Abstract
Heavy metal pollution in river sediments caused by industrialization and urbanization is a pressing environmental issue around the world. This issue is more serious in the rapidly industrializing countries like China. In this study, the contamination and sources of nine metals (Al, As, Cd, Cr, Cu, Hg, Ni, Pb and Zn) in 134 river sediments in Nantong, Eastern China have been analyzed using various statistical and spatial analysis techniques. Contamination level assessments using enrichment factor (EF), geo-accumulation index (Igeo), and ecological risk index (RI) revealed that rivers suffer severe heavy metal pollutions, especially for Pb and Zn. Four main potential sources of the metals were identified using principal component analysis (PCA) and cluster analysis (CA). They are: (1) industrial sources contributing As, Cr, Pb, Zn, and partly Cu, (2) parent materials contributing Al, Ni, and partly Cu, (3) municipal and domestic wastes associated with Hg, and (4) excessive fertilizer application responsible for Cd. The potential sources were further verified by comparing their spatial distributions and the locations of the actual local sources using GIS (Geographic Information System)-based spatial analysis. The results are useful for environmental protection agencies to target corresponding pollution sources for the contaminations of specific heavy metals.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heavy metals are toxic and persistent, and can accumulate in aquatic environment, so their distribution and contamination in rivers and river sediments are great environmental concerns around the world. They have been intensively studied, especially in the developing regions and countries that are experiencing rapid urbanization and industrialization, such as China, India, Bangladesh, Southeast Asia, and North Africa (Davutluoglu et al. 2011; Möller and Einax 2013; Nehme et al. 2014; Yuan et al. 2014; Azhari et al. 2016; Islam et al. 2016; Nguyen et al. 2016; Bhuyan et al. 2017, Krika and Krika 2017). When heavy metals are discharged into rivers by various anthropogenic activities, they are rapidly adsorbed by suspended matters. The suspended matters carrying heavy metals will eventually sink into the river sediments and then the heavy metals possibly become long-term pollution sources to surrounding environment (Chen et al. 2014). Heavy metals in river sediments can be taken into aquatic plants and seafood, such as fish, shrimps, mussels, and oysters, and accumulate, and then ultimately enter human body via the food chain and lead to health issues (Dhanakumar et al. 2015; Bhuyan et al. 2017). For example, ingestion of inorganic arsenic into human body can result in increased risks of internal cancers, including bladder and lung cancer (Cuzick et al. 1992; Smith et al. 1998; Chai et al. 2010). Cadmium is related to kidney damage, cancers, diabetes, and high blood pressure (Wang et al. 2011b; ATSDR 2018; Chowdhury et al. 2016). Among the heavy metals, As, Cd, Pb, Cr, Cu, Hg, and Ni are of particular concern because of their effects on human health and presence at relatively high concentrations in water, especially in developing countries (ATSDR 2018; Chowdhury et al. 2016). Thus, a good understanding of the contamination levels and sources of heavy metals in river sediments is crucial for watershed management, pollution control, and human health protection.
Heavy metals in river sediments come from both natural sources (e.g., parent materials) and anthropogenic sources (e.g., industrial, agricultural, and residential activities), while the change in heavy metal contamination is usually affected by the changing anthropogenic activities, and so the control of anthropogenic sources is the best way to control heavy metal contamination in river sediments. The contamination levels and sources of the heavy metals in river sediments are associated with the intensity and types of anthropogenic activities and the pollution control policies in the local areas. Thus, in order to understand and control the heavy metal pollution in river sediments for a specific area, it is necessary to understand the contamination levels and sources of heavy metals in the area.
Nantong is located in the Yangtze River Delta in eastern China. It has been experiencing the more rapid industrialization and urbanization than the majority areas in the world over the past nearly four decades. This area is also one of the most populous regions in China and an important agricultural region. The conflict between the rising demand of clean water and food associated with population growth and the increasing farmland loss and water pollution due to urbanization and industrialization has become a pressing issue in this area. Nantong district is a typical plain river network region with numerous crisscross rivers, which conveniently connect urban and rural areas, as well as the irrigation (or drainage) system of farmland. With this kind of river network, heavy metals from industrial and domestic waste discharges are more likely to be transported into river ecosystem and are of great harmfulness. Therefore, it is necessary to obtain the valuable information on concentrations, contamination levels, and sources of various metals in river sediments in this area. Systemic sampling along the entire river networks, contamination assessment through various statistical analyses, and spatial analysis on the relationship between heavy metal concentrations and anthropogenic activities can help scientists and government agencies to determine the contamination levels and to identify the sources of heavy metals and then to make effective policies to control the pollution. However, to the best of our knowledge, very few studies that involve intensive statistical and spatial analyses have been carried out in this area. Furthermore, previous studies in this area have been only focused on river sediments in urban or individual trunk rivers, as well as the Yangtze River estuary, not in the entire river networks that also cover agricultural areas (Wu and Shen 2012; Zhang et al. 2012; Wang et al. 2014; Liu et al. 2016; Han et al. 2017).
In the previous studies on heavy metals in river sediments around the world, enrichment factor (EF), geo-accumulation index (Igeo), and ecological risk index (RI) were widely used to assess the contamination levels and the potential biological effects (Wang et al. 2014; Azhari et al. 2016; Chen et al. 2016; Chai et al. 2017; Vu et al. 2017). Correlation analysis, cluster analysis (CA), and principal component analysis (PCA) were commonly used to identify the sources of heavy metals (Möller and Einax 2013; Wang et al. 2014; Azhari et al. 2016; Nguyen et al. 2016; Bhuyan et al. 2017; Liao et al. 2017; Lu et al. 2017). Most of the previous studies only identified the sources were either natural or anthropogenic, without specifying the detailed types of anthropogenic activities that might be responsible for different heavy metals. However, in order to effective control healy metal pollution, it is necessary to distinguish the specific anthropogenic activities (e.g. industrial, agricultural, or residential) for different metals. In addition, the contributing sources of heavy metals identified by previous studies are the potential ones assumed by the researchers based on the literature review and their general understanding of the chemical and physical properties of the heavy metals. Usually those potential sources were not verified by comparing them to the actual pollution sources in the local places, which might reduce the reliability of the findings.
