Abstract
The global energy system has to undergo a large transformation to achieve the climate targets as mentioned in the Paris Agreement. The share of electricity in all of the energy consumed by end users worldwide increases tremendously which would be about 40% in 2050 to achieve the decarbonised energy. Hydrogen could therefore be the possible link in the energy transition. Hydrogen from renewables could replace fossil fuel-based feedstocks in high-emission applications. Fuel cell electric vehicles (FCEVs) provide a low-carbon mobility option when the hydrogen is produced from renewable energy sources and offer driving performance comparable to conventional vehicles. FCEVs are complementary to battery electric vehicles and can also overcome some of the current limitations of batteries in terms of weight, driving range and refuelling time in the medium to high duty cycle segments. As the key hydrogen technologies are maturing and it has been understood that scale-up can yield the necessary technology cost reductions. Proton exchange membrane electrolysers and fuel cells are approaching technical maturity and economies of scale. Commercial deployment has started in several regions of the world. Initial efforts could focus on large-scale applications, with minimal infrastructure requirements, and on sectors where hydrogen from renewables stands out as the best-performing option to meet climate targets. Such applications include large-scale industry such as petrochemicals, steel and medium-to heavy-duty transport, medium to large passenger vehicles and commercial vehicles, large fleets of buses, trucks, trains, maritime, and aviation. However, hydrogen from renewable electricity is most likely to achieve cost competitiveness through high electrolyser utilisation rates combined with renewable electricity. The present document analyses the fuel cell technology, sustainability elements, the potential of using hydrogen as an alternative energy source and for identifying the possibilities of increasing the share of hydrogen energy in various applications. The Strengths–Weakness-Opportunities and Threats analysis was provided, giving in depth details about the technical challenges and hurdles for commercialisation. The recent developments in terms of International initiatives, targets, visions and also from the Indian perspectives, are discussed and a special reference is highlighted about International Advanced Research centre’s work in this direction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hydrogen and fuel cell technologies are still in the early stages of commercialisation and currently struggle to compete with alternative technologies, including other low-carbon options, due to high costs. A fuel cell works much like an electric battery, converting chemical energy into electrical energy using the movement of charged ions across an electrolyte to generate current. There they recombine with oxygen to produce water, a fuel cell’s only emission, with hot air. Today’s fuel cells compare favourably with internal combustion engine technology, which converts fuel into kinetic energy at roughly 25% efficiency. A fuel cell, by contrast, can mix hydrogen with air, electrochemically without any combustion, to produce electricity up to 60% efficiency.
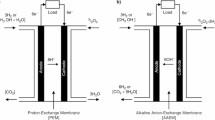
As an energy carrier, hydrogen can enable new linkages between energy supply and demand, in both a centralised or decentralised manner, potentially enhancing overall energy system flexibility (Hydrogen Council November 2017). Hydrogen being a clean and flexible energy carrier, it has got varied sources as well diverse applications as shown in Fig. 1.
The ‘hydrogen economy’ refers to the idea of transforming our existing hydrocarbon-based infrastructure—from static power generation to a full range of transportation applications—to run on hydrogen to cut carbon and carbon dioxide emissions (Rajalakshmi and Dhathathreyan 2005). This transformation can occur wherever hydrocarbons are used as fuel, but there is particular interest in the potential transport applications, where fuel cell electric vehicles are seen as a particularly viable alternative to conventional hydrocarbon-powered vehicles. All Government sectors are helping to accelerate the development and deployment of hydrogen and fuel cell technologies by ensuring funding for continued research, development and demonstration.
Fuel cells can be classified primarily based on the kind of electrolyte they employ and the type of fuel they use. Fuel cells can broadly be put under these categories (Vielstich et al. 2003):
-
Polymer electrolyte membrane fuel cells (PEMFC)
-
Direct methanol fuel cell (DMFC)
-
Direct ethanol fuel cell (DEFC)
-
-
Phosphoric acid fuel cells (PAFC)
-
Alkaline fuel cells (AFC)
-
Molten carbonate fuel cells (MCFC)
-
Solid oxide fuel cells (SOFC)
The various types of fuel cells and their characteristics are shown in Table 1.
In recent times several derivatives of these major fuel cell types are also in vogue. The performance of fuel cells is critically dependent on the materials used in the reaction and the temperature at which they operate. These affect their sensitivity to contamination from other gases, while there is an inverse relationship from a temperature between start-up time and efficiency gained from the thermal output. There are many potential applications in a wide range of industries, including residential combined heat and power, larger-scale stationary industrial power that includes combined heat and power, transport, and portable electronics. The stationary and transport application sectors are the most important energy consumers. Hydrogen fuel cell technology represents one of the alternative solutions for future clean energy systems. Proton exchange membrane (PEM) fuel cells are approaching technical maturity and economies of scale. Commercial deployment has started in several countries such as Japan, California, and Europe for number of applications. The PEMFC technology has received the highest score in the ranking in spite of its major challenges like durability, cost, sensitivity to impurities, and a complex water management system (both gas and liquid). This technology is also characterized by highly sensitive operating conditions and a high system mass.
In the transportation sector, fuel cell-based electric vehicles present relatively low barriers to entry in terms of societal changes, as they operate and perform similarly to conventional vehicles, refuelling at stations in minutes and driving for 500–600 kms on a single tank, all with no harmful emissions. Most observers of current events are aware of the growing transportation trend towards electric cars and Fuel cell vehicles, and many will certainly have heard the name Tesla and Mirai.
In this paper, we outline an overview of the fuel cell technology, sustainability elements, the potential of using hydrogen as an alternative energy source and for identifying the possibilities of increasing the share of hydrogen energy in various applications. The Strengths–Weakness-Opportunities and Threats (SWOT) analysis was provided giving in depth details about the technical challenges and hurdles for commercialisation. The recent developments in terms of International initiatives, targets, visions and also from the Indian perspectives, are discussed and a special reference is highlighted about International Advanced Research centre (ARCI)’s work in this direction.
SWOT analysis
As the name suggests fuelcells have got both strength and weakness which are opportunities and threats for implementation. The SWOT analysis functions on the concept of hydrogen energy implementation and at the same time evaluates the internal and external influence factors of the concept, as well as its position in the applicability environment to highlight the strengths and weaknesses of the concept, in relation to the opportunities and threats existing at the moment. The various steps used to perform the SWOT analysis are identification of the key issues, options and selection of strategy, which are strength and weakness, while external are opportunities and threats. To improve the performance, current strategies are analysed using new opportunities or by neutralizing the threats.
To start with analyzing the fuel, hydrogen, the favourable arguments are the following:
-
1.
Hydrogen as synthetic fuel
-
2.
Can be obtained from renewable sources
-
3.
Has the highest energy/mass unit of all the fuel types
-
4.
It is ecologically friendly
-
5.
It has the highest energy reserve factor
-
6.
The largest conversion factor into electricity
-
7.
Considered as the best of the fuels.
However, the barriers to overcome are, safety issues due to hydrogen burning in air, storage of hydrogen and high cost of hydrogen technologies processes, conversion in fuel cells, transportation cost etc., When analysing the value data reported over the last decade regarding the distribution of the technological transfer according to the fuel cell types, it is observed that proton exchange membrane fuel cell is dominant, due to the possibility for a wide range of applications that includes portable, stationary and transport, from small applications, micro-cogeneration systems to centralized power generation through high power applications. Initially, PEMFC was considered less suitable for stationary applications than the other fuel cell types. However, the opinion has changed by the rapid technical progress made and by the considerable reductions in projected manufacturing costs. Among the various applications of PEMFC two aspects remain similar viz., the electrolyte used and the electrode structure and the catalyst. However, depending on the applications, the various options available are, water and thermal management, inter connection of the cells, reactants to be used and pressure of the operation.
The lower operating temperature of PEMFC results in both advantages and disadvantages. Low-temperature operation is advantageous because the cell can start from ambient conditions quickly, especially when pure hydrogen fuel is available. Using appropriate bipolar plates and supporting structure, PEMFC should be capable of operating at pressures up to 3000 psi and differential pressures up to 500 psi, which can improve the current density. The PEMFC stacks are modular in nature, easy to pack them up, and hence they find a wide variety of applications ranging from microwatts to Megawatt level. The solid electrolyte in PEMFC exhibits good ionic conductivity, minimum gas crossover depending on the thickness of the membrane, and does not require replenishment of electrolyte, as it is a solid. The PEMFC can operate at very high current densities compared to the other fuel cells. These attributes lead to a fast start capability and the ability to make a compact and lightweight cell. Other beneficial attributes of the PEMFC include lower sensitivity to orientation. As a result, the PEMFC is particularly suited for vehicular power application (Rajalakshmi and Dhathathreyan 2008a).
The disadvantage is that the low-quality thermal output cannot be used effectively. Another disadvantage associated with PEMFC is that platinum catalysts are required to promote the electrochemical reaction. If a reformate is used as fuel, carbon monoxide (CO) binds strongly to platinum sites at temperatures below 150 °C, which reduces the active sites available for hydrogen chemisorption and electro oxidation. Because of the CO poisoning at the anode, only a few ppm of CO can be tolerated with the platinum catalysis at 80 °C. The reformed hydrocarbons contain about one percent of CO, hence a mechanism to reduce the level of CO in the fuel gas is needed. The low temperature of operation also means that little heat, if any, is available from the fuel cell for any endothermic reforming process to take place. The important elements that need to be considered regarding hydrogen fuel cell technology for sustainability are given schematically in Fig. 2, has been widely developed within the SWOT analysis and are referred to technological, environmental, social and economic factors.
Based on the most pertinent reports available during the last 2 decades, various pilot projects aiming at hydrogen technology are being validated, and the performances of fuel cell systems operating under real conditions are analysed. This analysis brought out reports on technology performance, progress and new challenges. In addition, this analysis also includes fuel cell assemblies of various types, namely proton exchange membrane fuel cells, solid oxide fuel cells, phosphoric acid fuel cells and molten carbon fuel cells, with power generation ranging from 5 kW up to 3 MW. Fuel cell systems are used in stationary applications for various purposes, such as back-up power supplies, power generation for remote locations, stand-alone power stations, distributed generation for buildings. The stationary sector shows an increasing trend of technology transfer from the producer to the final consumer. This has been currently recognized as a feasible option compared to the conventional technologies of generator type, internal combustion engines or batteries. This technology transfer amounted to a value of 395,000 units in 2014, which increased to 575,000 units, during 2018. The residential fuel cell program “ENE FARM” has played the role of energy backup (back-up system), and found to be very successful fuel cell program in Japan. Asia is the region with the largest number of fuel cell units in practical applications over the last 5 years, with an increase due to the commercial development of micro-cogeneration fuel cells produced in Japan, so for in 2018 around 55,500 units, 26,000 units in USA, and around 9000 units in Europe (International Energy Agency 2019).
