Abstract
Coralline red algae are important components of numerous tropical and temperate carbonate systems throughout the world. The environmental factors such as light, water depth, temperature and ocean chemistry have been acknowledged by researchers worldwide to have an impact on the recruitment and diversity of shallow-water coralline algae in marine benthic environments. The potential of coralline red algae as marine climate archives has also been highlighted in many recent studies. A brief overview of the fossil coralline red algae from various sedimentary basins of India is presented herein as well as their palaeoecological applications. The shortcomings and future prospects of coralline algal studies in India pertinent to significant aspects such as palaeoecology, palaeoenvironmental reconstructions, climate dynamics and extinction episodes are also discussed succinctly.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The discipline of ecology is associated with numerous laws in the form of widespread, repeatable patterns existing in nature. However, to identify and understand such patterns in relic ecosystems, a plethora of biotic proxies and environmental variables need to be considered for polished palaeoecological evaluations. Temperature, light intensity, salinity, nutrient regime, hydrodynamic energy, water transparency, oxygen and CO2 concentrations, Mg/Ca ratio and water pH have all been acknowledged as important limiting factors regulating populations of marine communities (Hallock and Schlager 1986; Carannante et al. 1988; Hallock et al. 1988; Stanley and Hardie 1998). In addition, bathymetry of the sea floor and substrate requirements are equally important in determining the spatial distribution of benthic marine communities (Pomar and Ward 1999; Pomar et al. 2004).
Coralline red algae (Orders Corallinales and Sporolithales; Division Rhodophyta) are among the most common and ecologically important organisms occurring in a wide range of marine habitats, from the intertidal to the lower photic zone, and from the polar to the tropical regions (Nebelsick et al. 2013; McCoy and Kamenos 2015). Contrary to other groups of calcareous red algae (Peyssonneliales and Nemaliales), they are major carbonate contributors and framework builders in the tropical systems as well as temperate/cool-water settings (Bosence 1983; Carannante et al. 1988; Freiwald and Henrich 1994; Iryu et al. 1995; Basso 1998, 2012; Foster 2001; Pomar et al. 2004; Savini et al. 2012).
Corallines first appeared in the Early Cretaceous (Ghosh and Sarkar 2013a; Table 1) and displayed progressive diversification peaking in diversity in the Early Miocene. This was followed by a phase of stabilization during the Neogene (Aguirre et al. 2000a). Corallines are presumed to have played an important role in the carbon and carbonate cycles of shallow-water ecosystems since the Late Mesozoic and have shown to be important contributors to Cretaceous and Tertiary platform deposits (Carannante et al. 1995; Aguirre et al. 2000a, b, 2007; Bassi 2005; Halfar and Mutti 2005; Quaranta et al. 2012; Nebelsick et al. 2013). During the Burdigalian to Tortonian periods in particular, global coralline algal deposits reached their acme (Halfar and Mutti 2005). Coralline algae have the ability to colonize a wide range of habitats due to their variable adaptations to a number of environmental (for e.g. light, temperature, hydrodynamic exposure) conditions from Cretaceous to Recent (Aguirre et al. 2000a, 2010).
Coralline red algae are important in tropical ecosystems because they contribute significantly to the carbonate reef structure, provide nursery habitats for juvenile invertebrates, act as settlement cues for coral larvae and a host of molluscan invertebrates, and assist in the stabilization/development of reef structures (Kamenos et al. 2004; Tierney and Johnson 2012; McCoy and Kamenos 2015). In addition, coralline algae are among the highest sediment producers in photic carbonate factories. Case studies with a special emphasis on coralline red algae are gaining large-scale attention in the context of global environmental changes like ocean acidification (Martin and Gattuso 2009; Kamenos et al. 2013), and their indispensable roles in maintaining marine ecosystems across a wide spectrum of latitudes and habitat types (Burdett et al. 2011; McCoy and Kamenos 2015). Owing to their abundance in Cenozoic shallow, marine carbonates, coralline algae are finding increased application in palaeoecological studies and reconstruction of palaeoenvironments (Braga and Martín 1988; Perrin et al. 1995; Bassi 1998, 2005; Cabioch et al. 1999; Halfar et al. 2000; Braga and Aguirre 2001; Aguirre et al. 2007; Burdett et al. 2011; Williams et al. 2011; Ghosh and Sarkar 2013b, c; Sarkar 2015a, b).
