characteristics of soils prone to iron toxicity and management—a review. Agric Rev 26(1):50–58
Taylor SR, Mclennan SM (1995) The geochemical evolution of the continental crust. Rev Geophys 33:241–265
Tian K, Huang B, Xing Z et al (2017) Geochemical baseline establishment and ecological risk evaluation of heavy metals in greenhouse soils from Dongtai, China. Ecol Ind 72:510–520
Wang XF, Deng CB, Xu GP et al (2017) Heavy metals in soil and sugarcane accumulation in agricultural area along Huanjiang river in Guangxi, China. J Residuals Sci Technol 14(1):143–154
WHO/FAO. 2007. Joint FAO/WHO Food standard programme codex alimentarius commission 13th session. Report of the thirty eight session of the codex committee on food hygiene, Houston, United States of America, ALINORM 07/30/13
Zhuang P, Li Z, Zou B et al (2013) Heavy metal contamination in soil and soyabean near the Dabaoshan mine, South China. Pedosphere 23(3):298–304
Acknowledgements
The authors acknowledge UGC for financial support under UPE, CPEPA and DRS-SAP programmes and Head, Department of Botanical and Environmental Sciences, Guru Nanak Dev University, Amritsar for laboratory facilities.
Funding
This work was supported by the University Grants Commission, New Delhi India with Rajiv Gandhi National Fellowship for SC/ST students under Grant No. “F1-17.1/2012-13/RGNF-2012-13-SC-PUN-22652/(SAIII/Website)”, awarded to Sandip Singh Bhatti (First author).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Bhatti, S.S., Sambyal, V. & Nagpal, A.K. Analysis of Genotoxicity of Agricultural Soils and Metal (Fe, Mn, and Zn) Accumulation in Crops. Int J Environ Res 12, 439–449 (2018). https://doi.org/10.1007/s41742-018-0103-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41742-018-0103-1