Polymer Electrolyte Membrane Fuel Cells
The primary components of a PEMFC are anode, cathode and an ion-conducting electrolyte. The use of organic cation exchange membrane polymers in fuel cells was originally conceived by William T. Grubbs and the desired function of the ion membrane was to provide an ion conductive and a gas barrier. The basic cell (Fig. 3) consists of a proton-conducting membrane, such as a per fluorinated sulfonic acid polymer, sandwiched between two platinum impregnated porous carbon electrodes.
The electrodes were also made hydrophobic for proper water management without affecting the gas diffusion path to the catalyst layer. Laminating the anode, cathode and an ion-conducting electrolyte is called Membrane Electrode Assembly (MEA) and a single fuel cell is formed by passing a fuel such as hydrogen into the anode compartment and an oxidant, typically oxygen, into the cathode compartment with the help of gas flow distribution plates for reactants, and mechanical components such as end plates, current collectors, gaskets, bolts and nuts. The voltage of a fuel cell is small 1.23 V, which decreases when drawing a useful current. Hence many cells have to be connected in series to increase the voltage. Such a collection of fuel cells in series is known as a stack. A better method to do connect the cells is to use a “bipolar plate” where the entire face of the anode plate (opposite the gas distribution side) is in contact with the obverse of the cathode plate. Therefore, a major component of the fuel cell stack is the bipolar plate which provides the gas feeds to the cell and also transfers the current produced in each cell. Besides the gas bipolar plates, the fuel cell stack is interspaced with gas/water plates wherein on one side of the reactant gases is fed and to the other side a coolant is supplied which helps in maintaining the temperature of the stack (Dhathathreyan and Rajalakshmi 2009).
Membrane Electrolytes
PEMFC requires an ion-exchange polymer membrane in the form of a continuous pore-free sheet. The properties which characterize the ideal ion- exchange membrane fuel cell electrolyte are, high ionic conductivity, zero electrical conductivity, low gas permeability, dimensional stability, high mechanical strength, low transference of water by conducting ions, high resistance to degradation, chemical stability to oxidation and hydrolysis. The factors which affect the conductivity of the membrane are ionic size, charge and solvation. The most highly conducting membrane electrolytes for fuel cell application are those in which the mobile ion is the hydrogen ion and the solvate is water at saturation. Water transport is of importance among the various properties required by the ion exchange membranes, both with regard to electro-osmotic transport of water and back diffusion of water once a gradient is established (Vielstich et al. 2003).
DuPont’s Nafion® is the most advanced commercially available proton-conducting polymer membrane, which is produced in sheet form with thickness varying between 25 and 250 µm. Nafion membranes exhibit a protonic conductivity as high as 0.10 S cm−1 under fully hydrated conditions. For a membrane thickness of say, 175 μm (Nafion 117), this conductivity corresponds to a real resistance of 0.2 Ω cm2, i.e., a voltage loss of about 150 mV at a practical current density of 750 mA cm−2. Nafion® is the electrolyte against which other membranes are judged and is in a sense an ‘Industrial Standard’ for PEMFC. A lifetime of over 60,000 h under fuel cell conditions has been achieved with commercial Nafion membranes. The copolymer’s acid capacity is related to the relative amounts of co-monomers specified during polymerization and can range from 0.67 to 1.25 meq g−1 for 1500–800 EW, respectively. The traditional extrusion-cast membrane manufacturing process was developed for “thick” films, typically greater than 125 µm. The PTFE backbone imparts hydrophobicity and the sulfonic acid group imparts hydrophilicity. The hydrophilic regions around the clusters of sulfonated side chains can lead to the absorption of large quantities water as much as 50% from the dry weight (Smitha et al. 2005). Within these hydrated regions, the H+ ions are weakly attracted to the SO3- group and are able to move. As an electrolyte, the polymer membrane provides an environment for electrode reactions at the electrolyte electrode interfaces. As a result of the fast electrode reaction kinetics, the performance of PEMFC is high, especially at low noble metal loadings. The membrane also serves as a catalyst support and an effective gas separator. At 23 °C and 50% relative humidity (RH), for example, the tensile strength of Nafion® membranes is about 40 MPa and the elongation is larger than 200%. The permeability of both oxygen and hydrogen through the membrane is of the order of 10–11 to 10–10 mol cm−1 s−1 atm−1, corresponding to an equivalent current loss of 1–10 mA cm−2, about 1% of the performance (Dhathathreyan and Rajalakshmi 2009).
In spite of many advantages, Nafion® has some limitations. The primary limitation is its ability to proton conduction, only in a highly hydrated state, thereby leading to a requirement of secondary hardware system to humidify the gases before they pass into the stack. A further limitation is that it does not function well above 80 °C in PEMFC (under normal operating conditions). However, there is a necessity to operate PEMFC at higher temperatures as this would allow the fuel cell to tolerate much higher levels of carbon monoxide if the fuel is reformate hydrogen.
Dow Chemical Company (Eisman 1990) and Asahi Chemical Company (Miyake et al. 2008) have made advanced perfluoro sulfonic acid membranes with shorter side chains and a higher ratio of SO3H to CF2 groups. The lower equivalent weights of these membranes compared to Nafion account for their higher specific conductivities, which enabled significant improvements in PEMFC performance, i.e. about 50–100 mV increase in cell potential at 1 A cm−2 over that on the control Nafion® 115, with about the same thickness (~ 100 μm). Since the ohmic overpotential is predominant in PEMFC in the intermediate to high current density range (0.3–1 A cm−2), one logical approach to enhance power densities is to use membranes thinner than Nafion® 115. However, there have been problems of a small amount of cross-over of the reactant gases, which reduced the open circuit potential of the cell by about 0.1 V and also mechanical stability of the thin membranes, which have created hot-spots leading to cell failure.
One of the major problems with the perfluoro sulfonic acid membranes is their high cost (~ US$ 1000 m−2) (International Energy Agency (2019); Dhathathreyan and Rajalakshmi 2009). Thus, for a PEMFC operating at the desired power density of about 0.6 W cm−2, the cost of membrane alone will be about US$ 150/kW. According to DuPont and Asahi Chemical, increasing the production of perfluoro sulfonic acid membranes to that required for at least a million vehicles per year could make it possible to reduce the cost of the membrane by a factor of 10. The high cost of perfluoro sulfonic acid membranes is due to the expensive fluorination step: thus partially-fluorinated and non-fluorinated ionomer membranes are currently under study. The polymer membranes (other than the perfluorinated membranes) are classified into three groups viz., modified PFSA membranes,alternate sulfonated hydro carbon polymers and their inorganic composite membranes, and acid–base complex membranes and are explained in detail elsewhere (Ramya et al. 2006).
Electrodes, Electrode Structures and Electro Catalysis
Gas diffusion Electrode (GDL) have assumed a fundamentally important role in the technology of PEMFC. The PEM fuel cell consists of a gas diffusion electrode in which the reaction is considered to proceed through all of the following elementary steps (Vielstich et al. 2003). It starts with bulk flow and diffusion of reactant molecules through large electrode pores, adsorption of molecules on reaction site viz. platinum or platinum alloy catalysts, discharge of ionic species, proton to electrolyte, surface reactions between adsorbed molecules, discharged ions or radicals and desorption of products and transport into the electrolyte or pores. The surface of a gas electrode depends on the successful maintenance of a three-phase equilibrium involving the electrode, porous matrix, the reactant gas and the electrolyte. In addition, the gas diffusion layer is the electrical conductor that transports electrons to and from the catalyst layer. Typically, gas diffusion layers are constructed from porous carbon paper, or carbon cloth, with a thickness in the range of 100–300 μm. The gas diffusion layer also assists in water management by allowing an appropriate amount of water to reach, and be held at, the membrane for hydration.
Despite its many functions, Gas diffusion media has received very little development attention, as evidenced by the scarcity of publications on PEMFC diffusion media in the literature. Currently, available diffusion media do not meet long-term requirements for cost, and the development of less expensive materials is needed. Additionally, issues of flooding under steady-state and transient (e.g., start-up) conditions as well as low-current stability issues demand careful diffusion media design. Moreover, it is likely that durability is significantly impacted by diffusion-media substrate and treatment in ways that are not yet understood. There are many candidate materials and process variables that can be adjusted to develop optimum materials for a given application. In support of this, much remains to be done in terms of establishing characterization methods and property-performance relationships. The diffusion media will need to receive much more focused attention to development before the widespread commercialization of PEMFC fuel cells becomes a reality (Velayutham et al. 2008). The subject of gas diffusion electrode is dealt with in more detail elsewhere in this chapter. Recently Ozden et al. reviewed the gas diffusion layers for PEM fuel cells: ex- and in-situ characterization (Ozden et al. 2018) in terms of the required properties. The Manufacturing the Gas Diffusion Layer for PEM Fuel Cell Using a Novel 3D Printing Technique and Critical Assessment of the Challenges Encountered was reported by Jayakumar et al. (2017). Wei et al. reported an ultra-thin, flexible, low-cost and scalable gas diffusion layer composed of carbon nanotubes for high-performance fuel cells (Wei et al. 2020).
Electrodes, Electro Catalysis and Kinetics
There are two basic criteria which must be satisfied before a chemical reaction can be considered as a source of energy in a fuel cell. The first criteria are that at least one of the reactants must be ionisable at the operating conditions and the ionisable reaction system is the source of energetic electron flow that does work in the external circuit. During this process, the formed ions establish oxidation–reduction which when traversed by ions ultimately provides the energy that will be used at the terminals. For practical purposes, the high flow rate of electron flow is required, hence the rapid rates of electron supplying and consuming reactions is a second criterion. The first criterion is the static system and is the object of thermodynamic inquiry. The second criterion is dynamic in nature and is the subject of chemical kinetics. However, the equal rates of the backward and forward electrode reactions that occur at static conditions establish the equilibrium. Hence the first criterion may also be considered as dynamic and is also the subject of kinetics. Successful development of PEMFC depends on how well these kinetic criteria are met.
Activation energy must be supplied before these processes and the magnitudes depend on the properties of the reactants, products and intermediates for most physical and chemical reactions. If any one of the reactions listed is slower than the activation, thermal and electrical output will control the cell’s current–voltage characteristics. The slow step may occur before or after the electron transfer step and may not involve charged particles. If the products or reactants of the non electrical reaction participate in electron transfer, then potential will affect the kinetics by its effect on reactant activity. This potential dependent for the non current producing reactions are due to chemisorption prior to electron transfer and a surface reaction following the ion discharge.
In a fuel cell electrode process, the reactant is assumed to be adsorbed at an active site on the electrode prior to electron transfer or surface reaction (Mathias et al. 2003). The activation energy may often be low for gases such as hydrogen on a typical fuel cell catalyst such as platinum, the adsorption of a hydrocarbon may require as much as 10 kcal, thereby presenting a formidable barrier to higher currents. At high current densities, depletion of reactants at the electrode may occur when they are removed by the electrode process faster than that can be supplied through diffusion. Product water may also accumulate faster than that can diffuse away from the reaction sites, which results in electrode potential change. Generally, these potential losses are very large in comparison to those attributed to activation polarization. When the reactant activity approaches zero, the current density is limited by the rate of mass transfer and the electrode is being described as concentration polarized. In PEMFC, slow transport of gas through porous electrodes or ions through the electrolyte may result in concentration polarization. An improvement in diffusion layer characteristics, minimizing the mass transport problems, is particularly useful for the cathode, mainly working at elevate pressures in H2/air operation (Velan et al. 2014).