The purpose of this paper is to highlight the ecology of coralline red algae dwelling in shallow, marine benthic ecosystems with precise information pertaining to the environmental factors that influence their recruitment and diversity. The potential of coralline red algae in analyzing the anticipated consequences of climate change, especially global warming and ocean acidification, has been briefly discussed. A concise overview of the fossil coralline red algae recorded from different sedimentary basins of India is presented herein. The present drawbacks and future prospects of palaeoalgological studies in India emphasizing on coralline red algae are also highlighted.
Environmental Factors Influencing Coralline Red Algae
The principal environmental factors affecting coralline red algae are light, temperature, ocean chemistry and bathymetry. Bathymetry comprises the fluctuations in water depths that are associated with variations in hydrodynamic energy. These factors influence the growth and development of corallines in a multitude of benthic environments worldwide. Palaeoenvironmental evaluations of the ancient ecosystems depend on the examination of fossil species with documented ecological functions (Adey and Steneck 2001; Perry and Hepburn 2008; McCoy and Kamenos 2015). Since majority of fossil coralline red algae have living representatives with recognized environmental tolerances and roles in the marine ecosystems, these are typically used in the reconstruction of carbonate environments. Important aspects such as reef development (Payri and Cabioch 2004; Tierney and Johnson 2012) and community recovery dynamics related to various local/global disturbance events (Aguirre et al. 2007; Toth et al. 2012) can also be analyzed over time using coralline red algae in tandem with sedimentological inputs. Such reconstructions can provide valuable palaeoclimatic data regarding the depositional environment (Braga and Aguirre 2001). For example, species-specific bathymetric zonations can be used to reconstruct the sea-level changes or reef accretion at a given locality during different geological time slices (Cabioch et al. 1999; Yamano et al. 2001). Coralline algal ridges (also known as bioherms or mounds) provide very accurate estimates of sea-level, as they are restricted to the wave crest zone, and can assist in reconstruction of mm-cm scale sea-level changes (Adey 1986; Gherardi and Bosence 2005).
Water Depth and Light
Water depth (bathymetry) plays a key role in determining the composition of coralline red algal assemblages in different sub-environments within a given locality. The amount of incident light directly associated to the bathymetric levels is a very important ecological factor controlling the diversity and abundance of coralline algal assemblages. Water transparency is another important factor linked to water depth that is involved in the growth and development of coralline communities. Shading by other organisms (like macrophytes) and reflectivity of the sea-floor are other significant aspects regulating the algal assemblages (Kroeger et al. 2006).
Coralline red algae display strong patterns of zonation throughout the intertidal and subtidal zones of the marine benthic ecosystems depending upon their light, temperature, pH, desiccation, and grazing tolerances (Martone 2010; Guenther and Martone 2014; McCoy and Kamenos 2015). Phases of moderate disturbance in the surrounding environment frequently facilitate the development of coralline algal communities. Coralline red algae are often dominant in areas of high stress and disturbance potential where several other macrophytes are very limited or totally absent (Steneck 1986; Dethier 1994). This includes the habitats characterized by the preponderance of herbivores, sand scour, and low productivity potential such as the low photic zone, shaded understories of large macrophyte beds, and the intertidal zone vulnerable to action of storms/cyclones (Dethier 1994; Steneck and Dethier 1994; Dethier and Steneck 2001; McCoy and Kamenos 2015).