In this study, we conducted systemic sampling, contamination and risk assessment methods, multivariate statistics, and GIS-based spatial analysis approaches to assess the contamination levels and to identify the sources of heavy metals in river sediments distributed in the entire river network in Nantong district. It aims to (1) assess the contamination levels of heavy metals using EF, Igeo and RI; (2) identify the potential contributing sources of heavy metals using correlation analysis, CA, and PCA; (3) verify the identified potential sources by spatial analysis. This study can improve scientists’ understanding of the contamination levels and the anthropogenic sources of heavy metals in river sediments and provide reliable baseline information to environment protection agencies to develop heavy metal pollution control policies.
Materials and Methods
Study Area
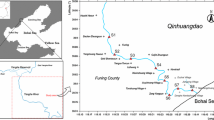
Nantong District, adjacent to Shanghai, lies on the north edge of the mouth of the Yangtze River. It has a total population of about 8 million with an area of 8000 km2. Located in the Yangtze River Delta, Nantong has the landform of alluvial plain with an average elevation of about 4 m. It has a typical fluvo-aquic soil developed on calcareous deposits in the alluvial plain. It contains over 570 rivers and channels with the total length of 866.9 km, which form extraordinary developing water network and finally connect to the Yangtze River and the Yellow Sea. The rivers are in microtidal environment and their hydrodynamics are controlled mainly by wind forcing and river flow. With an average annual precipitation of about 1040 mm (mainly falling between June and August) and an average annual temperature of about 15.1 °C, it has a typical warm north subtropical climate. With the warm and humid climate, abundant water supply, and rich soil, the study area is an important agriculture base to produce crops and vegetables, well known as “the land of fish and rice”.
On the other hand, Nantong is also one of the areas that have been experiencing the most rapid urbanization and industrialization over the past nearly 40 years in China. It has established various industries, including chemicals, metallurgy, engineering, shipbuilding, and textile. Thousands of textile mills on different scales are distributed in the study area. For example, the town of Jiangzhao has 918 industrial enterprises, of which 809 (88.1%) are home textile enterprises. Domestic textile enterprises contributed the industrial output value of 7.2 billion yuan in 2006. Its textile industry can be dated back to 1980. The town of Zhuhang is known as the country of wire rope because the wire rope industry has become its leading industry. The number of wire rope enterprises reached 248 in the prosperous period and its products are sold to all around the world. The effluents from those industries are discharged into the aquatic system with very little or improper treatment. In addition, in the process of urban development and city growth, a large number of floating population living in urban villages generate domestic effluents without any treatment before randomly discharged into rivers. The huge amount of effluents without much treatment from the industrial and domestic activities have caused serious water pollution issues in the area. For instance, Tongqi River is an important river crossing the study area in East–West direction. The total wastewater discharge in Tongqi River Basin reached 2.9 million tons in 2010, of which printing and dyeing industry and metal products industry accounted for 99.3% of the total amount. As a result, low level of dissolved oxygen in river water and high concentrations of heavy metals in sediment were found (NWCB 2010). The most serious polluted areas were in Guanyinshan and Zhuhang districts (NWCB 2010). Moreover, to enhance the yields of rice and to maintain the fertility of soil, the application of chemical fertilizers to farmlands has been increasing in recent years. For example, its use of chemical fertilizers reached 333 kg Ha−1 and some area attained 510 kg Ha−1 in 2007, which distinctly exceeded 255 kg Ha−1, the upper limit of the world mean allowance (Liu 2015). The chemical fertilizers, especially phosphatic fertilizers, generally contain high level of toxic metals such as Cd and Zn (Huang et al. 2007). These metals may ultimately enter river sediments by agricultural runoff and the erosion of agricultural soil. Thus, the river network is seriously exposed to the heavy metal pollution from the combination of industrial, domestic, and agricultural sources. It is quite urgent to gain a good understanding of the pollution level and sources of heavy metals in river sediments in the study area.
Sample Collection and Processing
Figure 1 shows the locations of the 134 sampling sites in Nantong. They are generally evenly distributed in the outlets of the rivers that accept runoff and sewage from urban, residential and industrial areas and that provide irrigation water for agriculture. Surficial sediment samples were collected in September 2014, using a self-made grab (15 cm × 15 cm × 23 cm) aboard a wooden boat. These samples were taken either in the middle or near the margins of the rivers’ active channels. To ensure the data to be representative and reproducible, we first took samples at 2–3 different spots within a distance up to 30 m in the river bed at each site, and then mixed them (Ridgway and Midobatu 1991).
Immediately after the sampling, we stored all the samples in polyethylene (PE) bags at 4–6 °C until laboratory analysis. Afterwards, we freeze dried the samples at − 45 °C for seven days in laboratory, then crushed (20 mesh) and divided the dry samples. 200 g sediment was then hand-ground to fine particles (< 0.074 mm) using an agate grinder, and then was stored in PE vials.
Analytical Procedure
We placed each dry sediment sample with the weight of about 1 g in a Teflon bomb and then treated it using solutions in the order of HCl (3 ml), HNO3 (9 ml), HF (2 ml), and HClO4 (8 ml). The graphite furnace was used to measure the concentration of Cd and the cold vapor atomic spectrometry was applied to determine the concentrations of As and Hg. The X-ray fluorescence spectrometry was employed to measure Al, Cr, Cu, Ni, Pb and Zn. Following the matrix correction method described by Franzini et al. (1975), we converted the X-ray counts to concentrations. The GBW07302 Standard Sediment was used to check the accuracy of the metal analysis. The analytical accuracy, relative standard deviation, and detection limit of the metal analysis are shown in Table 1. The accuracies of the nine metals were all higher than 94%. The relative standard deviations were low, ranging from 1.2 to 7.6%.