The electrochemical oxidation of hydrogen is extremely facile on a Pt group catalyst (Rajalakshmi et al. 2017). When operating with pure hydrogen at practical current densities, the anode potential is less than 0.1 V (vs. Reversible hydrogen electrode). Under such operating conditions, the cell potential is only slightly lower than the cathode potential and the fuel cell performance effectively reflects the cathode operation. The over potential for the hydrogen oxidation reaction is considerably lower than that for the oxygen reduction e.g., in a PEMFC operating at current densities of 1 A cm−2, the over potential at the hydrogen electrode is about 20 mV and at the oxygen electrode it is about 400 mV. About one-half of the over potential at the oxygen electrode is due to its loss at the open circuit. The departure of the potential of the PEMFC from the reversible value is due to the extremely low exchange current density (io) for oxygen reduction (about 10−9 A cm−2, very low if compared to that for the electro oxidation of hydrogen, 10–3 A cm−2) on smooth platinum electrodes. Even after over 50 years of research, a conclusive mechanism for the intermediate and the rate-determining steps for this reaction on different types of electrocatalysts have not been arrived. This is unlike in the case of the two-electron transfer hydrogen oxidation reaction, where there is definitive evidence for the reaction pathway.
One of the major problems with the Pt electrocatalysis for hydrogen electrode is its low tolerance to CO in H2 from reformed fuels (Gasteiger et al. 2005). The improvement in the ORR electrocatalysis on Pt alloy catalysts has been due to several factors such as electronic and structural effects. Usually, such carbon-supported Pt alloy catalysts were prepared by the impregnation of the second metal on Pt/C and then by alloying at temperatures above 700 °C under inert gas or hydrogen. This heat treatment at high temperatures gives rise to an undesired alloy particle growth, which may result in a decrease in Pt mass activity (MA) for the ORR. Also, the control of the particle size distribution with this preparation method is quite limited. Pt alloy catalysts could also be prepared by the co-reduction of the metallic salt precursors at low temperatures and that the obtained Pt alloy particle sizes are relatively small. Another alternative way to tailor the nanosized Pt-based alloys for different purposes is the use of organometallic compounds as precursors. By thermal decomposition or reduction treatment of precursors, small nanoparticles of metal or alloy with narrow size distribution could be obtained. Among the various precursors used, metal-carbonyl complexes are often employed for preparing carbon-supported metal or alloy catalysts. It is known that, among the various Pt-based alloy catalysts used for the ORR, the Pt–Cr alloy is stable in acidic and oxidizing media at high temperature, whereas the Pt–Cu and Pt–Fe alloys are unstable under fuel cell operating conditions. Pharkya et al. (Pharkya et al. 2005) have used a novel method (high energy ball milling) to prepare Pt–Co catalyst for oxygen reduction and find that the performance of this catalyst is superior to the conventional Pt–Co catalyst. A study on platinum supported on hydrous metal oxides (Swider-Lyons et al. 2003) has shown that these catalysts are at least six times more active than 20% Pt/C. These materials have open framework structures.
PEMFC face an efficiency loss, so called “oxygen gain”, when cathode gas is changed from oxygen to air. Prasanna et al. (2004) found that oxygen gain can be reduced by optimizing the surface area and porosity of the carbon support and by coating the catalyst on membrane than on the GDL. The ionomer content, solvent composition and evaporation rate during the preparation of catalyst ink also affects the catalyst layer microstructure which in turn the mass transport and kinetics of the reaction (Rajalakshmi et al. 2004a).
Catalyst Deposition and Membrane Electrode Assembly
Catalyst Deposition Electrochemical energy conversion for technical applications relies on a high catalytic reactivity. The electrocatalytic reactivity is strongly influenced by the structure and composition of the surface of the catalysts. In PEMFC the catalysts consist of Pt or Pt alloys, which are of nanometer size and often supported on carbon to optimize the surface area and the costs of the noble metals. However, the important relation between the reactivity and the structure is obscured in technical electrocatalysts by a variety of parameters, such as the properties of the carbon support, the preconditioning of the catalyst, and the structure of the interface between the electrolyte and the active layer (e.g. the Nafion® content (Rajalakshmi and Dhathathreyan 2007a). An excellent review on the PEM fuel cell electrodes is given by Litster and McLean (Litster and McLean 2004). The catalyst layer is in direct contact with the membrane and the gas diffusion layer, referred to as the active layer.
In both the anode and cathode, the catalyst layer is the location of the half-cell reaction. The catalyst layer is either applied to the gas diffusion media or to the membrane. In either case, the objective is to place the catalyst particles, platinum or platinum alloys, in close proximity of the membrane. There exist many methods for catalyst preparation and application to fabricate a GDL/catalyst assembly viz., Spreading, Spraying, Ionomer impregnation, Electro-deposition and Catalyst powder deposition, Impregnation reduction, Evaporative deposition, painting, dry spraying Catalyst decaling and Casting method, Colloidal method, controlled self assembly, graded catalyst layer, multiple layer sputtering, electro spraying, etc., which are described well (Vedarajan et al. 2019; Dhathathreyan et al. 2017).
Membrane Electrode Assembly
The membrane and electrode assembly (MEA) is the ‘heart’ of the PEMFC. Its structure and composition are of vital importance. They are to minimize all forms of over potential and maximize the power density, to minimize the noble metal loading (and thus, the cost per kW of the PEMFC) in the gas diffusion electrodes by high utilization of the surface areas of nano-sized particles of the electrocatalyst, for effective thermal and water management (the latter including operation at the PEMFC without external humidification and to attain lifetimes of PEMFCs. The design of MEA could vary depending on the application of the fuel cell. Major breakthrough in this development was achieved during the late 1980s and in the early 1990s. There was tenfold reduction in platinum loading from about 4 mg cm−2 leading to the demonstration of high power density PEMFCs with electrodes by optimization of not only the structure of the electrode but also by that of the MEA. In the late 1990s, other significant increases in power densities, with even further reduction in platinum loading (to a level of about 0.05 mg cm−2 for the hydrogen electrode and 0.1 mg cm−2 for the oxygen electrode) were achieved by deposition of thin active layers of the supported electrocatalyst and proton conductor on an un-catalyzed electrode or on the proton-conducting membrane. Recently Liu et al. reported the preparation of High Performance and Ultra-Low Platinum Loading Membrane Electrode Assembly for PEMFC Commercial Application (Liu et al. 2019), where a continuous ultrathin cathode catalyst layer that was 3 μm thick was successfully prepared. The performance of MEA-PtCoMn/C with an ultralow Pt loading of 0.147 mg cm−2 was evaluated using the single-cell test. The highest achieved power density of MEA-PtCoMn/C was 1.42 W cm−2. The corresponding amount of platinum was 0.1035 gPt/kW, which reaches the index of the Department of Energy (DOE).
An equally important advantage of such types of electrodes is the increase in platinum utilization from about 20–25% to 50–60%. It is worthwhile stressing at this point that minimizing ohmic over potentials is vital for attaining high power densities; and this was made possible using supported membranes (prepared by impregnation of Nafion into microporous Teflon mesh, invented by W.L. Gore and associates and by deposition of very thin active layers (about 10 nm), containing only the carbon-supported platinum nano crystallite and Nafion, directly on the supported membrane. This process reduced the contact resistance. Thus, this MEA has shown the best PEMFC performance to date and is being widely used by fuel cell developers in the USA and Japan (Yang et al. 2001). The degradation of MEA’s with respect to performance over a period of time has been studied by Cheng et al. (2004) and reported that the formation of metal oxides at the anode catalyst layer leads to large particle size and hence the decrease in catalytic activity. Lindermeir et al. (2004) have reported a tuned layer preparation and coating technology for making MEAs which is suitable for large scale production. The production sequence used is: wet ball milling, wet spray coating and calendaring. E Tek de Nora has developed a dual beam assisted platinum multiplayer deposition in high-temperature membrane V for improved mass transfer with less interfacial contact resistance (De Castro 2005). Similarly, 3 M has developed a technique for MEA fabrication in high volume with enhanced operating conditions (Debe 2005).
Bipolar Plates
In a fuel cell stack, bipolar also known as flow field or separator plates with certain topologies typically have four functions viz., distribution of fuel and oxidant within the cell, separation of the individual cells in the stack, facilitation of water and thermal management within the cell, and current collection. There are several types of materials that are being used in bipolar plates. The selection of a bipolar plate is based on the flow fields, system requirements and materials. Flow fields are generally machined, stamped on the bipolar plates. Bipolar plate materials include graphite, metallic plates with or without coating and a number of composite structures. Sheet metal, graphite foil and graphite polymer composites are potentially low-cost materials, and in principle suitable for mass production. High-purity electro graphite is an excellent material for machining prototype plates, but material costs and process costs are generally considered high for mass production.
Carbon–carbon composite is not expected to achieve cost price targets, and needs expensive post processing. Flexible graphite is a thin, low density, inexpensive material made from expanded natural graphite. Topologies can include straight, serpentine, or inter-digitated flow fields, rigid or flexible plates, internal or external manifolding, internal or external humidification, and integrated cooling (Millichamp et al. 2015). There are over 100 topology-material combinations and related fabrication options for PEMFC bipolar plates and their requirements have been grouped into four categories: stack performance related design criteria, system performance (for the vehicle, building, or other product or process needing power) related design criteria, manufacturing related design criteria, and environmental impact related design criteria. Most researchers have discussed the bipolar plate design for the former two categories, and very few investigations in manufacturing and environmental design requirements. Mehta and Cooper (2003) analyzed bipolar plate design focusing on requirements for the stack and automotive performance, Design for Manufacturing (DFM) and Life Cycle Design (LCD) and has arrived at lists of 51 requirements and 69 engineering characteristics which can be used for quantitative analysis or as a qualitative guide.
Recently Stein et al. reported the integrity maintenance of TiN PVD coating of AISI416, and the advantage of this combination lies in the fact that the coefficients of thermal expansion (CTE) of this alloy is close to the CTE of the TiN film (Stein and Ein-Eli 2019).