Bathymetric Affinities of Non-geniculate Coralline Algae
Mastophoroid Algal Assemblages
Mastophoroid corallines are commonly dominant in very shallow-water, tropical environments (Adey 1979; Iryu et al. 1995; Braga and Aguirre 2004). Mastophoroid-rich algal assemblages are usually characterized by crustose habit, and comprise superimposed encrusting and fruticose thalli overgrowing a variety of bioclasts (Braga and Aguirre 2004). They commonly grow in close vicinity of corals, foraminifera, gastropods and bryozoans (Braga and Aguirre 2004). In recent reef environments in the Indo-Pacific region, coralline algal assemblages dominated by mastophoroids characterizes reef crests and uppermost reef slopes exposed to intense wave action in water depths <10 m (Adey et al. 1982; Cabioch et al. 1999; Camoin et al. 2006), or even <3 m below spring low tides where vermetid gastropods are also associated with the coralline algae (Laborel 1986). Neogoniolithon brassica-florida and Spongites fruticulosus dominate the shallow-water assemblages in the western and central Mediterranean Messinian reefs (Braga et al. 2009). Species of Hydrolithon (H. craspedium, H. onkodes and H. reinboldii) have been reported from the very shallow-water reefal environments of the Andaman Islands with average depth 3–5 m (Sarkar and Sarkar 2015). The upper 15 m bathymetric zone in the Safarga Bay, northern Red Sea features corallines crusts dominated by thick encrusting H. onkodes and N. brassica-florida (Cabioch et al. 1999; Braga and Aguirre 2004). As per another study, N. brassica-florida is particularly restricted to the upper 10 m zone (Rasser and Piller 1997; Braga and Aguirre 2004). Mastophoroid assemblages dominated by H. onkodes encrusting Acropora (coral) colonies have been recorded from very shallow-water reef settings (<6 m) in Tahiti, French Polynesia (Montaggioni and Camoin 1993; Montaggioni et al. 1997).
Lithophylloid Algal Assemblages
Lithophylloid coralline algae are indicative of low to moderate energy environments and depths >10 m (Ringeltaube and Harvey 2000; Camoin et al. 2006). Coralline assemblages dominated by lithophylloid taxa with subordinate proportions of mastophoroids mostly occur in shallow-water environments of the temperate marine systems (Braga and Aguirre 2004). In numerous localities of the Mediterranean realm, coralline assemblages dominated by Lithophyllum are epilithic and compose the algal nodules in the upper 10–12 m bathymetric zone (Adey 1986; Di Geronimo et al. 1993; Braga and Aguirre 2004). Lithophyllum is common in ~20 m depth (Bosence 1991; Braga et al. 2009), but can also be found at deeper levels (Canals and Ballesteros 1997; Braga and Aguirre 2004). L. pustulatum has been reported from 10-m depth pertinent to the Heron Reef in the Great Barrier Reef (Ringeltaube and Harvey 2000; Braga and Aguirre 2004) and up to 45 m in the British Isles (Adey and Adey 1973; Braga and Aguirre 2004). This species also occurs as a very minor component (<1 %) of the rhodolith assemblages at 60-m depth on the eastern Australian shelf (Lund et al. 2000; Braga and Aguirre 2004). Lithophyllum tessellatum is commonly restricted to depths <10 m but has also been reported from deeper bathymetric levels in the Indo-Pacific region (Cabioch et al. 1999; Braga and Aguirre 2004). Lithophyllum incrassatum ranges from shallow littoral zone in South Africa (Chamberlain 1996) up to 60-m depth on the eastern Australian shelf (Lund et al. 2000; Braga and Aguirre 2004). Algal assemblages dominated by Lithophyllum kotschyanum occur in the intertidal and shallow subtidal back-reef settings in the northern Red Sea where the species thrive as coralline frameworks or rhodoliths (Piller and Rasser 1996; Rasser and Piller 1997; Braga and Aguirre 2004). L. kotschyanum has also been reported from similar bathymetric zones and sub-environments of One Tree Reef in the Great Barrier Reef (Braga and Davies 1993; Braga and Aguirre 2004). Thick encrusting Lithophyllum congestum are preponderant in the reef crests at St. Croix, Virgin Islands in the Caribbean (Steneck and Adey 1976; Bosence 1984) where they have been reported to show intergrowths with thick Hydrolithon thalli (Braga and Aguirre 2004). They are also found in the exposed algal-ridge and fore-reef habitats in Holandés Cays, Panama (Macintyre et al. 2001).