Statistical Analysis
We first standardized the metal concentrations to the Z score (mean = 0; standard variation = 1) and then applied the Ward’s method to classify them (Ward 1963; Mihailović et al. 2015). Afterwards, we analyzed the data using descriptive analysis, Pearson’s correlation analysis, CA, and PCA in SPSS. Varimax rotation with Kaizer’s normalization was applied in PCA (Micó et al. 2006; Mihailović et al. 2015). The CA and PCA results were used to identify different types of pollution sources.
Contamination and Risk Assessment Methods
Enrichment Factor
We calculated the enrichment factor (EF) for each metal to analyze its sources (anthropogenic or natural) and to evaluate the degree of anthropogenic contamination. The EF was calculated by normalizing the metal concentration to the concentration of reference element, Aluminum, which is usually associated with natural sources (Buat-Menard and Chesselet 1979). The equation of the EF calculation is shown as follows:
where the ratio of the studied metal concentration (M) to the Aluminum concentration in the samples was compared to that in the baseline. The soil background values (B n ) in Nantong district were used as the baseline in this study. It is difficult to establish the soil B n in the study area, due to the great geochemical variability of various districts and different anthropogenic impacts. B n values were obtained from 545 samples throughout the study area based on elimination of mean value plus or minus twice the standard deviation. These sampling stations, with the depths of 150–200 cm and rarely influenced by anthropogenic sources, were executed in a multi-objective geochemical survey of Jiangsu Province. Our choice appears reasonable by comparing our B n values with the “average shale” (Turekian and Wedepoh 1961) and the results reported by Wu and Shen (2012). Our B n values for Ni and Hg are much lower than the values from the previous two studies. In addition, the B n values from the previous two studies are also higher than those reported by Liao et al. (2011). Hence, the choice of B n in this study is reasonable (Table 2).
Geo-accumulation Index
The geo-accumulation index (Igeo) was originally defined by Muller (1969) for metal concentrations in fine fraction and developed for the global standard shale values, which is calculated as follows:
where C n is the measured concentration in the sediment for the metal n, B n is the geochemical background value of metal n, and 1.5 is used as a factor to minimize possible variation in the background value due to geogenic effects.
Ecological Risk Index
The potential ecological risk index (RI), originally developed by Hakanson (1980), was applied to evaluate the potential ecological risk of multiple elements. The sensitivity of the biological community can be reflected by RI when it considers the potential ecological risk factor and the toxicity response coefficient. RI is calculated as:
where \(C_{s}^{i}\) is the concentration of each metal in the sediment sample, \(C_{n}^{i}\) is the background value of each metal, \(C_{f}^{i}\) is the pollution factor of each metal, \(T_{r}^{i}\) is the toxicity response coefficient of each element (As = 10, Cd = 30, Cr = 2, Cu = Pb = Ni = 5, Zn = 1 and Hg = 40) (Hakanson 1980), and \(E_{r}^{i}\) is the potential ecological risk factor of each element (Yi et al. 2011).
The contamination levels represented by different classes of EF, Igeo, and RI are presented in Table 3.
Spatial Analysis
The values of EF, Igeo, and RI were mapped and interpolated to show the spatial pattern in the contamination levels of the heavy metals. The scores of the four principal factors generated in PCA at the sampling sites were calculated and then were also mapped and interpolated to show their spatial distributions in the whole study area. The spatial distributions were compared to the locations of actual local pollution sources to verify the potential contributing sources of heavy metals identified by PCA and CA. The mapping and spatial analysis were made in MapGis 67 and GeoExpl 2010.
Results and Discussion
Metal Concentrations
Table 4 shows the descriptive statistics summary of the metal concentrations in river sediments and the background values compared to the values from other regions. The mean concentrations of metals are all higher than their background values. Hg has the lowest concentrations, ranging from 0.028 to 0.580 mg/kg, with the mean of 0.130 mg/kg. Cd concentrations vary from 0.096 to 186.01 mg/kg, with the mean of 2.53 mg/kg, which is 2.27 times higher than its background value and significantly higher than the concentration reported by Zhang et al. (2012). Extreme high concentrations of Cd are found at sampling site 4 (186 mg/kg), 5 (139.9 mg/kg) and 3 (34.4 mg/kg). Cr concentrations vary between 67 and 689 mg/kg, in which 7.5% of the samples have a concentration of over two times of its background value. Pb and Zn concentrations vary greatly from 15 to 20,737 mg/kg, and from 56 to 24,329 mg/kg, respectively. The largest difference between the concentration in a sample and the background value is observed for Pb. Its highest concentration is 942.6 times its background value. In addition, 18% of the samples have a Pb concentration of 5–20 times higher than the background value, which indicates that the river sediments have been widely contaminated with Pb. On the contrary, the mean concentrations of Al and Ni are only 1.01 and 1.04 times their background values, respectively, which indicate the natural origins of these two elements.
The mean concentrations of Pb, Zn, Cd, As and Cr are significantly higher than their medians, together with their high skewness values, suggesting that these metals have very high outliers at some sample sites. This result can also be verified by their variation coefficients (VCs), which are SD/Mean and used to reflect the degree of discrete distribution of different metal contents, and to indicate indirectly the activeness of the selected element in the examined surrounding. The VCs of these five heavy metals, Hg, and Cu are higher than 0.7, indicating that the concentrations of these metals vary largely among the sampling sites. High VCs are a good indicator of anthropogenic contributions, so this result suggests that these metals are directly related to anthropogenic sources (Manta et al. 2002; Huang et al. 2007). In contrast, Al and Ni have a VC lower than 0.3, which indicates that they are more homogenously distributed and are more likely associated with natural sources.
To get a comprehensive understanding of the degree of pollution in Nantong district, a comparison of heavy metal concentrations with other regions was performed (Table 4). The result of the present study shows that the heavy metal concentrations of river sediments in Nantong district are generally lower than those reported in Xiangjiang River, Duliujiang River and Xiaoqingling River in other regions of China (Table 4). Cd value is similar to that from Mwanza Gulf, but is lower than that reported from Xiangjiang River. The concentrations of Pb and Zn obtain in this study are much high than other regions (Table 4).