Reactant Flow Field
To supply the fuel and oxidant to the fuel cell the bipolar plates have flow fields which are macroscopic channels running along with the bipolar plates. Computer simulation studies are being carried out extensively in recent times to optimize the design of the flow field. The feed channels can vary from ‘through’-channels, to dead-end channels where all the reactants are expected to be consumed within the cell, except for reformate fuel and air oxidant. In the simplest case at constant inlet flux, ideal humidification of membrane, and negligible losses on hydrogen side, the lower the stoichiometry ratio on the cathode side, the more oxygen will be consumed close to the inlet and the less left to consume close to the outlet. This will result in a highly nonuniform current distribution, an aspect which is overlooked in most fuel cell literature in which average current densities are reported (Gopi et al. 2020). If the downstream part of the channel is thus starving, then this later region of the cell will underperform or not perform at all. Similarly higher stoichiometry will lead to a more uniform reactant distribution, but this has the consequence of reducing the overall efficiency of the system. There need to be a balance between the overall reactant utilization, and the current distribution. Increasing the reactant utilization seems to require greater non uniformity of current distribution and hence greater energetic losses within the fuel cell. Minimization of energetic losses within the fuel cell requires higher stoichiometry which in turn lowers the overall fuel utilization. Understanding the laws of fuel consumption helps to rationalize the working regime of the fuel cell and provides hints towards promising cell and stack structural modifications.
In a fuel cell the current density distribution on all scales is of high interest for the understanding and the optimization of cell structures with respect to power density (Rajalakshmi et al. 2002). The size and ratio of the flow field ribs and the anisotropic electric conductivity of the GDL are the major issues on a sub millimeter scale. To examine and optimize these parameters, the processes occurring on this scale have to be investigated. The problem is not trivial, because the fuel cell local current generation is not constant over the MEA area covered by flow field ribs and the area covered by channels. Local current density on this scale can be modeled by accounting for the reactant flow limited by diffusion and the electrochemical reaction limited by electric resistance, as done in numerous scientific publications. These calculations are mostly based on assumptions and neglect for example the anisotropy of the GDL conductivity. It is therefore highly necessary to conduct experiments for gaining input information on simulations. Factors that lead to in homogeneities in current density distribution are limitations due to electric resistance in the GDL and reactant gas diffusion. Furthermore, the presence of liquid water at the electrodes adds to the complexity (Rajalakshmi et al. 2004b).
PEMFC Stack Construction Methods
PEMFC stacks are constructed by connecting a number of cells in series or parallel depending on the voltage and current requirement with gas distributors (bipolar plates) that feed the fuel to the anode and the oxidant to the cathode. Fuel cell stack construction using bipolar plates gives very good electrical connection between one cell to the next (Rajalakshmi and Dhathathreyan 2008). However, it does have the problem that there are many joints and potential problems or reactant gases and coolant leaks and hence careful manufacturing practices need to be followed. The repeat units are: gasket – MEA—gasket – bipolar plates. The reactants to each cell can be supplied from a common gas manifold externally or internally. The non-repeat components in the stack assembly are: stack housing, endplates, tie rods, current collectors, insulators, etc., Endplates serve as structural members for the fuel cell stacks made from cast aluminum alloy. Two electrical insulators die cut from elastomer sheet are required to isolate the endplates from the electrically charged current collectors of the stack. Tie rods with associated lock washers are used to compress the fuel cell stacks for proper contact as well to prevent the leaks. In micro fuel cells, there are different methods employed to connect the cells in series or parallel which many times avoid using a bipolar plate. In another topology especially useful in micro fuel cells, is using a single compartment for the air and another compartment for fuel, the compartments being made from low-cost plastics. On a single sheet of polymer electrolyte, a number of electrodes matching each other on each side are placed and laminated. Edge connections using very thin platinum or gold wire are used in such cases to build the voltage. These are known as flat fuel cells and this concept is well explained by Henzel et al. (1998).
Hydrogen for Stationary Power Applications
Hydrogen and fuel cell technologies offer greater personal choice in the transition to a low-carbon economy, given their similar performance, operation and consumer experience to fossil-fuelled technologies. Hydrogen and fuel cells are seeing a new interest recently as can be seen from the large-scale production of fuel cell vehicles and hundreds of thousands of homes are now heated and powered by fuel cells. A key difference since the last hydrogen “hype cycle” in the 2000s is that manufacturing scale up and cost decreases mean hydrogen and fuel cells are being commercialised in several sectors, from portable electronics and backup power to fork-lift trucks. In addition, energy systems analyses have become more sophisticated in identifying the complexity of decarbonising heat and transport via full electrification, and thus the need for flexible and storable energy vectors. Internationally thirteen corporations have formed the Hydrogen Council to position hydrogen among the key solutions of the energy transition to address the challenges around its complexity and diversity. Stationary fuel cells have been labelled for a long time as a technology of the future. The overall objective of this application area for the mid-term is to improve the technology while reducing the total installed cost of fuel cell stacks and balance of plant components to the level required by the stationary power generation and CHP markets. This will be accomplished by bridging the gap between laboratory prototypes and pre-commercial systems. Stationary power covers any application in which the fuel cells are operated at a fixed location for primary power, backup power, or CHP. The stationary sector includes both large-scale (200 kW and higher) and small-scale (up to 200 kW) and a wide range of markets including retail, data centers, residential, telecommunications and many more (Weidner et al. 2019). The major commercially available stationary PEM-based fuel cells system for various ranges are shown in Table 2.
The stationary fuel cell industry has experienced healthy growth due to a surge in US and foreign governments interest in reliable and resilient energy sources. The sector is now at a point where, if, all government policy relevant to stationary fuel cells was carried out, the global market potential would increase to more than 50 GW by 2020. Further as per Pike research, nearly 2.5 million telecom towers will be supported by fuel cell-based back-up power system across the globe (Curtin and Gangi 2015).
PEMFCs are increasingly installed to provide reliable backup power to expand communication networks in rural, rugged and remote areas that may not have an established electrical grid or infrastructure. The backup and remote power fuel cell markets continued expanding into India with several companies making substantial sales and deliveries to telecommunications companies there, as well as in other parts of the world (Felseghi et al. 2019; Curtin and Gangi 2016).
Ballard Power delivered its three-thousandth ElectraGen fuel cell backup power system. The delivery was part of a 100-unit order of ElectraGen™-ME systems by Reliance Jio Infocomm Ltd., an Indian telecommunications company. Ballard also sold 50 of its ElectraGen™-H2 systems to Aditya Birla the Idea Cellular network in India. Intelligent Energy announced it will purchase contracts from GTL Limited to supply energy-management services across more than 27,400 telecom towers in India. Essential Energy India, Intelligent Energy’s partner intends to transition around 70% of GTL’s telecom towers from diesel power to hydrogen fuel cells throughout the contracts’ tenure (Government of India 2016).
Hydrogen Powertrains for Transport Applications
Fuel cell electric vehicles (FCEVs) predominantly use PEM fuel cells, due to its various advantages such as instant start-up, high efficiency, and modular nature,. A 60 kW fuel cell is typical for European cars, which is substantially larger than for residential fuel cells. Competing powertrains include conventional internal combustion engines (ICEs), battery electric vehicles (BEVs) and plug-in hybrid vehicles (International Energy Agency 2015).
Recently expert assessments of the cost and expected future performance of proton exchange membrane fuelcells for vehicles have been reported by Whiston et al. (2019). This study characterizes the uncertainty associated with PEMFCs’ future trajectory, identifies barriers to improving cost and performance, and prioritizes research and development (R&D) areas. These results could be used to inform technology roadmaps and future R&D funding. Experts suggested that PEMFCs would meet the ultimate cost and performance targets of the US Department of Energy (DOE) but would fall short of the DOE’s 2020 cost target and could also serve as inputs into cost models.
Fuel Cell Vehicle stock exceeded 12,900 vehicles as of end 2018 showing 80% increase in 2018, around 46% of the vehicles are in the US, followed by Japan (23%) and China (14%). Most of the vehicles being passenger cars in the rest of the world, and the Chinese stock is dominated by commercial vehicles. The distribution of vehicles worldwide is shown below in Fig. 4.
If Hydrogen powertrains are compared to alternatives they differ in the following ways.
-
1.
Higher Capital cost and operating cost ($65 K for the Toyota Mirai or Hyundai and $30 K for BEV). Cost reduction is possible as manufacturing volumes rise.
-
2.
Higher Range and less refuelling time: (ca. 500 miles and 3 min).
-
3.
Infrastructure requirements: Hydrogen filling stations can serve substantially more vehicles than EV chargers, and a wider radius due to greater FCEV range. However, Hydrogen refuelling stations are currently more expensive than electric charging stations ($1.5 m versus $1000 for slow chargers), and are expected to fall by two-thirds once the technology matures.
-
4.
Lifetime: Battery lifetimes are affected by local climate, overcharging, deep discharge and high charging/discharging rates (Tesla expect batteries to last 10–15 years, yet most BEVs are 5 years old so such lifetimes are unproven).
Hydrogen tanks can undergo fast refilling and frequent, deep discharging without compromising lifetime, and fuel cell stacks are expected to outlive other drivetrain components.
-
5.
User experience: FC Vehicles offer a smoother driving experience than ICEs (quieter, less vibration and no gear shifting). However, hydrogen tanks are large and inconveniently shaped, potentially restricting luggage space.
-
6.
Emissions: FCEVs have zero emissions at the point of use and are low-carbon at the point of production if made from renewable-powered electrolysis, biomass or fossil fuels with CCS. The same is true for BEVs, whereas there is limited decarbonisation potential for ICEs. Blending biofuels with petrol and diesel can reduce CO2 emissions, but not improve local air quality.
-
7.
Network requirements: The refuelling infrastructure does not need the electricity network upgradation as required for significant BEV penetration, and offer valuable grid-balancing services.
-
8.
Safety: FCEVs have comparable, but different, safety considerations to BEVs and ICEs. Hydrogen is flammable (more so than petrol) but hydrogen fires can cause little damage to the vehicle due to their localised nature.
On a future vision, the international Energy Agency (IEA) concludes that FCEV sales could reach 8 million by 2030 in developed nations, and 150 million sales and a 25% share of road transport by 2050 (Antoni et al. 2019).
Worldwide there are 380 hydrogen refilling stations as of 2018, and the various European H2Mobility programs have suggested a rollout of refuelling stations at critical locations, with a network of 65 refuelling stations for the UK by 2020 to start the market, growing to 1150 stations by 2030 to cover the whole country. The distribution of hydrogen refilling stations are given below in Fig. 5.
The Hydrogen Council targets 3000 refilling stations globally by 2025, sufficient to provide hydrogen for about 2 million FCEVs, after which refuelling infrastructure should be self-sustaining. Return-to-base fleets such as delivery vans and taxis, or passenger cars in a future car-sharing economy will see high utilisation and benefit from single refuelling depots with fast, infrequent refuelling.
International Scenario—Targets, Visions and Projections
The Hydrogen Council is the largest industry-led effort to develop the hydrogen economy. Launched in January 2017 at the World Economic Forum, its members include leading companies that invest along the hydrogen value chain, including transportation, industry, and energy exploration, production, and distribution. The vision for hydrogen economy in 2050 is: Hydrogen is a central pillar of the energy transformation required to limit global warming to 2OC and to achieve this, the world is required to make dramatic changes and decrease energy-related CO2 emissions by 60% until 2050. Achieving this vision would create significant benefits for the energy system, the environment, and the global economy (International Energy Agency 2015). The various countries targets for fuelcell vehicles are shown in the following Fig. 6.