Melobesioid and Sporolithoid Algal Assemblages
Melobesioid and sporolithacean coralline algae commonly dominate the coralline assemblages in deep tropical and shallow to deep temperate settings ranging >20 m and reaching up to depths of 110–120 m depending on the local environmental parameters (Adey 1979, 1986; Iryu et al. 1995; Lund et al. 2000; Braga and Aguirre 2004; Camoin et al. 2006). Sporolithon (Family Sporolithaceae) commonly shows association with melobesioids in deep-water bathymetric levels corresponding to the low-latitude regions (Braga and Aguirre 2004). In modern-day settings, melobesioids and Sporolithon are significant biotic components below 10–15 m zone in One Tree Reef at the southern end of the Great Barrier Reef region (Braga and Davies 1993; Braga and Aguirre 2004) and in the Safarga Bay reefs in the Red Sea (Rasser and Piller 1997; Braga and Aguirre 2004). This assemblage is also recorded from the reef bases <15 m in the Sulawesi Archipelago in Indonesia (Verheij and Erftemeijer 1993; Braga and Aguirre 2004). Lithothamnion superpositum is a melobesioid coralline species common in the subtidal zone of South Africa (Keats et al. 2000). Melobesioid taxa Mesophyllum and Lithothamnion, together with Sporolithon, are the main rhodolith components on the outer platform off Fraser Island, Queensland (Lund et al. 2000; Braga and Aguirre 2004).
Temperature
Temperature is one of the most important factors controlling carbonate sedimentation and species composition of coralline algal assemblages in different geographic realms. The distribution of coralline algal species in the North Atlantic has been correlated with temperature/habitat boundaries (Adey and Adey 1973; Wilson et al. 2004). Melobesioid species Lithothamnion corallioides presents an interesting example pertaining to the impact of temperature on coralline algae. Absence of this species from Scotland is either because winter temperatures occasionally drop below the minimum survival temperature of this species (2–5 °C) or because threshold temperatures do not sustain long enough to support their endurance (Adey and McKibbin 1970; Wilson et al. 2004). Laboratory experimental studies have shown that Phymatolithon calcareum survived up to 2 °C, died at 0.4 °C, and the optimum temperature for growth was 15 °C (Adey and McKibbin 1970; Wilson et al. 2004). According to a recent study, elevated temperature has a stronger negative impact on coralline algae by means of photodamage in comparison to reduced ocean water Ph (Vásquez-Elizondo and Enriquez 2016). It also implies that out of the two critical worldwide phenomena - global warming and ocean acidification, the former has a greater influence on the physiology of coralline algae. It is however difficult to relate changes in sedimentary environments and skeletal association to absolute temperature values. This is mainly because the temperature values usually refer to the sea surface temperatures (SSTs) whereas several biogenic components of a marine ecosystem including coralline red algae thrive at various bathymetric levels/zones. The exact position of thermocline and relative decrease of temperature with increasing depths in the ancient environments may be difficult to reconstruct only from study of fossil material. The minimum winter temperature and maximum summer temperature should both be carefully analyzed to understand the impact of temperature on coralline taxa (Kroeger et al. 2006). In addition to water depth, other control factors operating in the seascape should also be considered in order to evaluate the minimum threshold temperatures for various coralline algal genera and species.
Non-geniculate coralline red algae feature a high-Mg calcite skeleton with distinct annual (for e.g., Lithothamnion glaciale, L. muelleri, Clathromorphum compactum) and sub-annual (for e.g., P. calcareum) growth bands (Blake and Maggs 2003; Halfar et al. 2008; Kamenos and Law 2010; Burdett et al. 2011). The frequency of these bands has been reviewed in detail by Foster (2001). Several encrusting coralline species do not show rhythmic growth bands, possibly due to high incidence of herbivory (McCoy and Kamenos 2015). Since the geniculate species grow primarily by forming new apical segments, they do not form growth bands. In rhodoliths, factors like reduced light availability and lower temperature during winter, reduced water movement, burial, monthly/lunar growth cycles driven by tidal patterns and possible large-scale climate patterns (for e.g., El Nińo) induce the formation of growth bands (Foster 2001; McCoy and Kamenos 2015). In L. glaciale a negative correlation exists between calcite density of calcified cells and temperature (Kamenos 2010), and also temperature and light availability (Burdett et al. 2011). Positive relationships with instrumental SSTs were generated by averaging the growth bands of multiple C. compactum or C. nereostratum thalli, enabling reconstruction of SSTs (Halfar et al. 2010, 2011). Smaller, better calcified cells are deposited within each annual growth band during the winter period and larger, less calcified cells are deposited during summer (Freiwald and Henrich 1994; Foster 2001; Burdett et al. 2011). The application of densitometric algochronology based on the study of the coralline band widths has proven to be useful in understanding the cloud cover histories and their linkage to the irradiance-temperature relationships (Burdett et al. 2011).