Table 4 also compares our results with the reference values established in different sediment quality guidelines (SQGs). The concentration of As is slightly higher than the reference risk value given by the Canadian Council of Ministers of the Environment (CCME)’s interim sediment quality guideline (ISQG) (CCME 1999), but lower than Sediment Quality of China (GB 18668-2002). Similarly, Cu concentration falls between the lower and upper of Sediment Quality and CCME. Hg value is much lower than any of the values listed in the guidelines. Cr is slightly than the limit of ISQG, PEL, Sediment Quality I, but lower than Sediment Quality II. The mean concentrations of Cd, Pb and Zn are greatly higher than Sediment Quality, CCME’s probable effect level (PEL), but they are lower than the SQG-low in Xiangjiang sediments by Liu et al. (2017). In summary, average concentrations of the heavy metals in this study exceed the reference limits in different SQGs to a certain degree.
Contamination and Risk Assessment
To assess the degrees of contamination, metal concentrations are converted into Igeo values. Igeo values of Al and Ni range from − 0.8 to 0.3, and from − 1.1 to 0.8, respectively, which fall in class 0 or class 1, indicating that they are entirely from crustal material or natural weathering process (Table 3). For Cu, 84.3% of samples have constant values (− 1.68 to 0.93); only 21 samples are in the classes of moderately contaminated or/moderately to heavily contaminated. This result suggests that most samples of Cu are only partly influenced by anthropogenic activities. Igeo values of Pb and Zn vary between − 1.1 and 9.3, and between − 1.1 and 7.8, respectively, with a similar average value of 1.1. The Igeo values of Pb in 22.4% of samples, and Zn 29.1% samplers fall in the classes of heavily contaminated to extremely contaminated, suggesting that these two metals are largely affected by anthropogenic sources. The spatial patterns in the contamination levels reflected by Igeo for Pb, Zn, Hg, and Cd are illustrated in Fig. 2. Moderately contamination to heavily contamination levels for Pb and Zn are located in the southern part of the study area such as Zhuhang, Zhangzhishan, and Jiangzhao. Igeo of Hg varies from − 2.3 to 2.1, with an average value of 0.4, showing its relatively even spatial distribution. It is practically uncontaminated or uncontaminated to moderately contamination for the majority of the study area (Fig. 2). Similarly, Igeo of As has a low range of − 1.8 to 3.4, with an average 1.0. For Cd, the uncontaminated and moderately contamination level covers a large part of the study area, and the heavily and extremely contaminated levels are only found in small areas next to the towns. Overall, the average accumulation of the metals decreased in the following order: Pb(1.1) = Zn(1.1) > Cd(1.0) > As(0.1) = Cu(0.1) > Cr(−0.1) > Hg(−0.3) > Ni(−0.5) > Al(−0.6).
EF values of Ni range between 0.6 and 2.9, revealing deficiency to minimal enrichment. 79.9% of samples fall in the slight enrichment (EF ≤ 5), only 12 samples exhibit moderate enrichment in Ni. The spatial patterns in the contamination levels reflected by EF for Pb, Zn, Hg, and Cd are shown in Fig. 3. Pb and Zn range widely from 0.7 to 83.1, and from 0.8 to 38.6, with average values of 19.3 and 10.6, respectively. This result suggests that most samples suffer significant enrichment in Pb and Zn. They are mostly located in Zhuhang, Zhangzhishan and Jiangzhao (Fig. 3). Generally, Hg, As and Hg have median EF values (0.4–19.5), with the average values 1.5, 1.7 and 2.2, suggesting their moderate enrichment. Among these elements, Hg shows an even spatial distribution and only moderate enrichment next to towns (Fig. 3). The EF values of Cd show a slightly different spatial pattern from other elements. Cd has extremely high enrichment EF values mainly next to Xiaohai, but also shows significant enrichment in Zhuhang and Zhangzhishan (Fig. 3). The EF values of the sediments in the study area, from largest to smallest values, are in the order of Pb > Zn > Cd > As > Cu > Hg > Cr > Ni. This spatial pattern is similar to that observed using Igeo.
The heavy metal contamination in the study area is verified by the RI results that consider the ecological risks caused by multiple heavy metals. A considerable level of ecological risk is observed (Fig. 4). The low-risk level is only in a small area (Xianfeng) in the north. The moderate risk level occupies almost half of the study area, mainly located in the north. The majority of the middle and southern parts of the study area (Zhuhang, Zhangzhishan, Jiangzhao and partly Xiaohai) are covered by considerable risk or severe risk levels.
Correlation Analysis and Cluster Analysis
Table 5 shows the result of Pearson’s correlation analysis on the metal concentrations. A significant positive correlation exists in most pairs of Pb, Zn, As, and Cr at p < 0.01. For example, the correlation coefficients for Pb and Cr, Zn and Cr, Pb and As, Zn and As, and Zn and Pb are 0.874, 0.674, 0.632, 0.636 and 0.649, respectively. These results, together with the relatively high concentrations and VCs of these metals, indicate common contamination sources for them. Hg has a significant positive relationship with As (r = 0.512, p < 0.01), Cr (r = 0.307, p < 0.01), and Ni (r = 0.278, p < 0.01), but has a weaker positive relationship with Pb (r = 0.183, p < 0.05) and Zn (r = 0.132, p > 0.05). Cd does not show a significant relationship with all the other metals except Ni, which implies its sole source. Cu is significantly positively related to As (r = 0.271, p < 0.01), Cr (r = 0.353, p < 0.01), Ni (r = 0.520, p < 0.01), Pb (r = 0.368, p < 0.01), and Zn (r = 0.310, p < 0.01), but has no significant relationship with other metals. The only metal that has a significant positive relationship with Al is Ni (r = 0.553, p < 0.01), indicating the common natural origin for these two metals.