Many of the required technologies are already available today and now this is the is the time to deploy hydrogen infrastructure and scale-up manufacturing capacities and to achieve competitive costs and mass-market acceptance. In the transportation sector, hydrogen-powered FCEVs could complement BEVs and are best suited for applications with long-range requirements, heavier payloads, and a high need for flexibility. Decarbonizing this segment is particularly important as they consume a large share of total energy—while trucks and buses would account for only 5% of all FCEVs in 2050, they could achieve more than 30% of hydrogen’s total CO2 abatement potential in the transport sector (Adria et al. 2017; https://www.energy.gov/sites/prod/files/2017/10/f37/fcto2016_market_report.pdf).
FCEV buses, medium-sized cars, and forklifts are commercially available today throughout the world and the projections for various countries are given below in Table 3.
The next 5 years will see the introduction of more models in medium-sized and large cars, buses, trucks, vans, and trains, and it is likely that additional segments such as smaller cars and minibuses 2030. To realize the Hydrogen council vision of 1 in 12 cars sold in California, Germany, Japan, and South Korea by 2030, sales also starts ramping up in the rest of the world. More than 350,000 hydrogen trucks could be transporting goods, and 50,000 hydrogen buses, thousands of trains and passenger ships could be transporting people without carbon and local emissions. Towards 2050, the vision also includes hydrogen will be a carrier for renewable fuels for commercial aviation and freight shipping. Industries would like to bring down the costs of hydrogen and applications through the scale of the economy. Significant cost of refueling stations and fuel cell stack production has been cut in half in the last ten years, and expect major reductions in the coming years from scaling up manufacturing to industrial levels. Further cost reductions are also possible through cost reductions in the hydrogen production and renewable power generation for electrolysis.
National Mission on PEMFC Research in India and ARCI Programmes
India with a population of approx. 1.4 billion, with significantly increased energy access for citizens, availability of cleaner cooking fuel and growing penetration of renewables, the country is advancing on a faster growth path. India’s per capita energy consumption stands at 30% of the world’s average. Major consumption is in the Industrial sector (42%) followed by residential (29%), transport (17%) and services (12%). With the rise in energy demand the major impact will be on the environment. The energy sector is considered to be the major source for environmental concerns, including the local air pollution, GHG emissions and thus accounting for the implications associated with climate change. The Government of India has laid several policies and is targeting to reduce emissions and lay down a path for economic growth. The country is determined to contribute under the Paris agreement towards reducing the emissions and increasing the share of non-fossil fuels in the power generation sector.
The National Action Plan on Climate Change was launched in 2008 to address the development objectives as well to address the climate change issues effectively. The Government of India has initiated several National missions, viz., National Mission for Enhanced Energy Efficiency, National Solar Mission, National Electric Mobility Mission, National Smart Grid Mission, National Mission on Advanced Ultra Super Critical Technology, National Mission on Transformative Mobility and Battery Storage, etc., Ministry of New and Renewable Energy constituted a high-level committee to come up with Hydrogen energy roadmap. The National Hydrogen Energy Roadmap was laid in the year 2006 to provide a blueprint for the long-term public and private efforts which was required for hydrogen energy development in the country. Department of Science and Technology has taken up the development of hydrogen and fuel cell technologies in a mission mode. The focus is towards creating volumes and infrastructure, towards demonstration of niche applications, provide facilitative policy support and provide symbiotic international linkages. The hydrogen mission will be directed towards the advancement of hydrogen and fuel cell technologies through R&D and validation, to make technologies competitive in cost and performance, to reduce institutional and market barriers towards commercialization (Mission Innovation and document 2020).
In India, a large number of groups are engaged in the research, development and demonstration activities of PEMFC and yet to reach the manufacturing capability and commercialization. A few Government organizations such as CSIR labs, ARCI, NMRL, VSSC and BHEL are engaged in complete development of PEMFC system. There exists a lot of scope for improving the product with sufficient engineering inputs, and capable of producing in large numbers (Basu 2006). The Energy and Resources Institute (TERI), under the Energy Transitions Commission India work programme, has been assessing technological and economic trends in various energy-producing and consuming and concluded that green hydrogen (produced from renewable electricity) has huge potential in India’s energy transition. In transport, this can be used to fuel longer-range vehicles and heavier-duty trucks, in an industry largely as a chemical feedstock, and in the power sector, to provide longer-term energy storage (Hall et al. 2020).
International Advanced Research Centre for Powder Metallurgy and New Materials (ARCI) is an Autonomous Research and Development Centre of Department of Science and Technology (DST), Government of India with the main campus at Hyderabad and with operations in Chennai and Gurgaon. ARCI's mandate lies in the development of High-Performance Materials and Processes for Niche Markets, Demonstration of Technologies at Prototype/pilot scale and Transfer of Technology to Indian Industry. Centre for Fuel Cell Technology, a unit of ARCI is located at IIT-M Research park in Chennai. CFCT works on several aspects of hydrogen and fuel cell technologies with the major objective at present being the development of polymer electrolyte membrane fuel cells (PEMFC), one of the low-temperature fuel cells, in its entireness and demonstrate its application in Decentralised power generation systems and transportation applications. The work includes several aspects of materials development, stack design and engineering, the balance of system development, system design and engineering and carrying out field trials. In addition to the PEMFC development, the Centre is also engaged in other types of fuel cells and also developing hydrogen generation and storage materials and technologies. One of the mandates of the Centre is to include industries in its development as also eventual commercialization. Knowledge dissemination and manpower generation are other objectives of the Centre.
The Centre has been at the forefront of PEMFC development in the country. During the last 15 years, the Centre has developed process know-how for the various components used in the fuel cell stacks, built fuel cell stacks modules of up to 10 kW capacity, integrated system of upto 20 kW capacity, demonstrated fuel cell-based power packs with the required Balance of systems for Decentralised power generation and demonstrated fuel cells in an electric vehicle for use as range extenders. As part of the hydrogen technology development, the Centre has developed technology for hydrogen generation. The R&D activities address the issues of performance improvement and cost reduction. From the fundamental R&D side ARCI has focussed on selecting good catalysts with high electrochemical surface area by various deposition methods. Ramya et al. (2006) studied the ePTFE membrane for fuelcells and the SEM image is shown in Fig. 7.
SEM micrographs of: a porous PTFE membrane, b Nafion impregnated PTFE at low magnification, c Nafion® impregnated PTFE at high magnification and d Nafion® impregnated PTFE with hexane solvent. e Transverse section of Nafion® impregnated porous PTFE (Ramya et al. 2006)
For PEMFC air electrode, the hydrophobic coating on the substrate materials (GDM) plays an important role in the performance. High loading of PTFE will increase the resistance of the cell and with low PTFE loading the resistance is low but the mechanical strength of the electrode is poor (Velayutham et al. 2007). Initially, studies were carried out by varying the PTFE loading on the GDM from 7 to 30 wt.%, keeping all the other parameters constant. The current–voltage polarisation curves measured at 55 °C and at ambient pressure of hydrogen and air are shown in Fig. 8. Functionalisation of carbon materials used for gas diffusion electrodes has also been analysed at ARCI (Lakshmi et al. 2006).
The systematic study of the performance of three different types of fuel cells with low loadings of nano-crystalline Pt–Ru and Pt–Sn electrocatalysts at the anode (0.25 mg cm−2 for H2–O2, 2.5 mg cm−2 for CH3OH–O2 and C2H5OH–O2) and with Pt-free Co-PPY-MWCNT (Co loading: 0.5 and 2.5 mg cm−2, respectively for PEMFC and DAFC) at the cathode with the fuel injection accomplished at atmospheric pressure highlights the advantages of using the emerging nanotechnology to the fuel cell technology with CNT supported nanocrystalline electrocatalysts has been studied (Reddy et al. 2008). The SEM/TEM pictures of some of the catalysts developed are shown in Fig. 9.
The catalyst coating either on the substrate or on the membrane determines the efficiency of the fuel cell. Compared to conventional spraying, brushing, ARCI has adopted a new process of coating on the membrane or by decal process (Rajalakshmi and Dhathathreyan 2007b). The coating procedure consists of positioning the substrate layer under the silkscreen mesh, which is not masked, and squeegee on top of the mesh at one side. The schematic of the coating facility is shown in Fig. 10. The appropriate amount of the ink is micropipetted near the squeegee and the slurry is spread across the mesh and then pushed through the mesh to the substrate layer by moving the squeegee quickly. The hot air heater is turned on to dry the catalyst layer coated on the substrate. The same procedure was repeated until the pipetted volume of catalyst ink is being transferred to the substrate layer.
The other methods of coating include pulsed electrodeposition directly in membrane, as reported by Rajalakshmi et al. (2008b). Platinum supported on carbon is generally used as a catalyst in fuel cells. Carbon is most widely used as a catalyst support as it has a very high surface area and very good electronic conductivity. In addition to surface area, the charge–discharge properties of the catalyst support also play a critical role. Durability of the catalysts in PEMFC for transport applications is one of the major issues as the oxidation of carbon catalyst supports causes degradation in catalyst performance during cycling in PEMFC. Alternative supports that can withstand the corrosive environment of PEMFC’s are currently required. ARCI has done extensive work on using different support materials starting from graphene, TiO2, MnO2, ZnO, CNT, MWCNT, N doped MWCNT, etc., (Vinayan et al. 2012; Karthika et al. 2012, 2014; Jafri et al. 2009, 2010a, b, 2015; NeetuJha et al. 2011; Aravind et al. 2011; Jyothirmayee Aravind et al. 2010; Mohana Reddy et al. 2010; Jha et al. 2008; Rajalakshmi et al. 2008a; Shaijumon et al. 2005; Manikandan et al. 2015). Some of the SEM pictures of these alternative catalyst support materials with their performance is shown in Figs. 11 and 12.
ARCI has also done research on Pt electrocatalysts without any support (Prithi et al. 2017b; Karthika et al. 2013a,b) such as mesoporous platinum and also mesoporous Pt with Ru, CO and Ni as various oxidation catalysts that includes hydrogen, and methanol,. A typical mesoporous Pt/Ni with its methanol oxidation characteristics is shown in Fig. 13.
The major concern for PEMFC is the requirement of pure hydrogen and air from atomosphere. ARCI has taken up mitigation studies at both single-cell level, stack level, high-temperature PEMFC (Prithi et al. 2017a, 2017c; Jayaraj et al. 2014; Unnikrishnan et al. 2018; Kakati et al. 2016; ViswanathSasank et al. 2016; PrithiJayaraj et al. 2014) experimentally as well by modelling for various contaminants such as S, Chlorine, Marine atmosphere and the possible recovery mechanisms are well explained. The sulphur coverage in mesoporous Pt and Pt black is shown in Fig. 14 and chlorine contamination and the recovery is shown in Fig. 15
The schematic of marine environmental condition and the PEMFC stack before and after recovery is shown in Fig. 16.