Figure 5 illustrates the result of the explorative hierarchical cluster analysis (CA) on the metal concentrations, which can help us to differentiate the heavy metals into distinct groups of natural or anthropogenic sources. A distinct cluster is formed for As, Cr, Pb and Zn at the level of similarity 9, indicating the significant association among them and their similar sources. This result, verified with the results of descriptive statistics and correlation analysis, suggests these four metals are dominated by a similar anthropogenic input. Al and Ni form a distinct cluster and then join with Cu, which may imply natural inputs of these three elements in the river sediments. Cd and Hg build a cluster that has a long distance with other elements. These nine metals do not form very simple and distinct clusters, which imply that the heavy metals in the river sediments are contributed by various sources.
Principal Components Analysis
PCA was conducted on the concentrations of the nine metals to identify their sources. The results are shown in Table 6. The first four components (factors) have an eigenvalue higher than 1.0, so they were considered to have dominant influences and were retained. They explain 80.2% of the total variance in the samples. The loadings of the metals on these four components are plotted as 3D diagrams to assist in the interpretation of the components (Fig. 6).
Factor 1 (F1) explains 33.6% of the total variance, and it has high positive loadings on As (0.705), Cr (0.888), Pb (0.937), and Zn (0.842). This factor might be associated with effluents of industry (e.g., textile, metal processing) because these metals have been reported to come from discharges from textile, metal processing in many previous studies (Siegel 2002; Aslan 2009). It is generally known that Cr is widely added in the metalized synthetic dyes, pigments, antibacterial, and fungicide (Jozwiak et al. 2007; Aksu and Karabayir 2008). Pb and Zn are usually used in garment accessory, and antimicrobial, respectively (Blanquez et al. 2004). The concentrations of these elements in this study are very close to that in dyeing sludge in Nantong district reported in Tu et al. (2015). Acid washing and phosphating wastewater are produced in the production process of steel wire rope, in which high concentrations of Pb and Zn, even up to hundreds of mg/L, can be found (Zhang et al. 2012; Zhang and Shen 2012). Once the wastewater with high concentrations of Pb and Zn are discharged into rivers without treatment, certainly the sediments in river basin will carry high amounts of these two metals. Pb in soils and river sediments was also considered to be emitted from the factories processing steel wire rope in Nantong district by Teng et al. (2006) and Wu and Shen (2012). Moreover, this factor also has a medium positive loading (0.353) on Cu, which can be explained by the fact that Cu is usually added in the synthetic dyes, the same as Cr (Aksu and Karabayir 2008). Therefore, these metals with high positive loadings in F1 can be attributed to wastewater effluents from textile and metal processing industries, and F1 represents the source of industrial wastewater discharge.
Factor 2 (F2) explains about 20% of the total variances in the data. It has high positive loadings on Ni (0.897), Al (0.797) and Cu (0.524). Combined with the results of descriptive statistics, Igeo, EF calculation, cluster analysis, and correlation analysis, the PCA result indicates that F2 represents the natural source of parent material (sandy alluvial deposit), which contributed these three metals to the river sediments. The similar result was also found for top soils and dusts of Nantong City by Qian et al. (2012). In addition, Cu also has a relatively higher loading in F1, and it has significant positive correlations with As, Cr, Pb, and Zn, which suggests that it is also slightly influenced by industrial sources.
Factor 3 (F3) explains 14.4% of the total variance. As shown in Table 6 and Fig. 6, it exhibits a high positive loading for Hg (0.960), significantly higher than the loadings for other metals. This factor might represent domestic effluents and waste incineration sources. Spatial variability in Hg concentrations in river sediments among sampling sites shows that they are evidently controlled by the effluents sources. For example, Hg has high concentrations in the sediments near the Nantong municipal effluents. In addition, it also has high concentrations at sampling sites in the river segments by towns such as Zhangzhishan and Xianfeng. Many previous studies also found that municipal effluent discharges led to Hg contamination of sediments and soils (Mirlean et al. 2003; Wang et al. 2011a). As seen during the sampling and field survey, domestic solid wastes from individual homes were piled up in rectangular chambers in the open air by the residents along the river. The wastes contain high Hg items such as battery and fluorescent lamp. After they are simply incinerated without proper classification, some Hg retains in ashes (Pirrone et al. 1996; Streets et al. 2005; Wang et al. 2011a). During heavy rains, the mercury-contained particles are washed out from incineration chambers and discharged into nearby rivers. All these activities may enhance Hg concentrations in river sediments to some extent. Because no evident coal burning activities (coal-fired power stations, coal burning furnaces, etc.) were found in our field survey, Hg is considered more likely to be associated with domestic effluents and incineration of solid waste in the study area. Thus, F3 represents municipal and domestic waste sources.
As shown in Table 6 and Fig. 6, Factor 4 (F4) can only explain 12.3% of the total variance in the data. It has a significantly high positive loading on Cd (0.912). Cd tends to have a higher content in phosphatic fertilizers because it is inherited from the phosphatic rocks. The Cd contents in phosphatic fertilizers ranged from 0.1 to 2.9 mg/kg with a mean 0.60 mg/kg according to Lu et al. (1992). A study in USA found much higher Cd contents in fertilizers ranging from 2 to 91 mg/kg, with most values of about 7 mg/kg (Motvedt 1986). The increasing application of phosphatic fertilizers in farmlands over years has accelerated the accumulation of Cd in soils, and then resulted in greater overall movement of Cd to aquatic ecosystems, which ultimately increased Cd concentrations in river sediments to some extent.
Spatial Analysis of Factor Scores
To verify the potential contributing sources of heavy metals in the river sediments identified in PCA, the spatial distributions of the scores for the four factors are displayed in Fig. 7 to see how they match the locations of actual sources in the study area. As identified by PCA, F1 represents the industrial sources of the metals, which is consistent with the spatial distribution of its scores. High scores of F1 appear at samplings sites in the area with a high density of textile mills, especially at sampling sites 129 and 131 in Jiangzhao that are surrounded by over 20 small textile and leather mills. High scores of F1 are also found in Zhuhang, where many metal processing plants are distributed. The discharges from metal processing plants were the cause of the serve Pb and Zn pollution in atmospheric depositions and surrounding environment as found in previous studies (Wu et al. 2011; Teng et al. 2006). The good match between the spatial distribution of F1 and the locations of textile, leather, and metal processing plants suggests that As, Cr, Zn, and Pb from the industrial activities have contaminated the river sediments.
As identified in PCA, F2 represents the natural sources. The factor scores of F2 are generally low all over the study area, which is consistent with the natural origin. However, the scores are slightly higher around sampling sites 129 and 131 in Jiangzhao, which can be explained by the high loading of Cu on this factor. Cu is contributed by mixed natural and industrial sources. Cu has the highest concentrations at sampling site 131 (782 mg/kg) and site 129 (475 mg/kg), which are surrounded by industrial sources such as textile mills. These two sites also have high concentrations of Pb, Zn and As, which are represented by F1.
As identified by PCA, F3 represents the municipal and domestic waste sources of the heavy metals. This finding can be also confirmed by the spatial distribution of its factors. The high scores of F3 are located near the outlets of municipal and domestic effluents (Fig. 7c). For example, the sampling sites that are adjacent to discharge outlets from Nantong city and towns (Xianfeng, Xiaohai, and Zhangzhishan) have relatively high scores of F3. The highest Hg concentration of 0.58 mg/kg is observed at sampling site 104 near the outlet, where the effluent from Zhangzhishan town is discharged into the rivers.
F4 is identified to be associated with phosphatic fertilizer application in PCA. The scores of F4 are more homogenously distributed in the study area. This spatial pattern matches the spatial distribution of farmlands, which exist all over the study area. Samplings with the highest scores of F4 are located at sampling sites 4 and 131, which are close to farmlands.
Therefore, the spatial distributions of the four factors are consistent with the spatial distributions of the corresponding natural and anthropogenic sources of heavy metals in the local area, which further verifies the potential sources identified by statistical analyses.
Conclusions
This study assessed the contamination of heavy metals in river sediments and identified their sources in Nantong, Eastern China using multivariate statistical techniques including CA and PCA combined with descriptive statistics, contamination and risk assessments (Igeo, EF, and RI), correlation analysis, and spatial analysis.
The results of descriptive statistics and risk assessment show the contamination level of heavy metals is in the order of Pb > Zn > Cd > As > Cu > Cr > Hg > Ni. The river sediments are heavily contaminated with Pb and Zn adjacent to the towns where lots of textile plants and wire rope enterprises are located.
The results of correlation analysis and CA show that two metals, Al and Ni, are mainly from natural sources; Cu are contributed by both natural and anthropogenic sources; while other heavy metals (Hg, Cr, As, Zn, Pb, and Cd) are mainly from anthropogenic sources. Four specific sources of the metals in river sediments are identified using PCA and spatial analysis. They are (1) industrial sources (i.e., textile and metal processing industries) that mainly contribute As, Cr, Pb and Zn, and also partly Cu, (2) natural sources (i.e., parent materials) in which Ni, Al, and Cu originate from, (3) municipal and domestic wastes that mainly contribute Hg, and (4) fertilizer application that is responsible for Cd in the river sediments. This study identified more specific anthropogenic activities that are associated with different heavy metals than those found in most of previous studies. More importantly, the potential contributing sources of the heavy metals were verified by comparing the spatial distribution in the scores of factors that represent different sources to the locations of actual local sources. Thus, the sources identified in this study are more reliable. To the best of our knowledge, very few studies have ever used this method (Huang et al. 2009).
The results and findings of this study can provide useful information for governments, environmental protection agencies, and other decision makers to implement appropriate policies and regulations to control the heavy metal pollution in the river sediments caused by the rapid urbanization and industrialization in the study area. The approaches that integrate descriptive statistics, correlation analysis, multivariate analysis, contamination and risk assessments, and spatial analysis can be also useful for environmental scientists to examine various environmental issues beyond the study area.
References
Aksu Z, Karabayir G (2008) Comparison of biosorption properties of different kings of fungi for the removal Grayfalan Black RL metal-complex dye. Bioresour Technol 99:7730–7741
Aslan A (2009) Determination of heavy metal toxicity of finished leather solid waste. Bull Environ Contam Toxicol 82:633–638
ATSDR (Agency for Toxic Substances and Disease Registry) (2018) Toxic substances portal. https://www.atsdr.cdc.gov/substances/index.asp Accessed 20 Apr 2018
Azhari AE, Rhoujjati A, Hachimi MLE (2016) Assessment of heavy metals and arsenic contamination in the sediments of the Moulouya River and the Hassan II Dam downstream of the abandoned mine Zeïda (High Moulouya, Morocco). J Afr Earth Sci 119:279–288
Bhuyan MS, Bakar MAl, Akhtar A, Hossain MB, Ali MM, Islam MS (2017) Heavy metal contamination in surface water and sediment of the Meghna River, Bangladesh. Environ Nanotechnol Monit Manag 8:273–279
Blanquez P, Casas N, Font X, Gabarrell X, Sarra M, Caminal G, Vicent T (2004) Mechanism of textile metal dye biotransformation by Trametes versicolor. Water Res 38:2166–2172
Buat-Menard P, Chesselet R (1979) Variable influence of the atmospheric flux on the trace metal chemistry of oceanic suspended matter. Earth Plant Sci Lett 42:398–411