Modelling of HT PEM studies were also done at ARCI, in collaboration with IIT Hyderabad (Unnikrishnan et al. 2017, 2019; Anusree Unnikrishnan et al. 2018), as shown in Fig. 17 and electrochemical impedance spectroscopy has been used as a tool for the evaluation of flow field geometry as well for porous electrodes (Prithi et al. 2017a; Kakati et al. 2016) and is shown in Fig. 17.
The cost–benefit analysis of two different grades of graphite plates were studied by Rajalakshmi et al. (2006) and the piechart in Fig. 18 shows the analysis.
It has been concluded that these benefits such as cost and volume can be valuable in transport applications as there was not much advantage in weight. Pandian et al. (2008) studied the thermal heat evaluation from a fuel cell stack and is shown in Fig. 19. This study is aimed to know the ability of the heat fluxes to cross through the various layers of the cells and to quantify the heat by taking in to consideration of the operating current, pressure, flow rate, channel dimensions, coefficient of heat transfer coolant volume, etc. The distribution of temperatures is obtained for different current densities. An analytical method has been developed to find out the change in current and resistance due to unit change in temperature at a particular current density, without changing the coolant flow rate. It has been observed that the thermal and electrical energy output is directly related to the variables in the process as well as the process also. This approach will be helpful in identifying the thermal and electrical output for a PEM-based fuel cell power plant of large capacity for cogeneration.
To have an efficient thermal management system, two-loop closed thermal management configuration was designed specifically for transport application at ARCI (Bethapudi et al. 2012). The thermal management configurations using a plate heat exchanger and radiator fan assembly have been developed, which are helpful for effective heat management in the fuel cell. The schematic and the thermal power in the primary and secondary circuit is shown in Fig. 20.
The cumulative heat transfer from all the configurations, parameters such as flow rate of coolant and coolant inlet temperature play a major role in the optimum design of the system. It has been observed from these studies that the configuration Rp with radiator/fan assembly positioned in the primary loop resulted in the maximum heat removal from the fuel cell for any given flow rate of coolant.
To reduce the fuel cell system complications due to humidity, reactants stoichiometry, coolant requirements for thermal management, pressure drop in the manifold, flow field design, uniform current collection, MEA lamination, etc., it has been decided to develop stacks smaller in size followed by connecting them in series or parallel depending on the power requirement. Rajalakshmi et al. (2008b) has discussed the various concepts, design, prototype manufacturing and short term evaluation of small stacks in modular architecture and describes the results in the development of a water cooled 250 W modular PEMFC stack for operation with Hydrogen and air and is shown in Fig. 21. Design for Forced air cooling stacks, water cooled stacks along with pressure drop analysis by numerical simulation as well by CFD analysis has also been studied at ARCI (Gopi et al. 2020; Dhathathreyan et al. 2012; Pandiyan et al. 2013).
A fuel cell stack for use in transportation or in applications which involves the positioning of such systems in a location of high vibration and shock is subjected to accelerated stress screening to ascertain the reliability of the stack, mechanical integrity and also to assess the mounting requirements. ARCI has done the vibration test analysis on a 500 W Proton Electrolyte Membrane (PEM) fuel cell stack developed at the centre by simulating some of the vibration, shock and resonance in the stack and the likelihood of the stack to undergo in any application and evaluated the robustness of the stack (Rajalakshmi et al. 2009a). An experimental setup was designed for this purpose consisting of subjecting the PEM fuel cell stack to random and swept-sine excitations on a vibrating platform in three axes and measuring the mechanical response using accelerometers fixed at various locations in the stack is shown in Fig. 22.
The fuel cell performance (pre-vibration test and post-vibration test) as obtained from polarization studies and the power–amperage curves does not show any significant damage effects, and a post-testing stack inspection showed a minor torque release, as shown in Fig. 23.
To reduce the number of experiments Taguchi analysis for electrode fabrication as well stack operation has been undertaken at ARCI. The performance of the fuel cell electrode depends on many factors: types of materials and their properties, composition, process parameters and fuel cell operation conditions. In the present paper, cathode electrode performance in a PEM fuel cell as a function of Teflon concentration in the substrate materials and in micro-layer carbon, pore former in the micro-layer, amount of carbon used in the diffusion layer and Platinum and Nafion loading in the catalyst layer are studied. These six factors each at two levels are considered (Velayutham et al. 2008). A full factorial design would have required 64 experiments to be carried out. With the use of Taguchi method, L12 designs, the number of experiments can be reduced to 12. The electrode current density values are taken as responses for the analysis. Statistical sensitivity analysis (ANOVA analysis) is used to compute the effects and the contributions of the various factors to the fuel cell electrode. Some graphic representations are employed to display the results of the statistical analysis made for different current values.
Similarly statistical analysis to a 2.5 kW proton exchange membrane fuel cell stack operation, by experimental design methodology, whereby robust design conditions were identified for the operation of fuel cell stacks (Rajalakshmi et al. 2008c). The function is defined as the relationship between the fuel cell power and the operating pressure and stoichiometry of the reactants. Four types of control factors, namely, the pressures of the fuel and oxidant and the flow rates of the fuel and oxidant, are considered to select the optimized conditions for fuel cell operation. The experimental data collected were analyzed by statistical sensitivity analysis by checking the effect of one variable parameter on the other.
Power conditioning is one of the important topic in developing a fuel cell system as fuelcell produces a variable DC power. An important objective is to reduce the ripple current, configurable with other power sources, and evaluate the dynamic performance under operating conditions. ARCI in collaboration with Anna University, Chennai has modelled and developed a few power conditioners using the multiphase converter, reconfigurable hybrid system, etc. (Latha et al. 2012, 2015; Kumar et al. 2013; Naveen Kumar et al. 2013). The simulation model with various flow rates of fuel and oxidant are shown in Fig. 24.
ARCI has got some expertise in hydrogen generation using different catalysts and membranes both cation as well as anion exchange membranes and also some depolarisers (Narreddula et al. 2019; Manjula Narreddula et al. 2018,2019). As a part of the Hydrogen economy ARCI has explored a few activated carbon materials such as corn cobs, Tamarind seeds, and Jute fibres,, for hydrogen storage (Ramesh et al. 2015, 2017, 2018; Rajalakshmi and Ramesh 2017; Rajalakshmi et al. 2014b). In addition, an off shoot of PEMFC technology, carbon-based electrodes have been used for supercapacitors and metal-air batteries (Ramesh et al. 2020; Imran et al. 2019). Based on the various R&D carried out at ARCI, many PEMFC prototype systems were developed for both stationary and transport applications with all the BoP systems.
The use of PEMFCs for providing reliable backup power for communication networks is increasing steadily in rural and remote areas that may not have access for electrical grid or infrastructure. The providing backup and remote power using fuel cell system for telecommunications sector are expanding in India and several companies making substantial sales. Ballard Power has delivered about three-thousand PEM-based fuel cell system for backup power application for telecommunication sectors. Among these systems, about 100 systems were installed by Reliance Jio Infocomm Ltd., and about 50 systems were installed at Aditya Birla the Idea Cellular network in India.
India is participating in various committees of international standard-making bodies such as ISO, UN-ECE. These committees are developing global standards for Hydrogen, that could be adapted in the future by India. In Parallel, the BIS committee on hydrogen standards is working towards developing the standards by adapting international standards with modifications for India-specific conditions. Government can play a very crucial role with policy support by creating infrastructure, risk reduction, creating hydrogen market and providing benefits over use of low carbon energy chain. After initial take-off, cost reduction could be achieved by manufacturing capability and with the scale of economy. The role that Government can play is towards creating a long-term policy framework which could build up confidence in private investment, provide incentives, create market demand with policy interventions, develop standards and regulations for the growth.
Conclusions
It is commonly accepted that the cost of PEM fuel cell systems will continue to drop, particularly as the market grows, volumes increase and production becomes more competitive. The other technical advance that may be expected is increased cell and membrane lifetimes which will improve life cycle costs. Future development may see different schools of thought developing on stack life; as stacks designed for more frequent replacement may have overall lower lifecycle costs provided the replacement cost is sufficiently low. High hybridisation of PEM fuel cells with renewable energies and batteries is also improving and will reduce the frequency of hydrogen refills and consequently the total cost of ownership.
Momentum for hydrogen is building. Around 20 countries such as Japan, Korea, Germany, California, and china, are initiating the market scale-up through public–private partnerships and incentives. To realize the ambitious vision, major step ups are required by all stakeholders like increasing the investments until 2030 to double hydrogen production, provide distribution infrastructure, and produce equipment such as FCEVs, trains, heating equipment, and components at a competitive cost.
References
Adria I et al (2017) DOE Hydrogen and Fuel Cells Program Record Record #: 17007 Fuel Cell System Cost - 2017 Originator: Adria Wilson, Gregory Kleen, and Dimitrios Papageorgopoulos
Antoni et al (2019) AFC TCP 2019 Survey on the Number of Fuel Cell Vehicles, Hydrogen Refueling Stations and Targets
Aravind SJ, Jafri RI, Rajalakshmi N, Ramaprabhu S (2011) Solar exfoliated graphene–carbon nanotube hybrid nano composites as efficient catalyst supports for proton exchange membrane fuel cells. J Mater Chem 21:18199
Basu 2015 -Proc Indian Natn Sci Acad Proton Exchange Membrane Fuel Cell Technology: India’s Perspective vol 81. pp. 865-890
Bethapudi VS, Rajalakshmi N, Dhathathreyan KS et al (2012)-Design and optimization of a closed two loop thermal management configuration for PEM fuel cell using heat transfer modules. IJCEA. 3(4):243–248 ISSN: 2010–0221. https://doi.org/10.7763/IJCEA.2012.V3.194
Cheng X, Chen L, Peng C, Chen Z, Zhang Y, Fanc Q (2004) Catalyst microstructure examination of PEMFC membrane electrode assemblies vs. Time. J Electrochem Soc 151:A48–A52
Curtin S, Gangi J (2015) Fuel Cell Technologies Market Report
Curtin S, Gangi J (2016) Technical report: business case for fuel cells. https://doi.org/10.2172/1375462
De Castro ES (2005) Integrated Manufacturing for Advanced MEAs DOE Hydrogen and FC Program Review-2005, DE-FC04-02AL67606
Debe MK (2005) Advanced MEA’s for enhanced operating conditions, Amenable to High Volume Manufacture, DOE Hydrogen and FC Program Review-2005, DE-FC04-02AL67606
Dhathathreyan KS, Rajalakshmi N (2009) Challenges in PEM Fuel cell development. Indian Assoc Nucl Chem Allied Sci Bull 3:214–228
Dhathathreyan KS, Rajalakshmiet N et al (2012) Challenges in PEM Fuel Cell Development, in “Fuel Cells” INCAS Bulletin. Int J Electrochem 2012(3):216494. https://doi.org/10.1155/2012/216494
Dhathathreyan KS, Rajalakshmi N, Balaji R (2017) Nanomaterials for PEM Fuel cells. Wiley publishers, Hoboken
DST Mission Innovation document (2020) India Country Status Report on Hydrogen and Fuel Cells DST, New DElhi
Eisman GA (1990) The application of Dow Chemical’s perfluorinated membranes in proton-exchange membrane fuel cells. J Power Sources 29(3–4):389–398
Felseghi RA, Carcadea E, Raboaca MS, Trufin CN, Filote C (2019) Hydrogen fuel cell technology for the sustainable future of stationary applications. Energies 12:4593–4621
Gasteiger HA, Kocha SS, Sompalli B, Wagner FT (2005) Activity benchmarks and requirements for Pt, Pt-alloy, and non-Pt oxygen reduction catalysts for PEMFCs. Appl Catal B Environ 56:9–35
Gopi KH, Nambi A, Rajalakshmi N (2020) Design and development of open cathode PEM fuel cell—flow analysis optimization by CFD. Fuel Cells 20(1):33–39
Government of India (2016) MNRE: Report on hydrogen energy and fuel cells in India a way forward-steering committee meeting report, MNRE
Hall W, Spencer T, Renjith G, Dayal S (2020) The potential role of hydrogen in India. A pathway for scaling-up low carbon hydrogen across the economy. The Energy and Resources Institute (TERI), New Delhi
Henzel A, Nolte R, Ledjeff-Hey K, Zedde M (1998) Membrane fuel cells—concepts and design. Electrochim Acta 43:3817–3820
https://www.energy.gov/sites/prod/files/2016/10/f33/fcto2015_market_report.pdf.