CCME (1999) Canadian sediment quality guidelines for the protection of aquatic life. Canadian Council of Ministers of the Environment, Winnipeg, Canada. http://www.ccme.ca/en/resources/canadian_environmental_quality_guidelines/. Accessed 26 Mar 2017
Chai L, Wang Z, Wang Y, Yang Z, Wang H, Wu X (2010) Ingestion risks of metals in groundwater based on TIN model and dose-response assessment—a case study in the Xiangjiang watershed, central-south China. Sci Total Environ 408:3118–3124
Chai L, Li H, Yang Z, Min X, Liao Q, Liu Y et al (2017) Heavy metals and metalloids in the surface sediments of the Xiangjiang River, Hunan, China: distribution, contamination, and ecological risk assessment. Environ Sci Pollut Res 24:874–885
Chen YM, Li HC, Tsao TM, Wang LC, Chang Y (2014) Some selected heavy metal concentrations in water, sediment, and oysters in the Er-Ren estury, Taiwan: chemical fractions and the implications for biomonitoring. Environ Mont Assess 186(11):7023–7033
Chen CF, Ju YR, Chen CW, Dong CD (2016) Vertical profile, contamination assessment, and source apportionment of heavy metals in sediment cores of Kaohsiung Harbor, Taiwan. Chemosphere 165:67–79
Chowdhury S, Mazumder MAJ, Al-Attas O, Husain T (2016) Heavy metals in drinking water: occurrences, implications, and future needs in developing countries. Sci Total Environ 569–570:476–488
Cuzick J, Sasieni P, Evans S (1992) Ingested arsenic, keratosis, and bladder cancer. Am J Epidemiol 7:117–124
Davutluoglu OI, Seckin G, Ersu CB, Yilmaz T, Sari B (2011) Heavy metal content and distribution in surface sediments of the Seyhan River, Turkey. J Environ Manag 92:2250–2259
Dhanakumar G, Soaraj G, Mohanraj R (2015) Heavy metal partitioning in sediments and bioaccumulation in commercial fish species of three major reservoirs of river Cauvery delta region, India. Ecotoxicol Environ Safe 113:145–151
Franzini M, Leoni L, Saitta M (1975) Revisione di una metodologia analitica per fluorescenza-X basata sulla correlazione completa degli effetti di matrice. Rend Soc Ital Mineral Petrol 31:35–378
Hakanson L (1980) An ecological risk index for aquatic pollution control. A sedmentological approach. Water Res 14(8):975–1001
Han D, Cheng J, Hu X, Jiang Z, Mo L, Xu H et al (2017) Spatial distribution, risk assessment and source identification of heavy metals in sediments of the Yangtze River Estuary, China. Mar Pollut Bull 115:141–148
Hao H, Gao B, Wang JK et al (2012) Distribution characteristic and potential ecological risk assessment of heavy metals in sediments of the Luanhe River. Rock Miner Anal 31(6):1000–1005
Huang SS, Liao QL, Hua M, Wu XM, Bi KS, Yan CY, Chen B, Zhang XY (2007) Survey of heavy metal pollution and assessment of agricultural soil in Yangzhong district, Jiangsu Province, China. Chemosphere 67:2148–2155
Huang SS, Tu J, Liu HY, Hua M, Liao QL, Feng JS, Weng ZH, Huang GM (2009) Multivariate analysis of trace element concentrations in atmospheric deposition in the Yangtze River Delta, East China. Atmos Environ 43:5781–5790
Islam SMD, Bhyiyan MAH, Rume T, Mohinuzzaman M (2016) Assessing heavy metal contamination in the bottom sediments of Shitalakhya River, Bangladesh; using pollution evaluation indices and geo-spatial analysis. Pollution 2(3):299–312
Jozwiak WK, Mitros M, Kaluzna-Czaplinska J, Tosik R (2007) Oxidative decomposition of Acid Brown 159 dye in aqueous solution by H2O/Fe2+ and ozone with GC/MS analysis. Dyes Pigments 74:9–16
Kishe MA, Machiwa JF (2003) Distribution of heavy metals in sediments of Mwanza Gulf of Lake Victoria, Tanzania. Environ Int 28:619–625
Krika A, Krika F (2017) Evaluation of the status of heavy metal pollution in surface water and sediments of the Nil River (North Eastern Algeria). Pollution 3(2):301–310
Liao QL, Liu C, Xu Y, Jin Y, Wu YZ, Hua M et al (2011) Geochemcial baseline values of elements in soil of Jiangsu Province. Geol China 38(5):1364–1378 (in Chinese)
Liao J, Chen J, Ru X, Chen J, Wu H, Wei C (2017) Heavy metals in river surface sediments affected with multiple pollution sources, South China: distribution, enrichment and source apportionment. J Geochem Explor 176:9–19
Liu QP (2015) Regional difference of NPK fertilizers application and environmental risk assessment in Jiangsu Province, China. J Appl Ecol 26(5):1477–1483 (in Chinese)
Liu R, Men C, Liu Y, Yu W, Xu F, Shen Z (2016) Spatial distribution and pollution evaluation of heavy metals in Yangtze estuary sediment. Mar Pollut Bull 110:564–571
Liu H, Zhang KJ, Chai LY et al (2017) A comparative evaluation of different sediment quality guidelines for metal and metalloid pollution in the Xiangjiang River, Hunan, China. Arch Environ Contam Toxicol 73:593–606
Lu RK, Shi ZY, Xiong LM (1992) Cadmium contents of rocks phosphates and phosphate fertilizers of China and their effects on ecological environment. Acta Pedol Sin 29(2):150–157 (in Chinese)
Lu J, Li A, Huang P (2017) Distribution, sources and contamination assessment of heavy metals in surface sediments of the South Yellow Sea and northern part of the East China Sea. Mar Pollut Bull 124:470–479
Manta DS, Angelone M, Bellanca A, Neri R, Sprovieri M (2002) Heavy metals in urban soils: a case study from the city of Palermo (Sicily), Italy. Sci Total Environ 300:229–243
Meng X, Zhang H, Shan BQ et al (2016) Pollution and ecology risk assessment of heavy metals in surface sediment of the Duliujian River drainage basin. Urban Environ Urban Ecol 29(4):36–41 (in Chinese)
Micó C, Recatalá L, Peris M, Sánchez J (2006) Assessing heavy metal sources in agricultural soils of an European Mediterranean area by multivariate ananlysis. Chemosphere 65:863–872
Mihailović A, Budinski-Petković LJ, Popov S, Ninkov J, Vasin J, Ralević NM, Vučinić Vasića M (2015) Spatial distribution of metals in urban soil of Novi Sad, Serbia: GIS based approach. J Geochem Explor 150:104–114
Mirlean N, Andrus VE, Baisch P (2003) Mercury pollution sources in sediments of Patos Lagoon estury, Southern Brazil. Mar Pollut Bull 46:331–334
Möller S, Einax JW (2013) Metals in sediments—spatial investigation of Saale River applying chemometric tools. Microchem J 110:233–238
Motvedt JJ (1986) Cadmium level in soil and plant tissues from long-term soil fertility experiments in US. Trans 15th Cong ISSS 111:870–871
Muller G (1969) Index of geoaccumulation in sediments of the Rhine River. Geo J 2:108–118
Muniz P, Danulat E, Yannicelli B et al (2004) Assessment of contamination by heavy metals and petroleum hydrocarbons in sediments of Montevideo Harbour (Uruguay). Environ Int 28:1019–1028
Nantong Water Conservancy Bureau (NWCB) (2010) Urban water system planning in Nantong. (in Chinese)
Nehme N, Haydar C, Koubaissy B, Fakih M, Awad S, Toufaily J et al (2014) The distribution of heavy metals in the Lower River Basin, Lebanon. Phys Procedia 55:456–463
Nguyen TTH, Zhang W, Li Z, Li J, Ge C, Liu J et al (2016) Assessment of heavy metal pollution in Red River surface sediments, Vietnam. Mar Pollut Bull 113:513–519
Pirrone N, Keeler GJ, Nriagu JO (1996) Regional differences in worldwide emissions of mercury to the atmosphere. Atmos Environ 30(17):2981–2987
Qian P, Zhou LM, Zheng XM, Jiang QF, Yan DZ (2012) Pollution and ecological risk assessment of heavy metals in the top soils and dusts of Nantong City, Jiangsu. Environ Chem 31(4):483–489 (in Chinese)
Ridgway J, Midobatu C (1991) Temporal variations in the trace element content of stream sediments: an example from a tropical rain forest regime, Solomon Islands. Appl Geochem 6:185–193
Siegel FR (2002) Environmental geochemistry of potential toxic metals. Springer, Berlin
Smith AH, Goycolea M, Haque R, Biggs ML (1998) Marked increase in bladder and lung cancer mortality in a region of northern Chile due to arsenic in drinking water. Am J Epidemiol 147(7):660–669
Streets DG, Hao J, Wu Y, Jiang JK, Chan M, Tian HZ, Feng XB (2005) Anthropogenic mercury emissions in China. Atmos Environ 39:7789–7806
Teng C, Xie P, Yang HP, Wan YP, Li XD (2006) Investigation of lead pollution from steel wire rope enterprises in Nantong district. J Nantong Univ (Med Sci) 26(4):271–273 (in Chinese)
Tu Y, Tian JR, Zhu HJ, Gé SF (2015) Status of dyeing sludge in Jiangsu Province. Acta Sci Circumst 35(2):527–534 (in Chinese)
Turekian KK, Wedepoh KH (1961) Distribution of the elements in some major units of the earth crust. Bull Geol Soc Am 72:175–192
Vu CT, Lin C, Shern CC, Yeh G, Le VG, Tran HT (2017) Contamination, ecological risk and source apportionment of heavy metals in sediments and water of a contaminated river in Taiwan. Ecol Indic 82:32–42
Wang JJ, Zhao HW, Zhong XP, Liu YS, Ceng H (2011a) Concentration levels and spatial distribution of heavy metal in soil surrounding a municipal solid waste incineration plant (Shenzhen). Environ Sci 32(1):298–301 (in Chinese)
Wang Z, Chai L, Wang Y, Yang Z, Wang H, Wu X (2011b) Potential health risk of arsenic and cadmium in groundwater near Xiangjiang River, China: a case study for risk assessment and management of toxic substances. Environ Monit Assess 175:167–173
Wang J, Liu R, Zhang P, Yu W, Shen Z, Feng C (2014) Spatial Variation, environmental assessment and source identification of heavy metals in sediments of the Yangtze River Estuary. Mar Pollut Bull 87:364–373
Ward JH (1963) Hierarchical grouping to optimize an objective function. J Am Stat Assoc 58:236–244
Wu JL, Shen HJ (2012) Assessment on heavy metal pollution and its potential ecological risk in the sediment on Nantong Hao River. Environ Monit For 4(4):46–50 (in Chinese)
Wu PF, Wu YJ, Yan YC (2011) Study on disposal and utilization of the sludge from steel wire rope enterprises in Nantong city. Environ Monit For 3(6):46–48 (in Chinese)
Xu XX, Zhao XQ, Sun BF et al (2017) Spatial distribution, ecological risk and source apportionment of heavy metals in sediments form Xiaoqinghe watershed of Jinan. J Southwest China Normal Univ (Nat Sci Ed) 42(2):78–84 (in Chinese)
Yi YJ, Yang ZF, Zhang SH (2011) Ecological risk assessment of heavy metals in sediment and human health risk assessment of heavy metals in fishes in the middle and lower reaches of the Yangtze River basin. Environ Pollut 159(10):2575–2585
Yuan X, Zhang L, Li J, Wang C, Ji J (2014) Sediment properties and heavy metal pollution assessment in the river, estuary and lake environments of a fluvial plain, China. Catena. 119:52–60
Zhang Q, Shen ZQ (2012) Investigation of heavy metal concentrations in soils in typical wire rope industry area. Environ Monit Manag Technol 25(6):19–23 (in Chinese)
Zhang Q, Liu YY, Chen M, Geng JS (2012) Evaluated on heavy metal pollution of inland river sediment in Nantong city by accumulation index method. Arid Environ Monit 26(1):27–30 (in Chinese)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Rights and permissions
About this article
Cite this article
Huang, S., Tu, J., Jin, Y. et al. Contamination Assessment and Source Identification of Heavy Metals in River Sediments in Nantong, Eastern China. Int J Environ Res 12, 373–389 (2018). https://doi.org/10.1007/s41742-018-0097-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41742-018-0097-8