https://www.energy.gov/sites/prod/files/2017/10/f37/fcto2016_market_report.pdf.
Hydrogen council (2017) A sustainable pathway for the global energy transition Hydrogen scaling up Hydrogen Council November 2017. https://hydrogencouncil.com/wp-content/uploads/2017/11/Hydrogen-scaling-up-Hydrogen-Council.pdf
Imran K, Ramya K, Ghosh PC, Sarkar A, Rajalakshmi N (2019) Ion immobilized bifunctional electrocatalyst for oxygen reduction and evolution reaction. ACS Appl Energy Mater 2(11):7811–7822
International Energy Agency (2015). https://www.iea.org/reports/technology-roadmap-hydrogen-and-fuel-cells
International Energy Agency (2019). https://www.iea.org/reports/the-future-of-hydrogen
Jafri RI, Sujatha N, Rajalakshmi N, Ramaprabhu S (2009) Au–MnO2/MWNT and Au–ZnO/MWNT as oxygen reduction reaction electrocatalyst or polymer electrolyte membrane fuel cell. Int J Hydrog Energy 34:6371–6376
Jafri RI, Rajalakshmi N, Ramaprabhu S (2010a) Nitrogen doped graphenenanoplatelets as catalyst support for oxygen reduction reaction in proton exchange membrane fuel cell. J Mater Chem 1–5
Jafri RI, Arockiados T, Rajalakshmi N, Ramaprabhu S (2010b) Nanostructured Pt dispersed graphene-multi walled carbon nanotube hybrid nanomaterials as electrocatalyst for proton exchange membrane fuel cells. J Electrochem Soc 157(6):B874
Jafri RI, Rajalakshmi N, Dhathathreyan KS, Ramaprabhu S (2015) Nitrogen doped graphene prepared byhydrothermal and thermal solid state methods as catalyst supports for fuel cell. IJHE 40:4337–4348
Jayakumar K, Pandiyan S, Rajalakshmi N, Dhathathreyan KS (2006) Cost-benefit analysis of commercial bipolar plates for PEMFC’s. J Power Sources 161(1):454–459
Jayakumar A, Singamneni S, Ramos M, Al-Jumaily AM, Pethaiah SS (2017) Manufacturing the gas diffusion layer for PEM fuel cell using a novel 3d printing technique and critical assessment of the challenges encountered. Materials (Basel) 10(7):796 (PMC5551839)
Jayaraj P, Jafri RI, Rajalakshmi N, Dhathathreyan KS (2014) Nitrogen doped graphene as catalyst support for sulfurtolerance in polymer electrolyte membrane fuel cells. Graphene 2:1–5
Jha N, Mohana Reddy AL, Shaijumon MM, Rajalakshmi N, Ramaprabhu S (2008) Pt–Ru multiwalled carbon nanotubes as electrocatalysts for direct methanol fuel cells. Int J Hydrog Energy 33:427–433
Jyothirmayee Aravind SS, Jafri RI, Rajalakshmi N, Ramaprabhu S (2010) Nitrogen-doped multi-walled carbon nanocoils as catalyst support for oxygen reduction reaction in proton exchange membrane fuel cell. J Power Sources 195:8080–8083
Kakati BK, Unnikrishnan A, Rajalakshmi N, Jafri RI, Dhathathreyan KS, Kucernak ARJ (2016) Recovery of polymer electrolyte fuel cell exposed to sulfur dioxide. IJHE
Karthika P, Rajalakshmi N, Imran Jaffri R, Ramaprabhu S, Dhathathreyan KS (2012) Functionalised 2D graphene sheets as catalyst support for proton exchange membrane fuel cell electrodes. Adv Sci Lett 4:1–6
Karthika P, Rajalakshmi N, Dhathathreyan KS, Abe H (2013a) Synthesis of mesoporousPt-Rualloy particles with uniform sizes by sophisticated hard templating method. Chem Asian J 8:902–907
Karthika P, Rajalakshmi N, Dhathathreyan KS, Abe H (2013b) Hard templating synthesis of mesoporous Pt based alloy particles with low Ni and Co contents. Chem Lett 42(4):447–449
Karthika P, Rajalakshmi N, Dhathathreyan KS, Arivuoli D (2014) Synthesis and electro-catalytic properties of platinum supported on graphene for methanol oxidation. J Nanosci Nanotechnol 15(12):97463. https://doi.org/10.1166/jnn.2015.10352
Kumar SN, Rajalakshmi N, Dhathathreyan KS (2013) Efficient power conditioner for a fuel cell stack-ripple current reduction using multiphase converter. Smart Grid Renew Energy 4:53–56
Lakshmi N, Rajalakshmi N, Dhathathreyan KS (2006) Functionalisation of various carbons for use in proton exchange membrane fuel cell electrodes—analysis and characterization. J Phys D Appl Phys 39:2785–3279
Latha K, Vidhya S, Umamaheswari B, Rajalakshmi N, Dhathathreyan KS (2012) Tuning of PEM fuel cell model parameters for prediction of steady state and dynamic performance under various operating conditions. Int J Hydrog Energy
Latha K, Umamaheswari B, Chaitanya K, Rajalakshmi N, Dhathathreyan KS (2015) A novel reconfigurable hybrid system for fuel cellsystem. IJHE 40:14963–14977
Lindermeir A, Rosenthal G, Kunz U, Hoffman U (2004) Improvement of MEAs for direct methanol fuel cells by tuned layer preparation and coating technology. Fuel Cells 4:78–85
Litster S, McLean G (2004) PEM fuel cell electrodes. J Power Sources 130:61–76
Liu S, Li S, Wang R, Rao Y, Zhong Q, Hong K, Pan M (2019) Preparation of high performance and ultra-low platinum loading membrane electrode assembly for pemfc commercial application. J Electrochem Soc 166(16)
Manikandan M, Vedarajan R, Kodiyath R, Abe H, Ueda S, Dakshnamoorthy A, Rajalakshmi N, Dhathathreyan KS, Ramesh GV (2015) Pt decorated free-standing TiO2 nanotube arrays: highly active and durable electrocatalyst for oxygenreduction and methanol oxidation reactions. J Nanosci Nanotechnol 15:1–10
Manjula Narreddula R, Balaji K, Ramya KS, Dhathathreyan N, Rajalakshmi AR (2018) Electrochemical methanol reformation (ECMR) using low-cost sulfonated PVDF/ZrP membrane for hydrogen production. J Solid State Electrochem 22:2757–2765
Mathias M, Roth J, Flemimg J, Lehnert W (2003) Diffusion media materials and characterization, Chapter 46. Handbook of fuel cells—fundamentals, technology and applications. In: Vielstich W, Gasteiger HA, Lamm A (eds) Fuel cell technology and applications, vol 3. John Wiley & Sons, Ltd, Hoboken
Mehta V, Cooper JS (2003) Review and analysis of PEM fuel cell design and manufacturing. J Power Sources 128:32–53
Millichamp J, Mason TJ, Neville TP, Rajalakshmi N, Jervis R, Shearing PR, Brett DJL (2015) Review-Mechanisms and effects of mechanical compression and dimensional change in polymer electrolyte fuel cells. J Power Sources 284:305–320
Mitsubhishi electric achieves domestic top level energy efficiency of 83% with PEFC cogeneration system using a Lossnay humidifier, www.global.mitsubishielectric.com. Release No. 2322
Miyake N, Wakizoe M, Honda E, Ohta T (2008) High durability of Asahi kasei aciplex membrane. Abstracts of 208th Meeting of the Electrochemical Society, Los Angels, CA, 2005
Mohana Reddy AL, Shaijumon MM, Rajalakshmi N, Ramaprabhu S (2010) Performance of PEMFC using Pt/MWNT-Pt/C composites as electrocatalysts for oxygen reduction reaction in PEMFC. J Fuel Cell Sci Technol 7:1–7
Narreddula M, Balaji R, Ramya K, Rajalakshmi N, Ramachandraiah A (2019) Nitrogen doped graphene supported Pd as hydrogen evolution catalyst for electrochemical methanol reformation. Int J Hydrog Energy 44(10):4582–4591
Narreddula M, Balaji R, Ramya K, Rajalakshmi N (2019) Hydrogen production by electrochemical methanol reformation using alkaline anion exchange membrane based cell. Int Journal of Hydrogen Energy. https://doi.org/10.1016/j.ijhydene.2019.08.202
Naveen Kumar S, Rajalakshmi N, Dhathathreyan KS (2013) Efficient power conditioner for a fuel cell stack—ripple current reduction using multiphase converter. Smart Grid Renew Energy 4:53–56
NeetuJha RI, Jafri N, Rajalakshmi SR (2011) Graphene-multi walledcarbon nanotube hybrid electrocatalyst supportmaterialfordirect methanol fuelcell. Int J Hydrog Energy 36:27284
Ozden A, Alaefour IE, Shahgaldi S, Li X, Colpan CO, Hamdullahpur F (2018) Gas diffusion layers for PEM fuel cells: ex- and in-situ characterization. In: Exergetic, energetic and environmental dimensions 2018, pp 695–727. https://doi.org/10.1016/B978-0-12-813734-5.00040-8
Pandian S, Jayakumar K, Rajalakshmi N, Dhathathreyan KS (2008) Thermal and electrical energy management in a PEMFC stack—an analytical approach. J Heat Mass Transf 51:469–473
Pandiyan S, Elayaperumal A, Rajalakshmi N, Dhathathreyan KS, Venkateshwaran N (2013) Design and analysis of a proton exchange membrane fuel cells (PEMFC). Renew Energy 49:161–165
Pharkya P, Alfantazi A, Farhat Z (2005) Fabrication using high-energy ball-milling technique and characterization of Pt-Co electrocatalysts for oxygen reduction in polymer electrolyte fuel cells. J Fuel Cell Sci Technol 2:171–177
Prasanna M, Ha HY, Cho EA, Hong S-A, Oh I (2004) Investigation of oxygen gain in polymer electrolyte membrane fuel cells. J Power Sources 137:1–8
Prithi JA, Rajalakshmi N, Ranga Rao G (2017a) Nitrogen doped mesoporous carbon support for oxygen reduction reaction in polymer electrolyte membrane (PEM) fuel cells. Int J Hydrog Energy 43(9):4716–4725
Prithi JA, Rajalakshmi N, Dhathathereyan KS (2017b) Mesoporous platinum as sulfur-tolerant catalyst for PEMFC cathodes. J Solid State Electrochem. https://doi.org/10.1007/s10008-017-3686-0
Prithi JA, Viswanath BS, Shmi NR, Dhathathreyen KS (2017c) Studies on PEMFC Stack for SO2Contamination at Air Cathode. Fuel cells. https://doi.org/10.1002/fuce.201600118
PrithiJayaraj P, Karthika N, Rajalakshmi KSD (2014) Mitigation studies of sulfur contaminated electrodes for PEMFC. Int J Hydrog Energy 39:12045–12051
Rajalakshmi N, Dhathathreyan KS (2005) Hydrogen energy technologies. In: The new energy economy. World Institute of sustainable energy
Rajalakshmi N, Dhathathreyan KS (2007) Catalyst layer in PEMFC electrodes—Fabrication, characterisation and analysis. Chem Eng J 129:31–40
Rajalakshmi N, Dhathathreyan KS (2008a) Present trends in fuel cell technology development. NOVA Publishers, Hauppauge
Rajalakshmi N, Dhathathreyan KS (2008b) Pulsed electrodeposition of catalyst layer of PEMFC electrodes. Int J Hydrog Energy 33:5672–5677
Rajalakshmi N, Ramesh T (2017) Activated carbons-promising materials-hydrogen perspective. Agric Res Technol 10:1–2
Rajalakshmi N, Raja M, Dhathathreyan KS (2002) Evaluation of current distribution in PEMFC by segmented cell approach. J Power sources 112:331–336
Rajalakshmi N, Ryu H, Dhathathreyan KS (2004a) Platinum catalysed membranes for proton exchange membrane fuel cells—higher performance. Chem Eng J 102:241
Rajalakshmi N, Jayanth TT, Thangamuthu R, Sasikumar G, Sridhar P, Dhathathreyan KS (2004b) Water transport characteristics of polymer electrolyte membrane fuel cell. Int J Hydrog Energy 29:1009–1014
Rajalakshmi N, Lakshmi N, Dhathathreyan KS (2008) Nano titanium oxide catalyst support for proton exchange membrane fuel cells. Int J Hydrog Energy 33(24):7521–7526
Rajalakshmi N, Pandiyan S, Dhathathreyan KS (2008a) Design and development of modular fuel cell stacks for various applications. Int J Hydrog Energy 33:449–454
Rajalakshmi N, Velayutham G, Dhathathreyan KS (2008b) Statistical analysis of a PEMFC stack—2.5 kW system operating condition. J Fuel Cell Sci Technol
Rajalakshmi N, Pandian S, Dhathathreyan KS (2009) Vibration tests on a PEM fuel cell stack usable in transportation application. Int J Hydrog Energy 34:3833–3837. https://doi.org/10.1016/j.ijhydene.2009.03.002
Rajalakshmi N, YaminiSarada B, Dhathathreyan KS (2014) Porous carbon nanomaterial from corncob as hydrogen storage material. Adv Porous Mater 2:1
Rajalakshmi N, Jafri RI, Dhathathreyan KS (2017) Recent advances in low temperature fuel cells. Wiley, Hoboken
Ramesh T, Rajalakshmi N, Dhathathreyan KS (2015) Activated carbons derived from tamarind seeds for hydrogen storage. J Energy Storage 4:89–95
Ramesh T, Rajalakshmi N, Dhathathreyan KS (2017) Synthesis and characterization of activated carbon from jute fibres for hydrogen storage. Renew Energy Environ Sustain 2:4
Ramesh T, Rajalakshmi N, Dhathathreyan KS, Gopal Reddy LR (2018) Hierarchical porous carbon microfibers derived from tamarind seed coat for high-energy supercapacitor application. ACS Omega 3(10):12832–12840
Ramesh T, Vedarajan R, Rajalakshmi N, Reddy LRG (2020) Dynamic electrochemical impedance spectroscopy as a rapid screening tool for supercapacitor electrode materials. J Mater Sci Mater Electr 31(2):1681–1690
Ramya K, Velayutham G, Subramaniam CK, Rajalakshmi N, Dhathathreyan KS (2006) Effect of solvents on the characteristics of Nafion®/PTFE composite membranes for fuel cell applications. J Power Sources 160:10–17
Reddy ALM, Rajalakshmi N, Ramaprabhu S (2008) Cobalt-polypyrrole-multiwalled carbon nanotube catalysts for hydrogen and alcohol fuel cells. Carbon 46(1):2–11
Sasank BV, Rajalakshmi N, Dhathathreyan KS (2016) Performance analysis of polymer electrolyte membrane (PEM)fuel cell stack operated under marine environmental conditions. J Mar Sci Technol. https://doi.org/10.1007/s00773-016-0369
Shaijumon MM, Ramaprabhu S, Rajalakshmi N (2005) Multiwalled carbon nanotubes-platinum/carbon composites as electrocatalysts for oxygen reduction reaction in proton exchange membrane fuel cell. Appl Phys Lett 88:253105
Smitha B, Sridhar S, Khan AA (2005) Solid polymer electrolyte membranes for fuel cell applications—a review. J Membr Sci 259:10–26
Stein T, Ein-Eli Y (2019) Proton exchange membrane (PEM) fuel cell bipolar plates prepared from a physical vapor deposition (PVD) titanium nitride (TiN) coated AISI416 stainless-steel. SN Appl Sci 1:1420
Swider-Lyons K, Bouwman P, Urgate N, Dmowski W (2003) Low platinum hydrous metal oxides for PEMFC cathodes. DoE Review, Berkeley
Unnikrishnan A, Rajalakshmi N, Janardhanan VM (2017) Electrochemical modeling of HTPEM fuel cells using elementary step kinetics. ECS Trans 80(8):57–64
Unnikrishnan A, Janardhanan VM, Rajalakshmi N, Dhathathreyan KS (2018) Chlorine-contaminated anode and cathode PEMFC-recovery perspective. J Solid State Electrochem 22(7):2107–2113
Unnikrishnan A, Rajalakshmi N, Janardhanan VM (2018) Mechanistic modeling of electrochemical charge transfer in HT-PEM fuel cells. Electrochim Acta 261:436–444
Unnikrishnan A, Rajalakshmi N, Janardhanan VM (2019) Kinetics of electrochemical charge transfer in HT-PEM fuel cells. Electrochim Acta 293:128–140
Vedarajan R, Prithi J, Rajalakshmi N (2019) Advanced nanocatalysts for fuel-cell technologies. In: Naushad M, Saravanan R, Raju K (eds) Nanomaterials for sustainable energy and environmental remediation. Elsevier, Amsterdam, p 165 (ISBN: 978-0-12-819355-6)
Velan VS, Velayutham G, Rajalakshmi N, Dhathathreyan KS (2014) Influence of compressive stress on the pore structure of carbon cloth based gas diffusion layer investigated by capillary flow porometry. Int J Hydrog Energy 39:1752–1759
Velayutham G, Koushik J, Rajalakshmi N, Dhathathreyan KS (2007) Effect of PTFE content in gas diffusion media and microlayer on the performance of PEMFC tested under ambient pressure. Fuel cells 1:1–5
Velayutham G, Dhathathreyan KS, Rajalakshmi N, Sampangi Raman D (2008) Assessment of factors responsible for polymer electrolyte membrane fuel cell electrode performance by statistical analysis. J Power Sources
Vielstich W, Gasteiger HA, Lamm A (eds) (2003) Handbook of fuel cells—fundamentals, technology and applications vol 3. Fuel cell technology and applications, vol 3. John Wiley & Sons Ltd, Hoboken
Vinayan BP, Rupali Nagar N, Rajalakshmi SR (2012) Novel platinum-cobalt alloy nanoparticles dispersed on nitrogen-doped graphene as a cathode electrocatalyst for PEMFC applications. Adv Funct Mater 22:3519–3526
Wei J, Ning F, Bai C, Zhang T, Lu G, Wang H, Li Y, Shen Y, Fu X, Li Q, Jin H, Zhou X (2020) An ultra-thin, flexible, low-cost and scalable gas diffusion layer composed of carbon nanotubes for high-performance fuel cells. J Mater Chem A 8(12):5986–5994
Weidner E, Cebolla RO, Davies J (2019) Global deployment of large capacity stationary fuel cells. European commission. JRC Technical report. https://ec.europa.eu/jrc/en/publication/global-deployment-large-capacity-stationary-fuel-cells
Whiston MM, Azevedo IL, Litster S, Whitefoot KS (2019) Expert assessments of the cost and expected future performance of proton exchange membrane fuel cells for vehicles. Constantine Samaras and Jay F Whitacre. PNAS 116(11):4899–4904
Yang C, Costamagna P, Srinivasan S, Benziger J, Bocarsly AB (2001) Approaches and technical challenges to high temperature operation of proton exchange membrane fuel cells. J Power Sources 103:1–9
Acknowledgements
The authors would like to thank Dr. G. Padmanabham, Director, ARCI, for his constant support and encouragement. Technical Research Centre (TRC) (AI/1/65/ARCI-2014), Department of Science and Technology, Government of India, for financial assistance is also acknowledged herewith. The authors would also like to acknowledge all the senior scientists, project Scientists, students and technical assistants, of CFCT ARCI, who made a lot of contribution in this fuelcell technology. Mr.Ramakrishnan and Mr.Sriharshas help in getting the copyrights and figures is acknowledged herewith.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rajalakshmi, N., Gopalan, R. Recent Trends in Science and Technology of Hydrogen and Polymer Electrolyte Membrane Fuel Cells. Trans Indian Natl. Acad. Eng. 6, 189–218 (2021). https://doi.org/10.1007/s41403-021-00206-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41403-021-00206-1