Some coralline species have been reported to continue their growth cycle during dark winters by means of physiological adaptations like utilization of starch grains stored in the perithallial cells (Freiwald and Henrich 1994) and CO2 dark assimilation (Kremer 1981). Calcite chemistry and growth band architecture reveals important information pertaining to the responses of coralline algae to the ambient environmental conditions. This enables them to act as palaeoenvironmental proxies, with the longest temperature reconstruction extending over 650 years (Kamenos 2010; Burdett et al. 2011; McCoy and Kamenos 2015).
Response to Ocean Acidification
During the last few decades scientific investigations pertaining to the appalling impacts of ocean acidification on calcifying marine biota have witnessed a significant rise. This is mainly aimed towards understanding the sensitivity of organisms and biomes to this large-scale global phenomenon. Increasing levels of pCO2 in the surface ocean causes major shifts in the seawater acid–base gradient and is likely to decrease the overall pH by 0.2.–0.4 units over the course of the 21st century (Caldeira and Wickett 2005; Martin and Gattuso 2009). Coralline red algae are one of the calcifying organisms that are highly sensitive to ocean acidification and absent in naturally acidified seawater where several other calcifying organisms can survive and proliferate (Hall-Spencer et al. 2008). Their susceptibility is related to the solubility of their Mg-calcite thalli which is the most soluble form of CaCO3 (Milliman 1974; Hall-Spencer et al. 2008; Kuffner et al. 2008; Martin and Gattuso 2009). Several facets of coralline algal ecology like recruitment (Kuffner et al. 2008), thallus growth (Jokiel et al. 2008) and calcification (Anthony et al. 2008) are all negatively affected under high pCO2 conditions. Occurrence of surface lesions (Martin and Gattuso 2009), epithelial cell damage (Burdett et al. 2012) and modeled structural stress (Ragazzola et al. 2012) are some other negative impacts of high pCO2 levels on coralline algae. Conversely, some studies have also shown non-negative responses. For example, intracellular concentrations of the algal antioxidant dimethylsulphoniopropionate (DMSP) did not increase under conditions of gradual change to lower pH (Burdett et al. 2012). A recent study by Kamenos et al. (2013) has demonstrated that the coralline algal structure is more sensitive to the rate of ocean acidification rather than its magnitude. Changes in the balance between coralline carbonate production and active dissolution induced by elevated pCO2 and temperature may have severe harmful impact by affecting the carbonate chemistry of the water column and the capacity of the marine bodies to take up atmospheric CO2 (Andersson et al. 2005; Martin and Gattuso 2009). Considering the wide spectrum of ecological services coralline algae are associated with, any change in their structural integrity may have long-term detrimental consequences for the ecosystem as a whole. Although some evidences of increased calcification under ocean acidification have been demonstrated (Martin et al. 2013; Kamenos et al. 2013) this does not necessarily result in repair of the already damaged coralline skeletons or imply that the newly calcified skeleton possess similar functionality as that deposited under higher pH conditions (Kamenos et al. 2013).
Other Factors
Despite being an important ecological factor, detailed information pertaining to the trophic tolerances of coralline red algae is lacking. Non-geniculate coralline algae are generally susceptible to nutrient input (Kroeger et al. 2006). Any nutrient equilibrium shift towards eutrophy normally triggers bloom in the populations of rapidly growing organisms and favors them in competition for space. It is therefore very unlikely that slow growing coralline red algae with average growth of 0.015–2.5 mm/year (Freiwald and Henrich 1994; Blake and Maggs 2003; Burdett et al. 2011) will proliferate in high nutrient conditions. Non-geniculate coralline red algae tolerate short periods of annual eutrophication as a consequence of nutrient upwelling (Halfar et al. 2004; Kroeger et al. 2006). It is noteworthy that in contrast to the non-geniculate algae, geniculate coralline taxa are more tolerant of eutrophic conditions as well as variable salinities (Johansen 1981; Kroeger et al. 2006). A recent experimental study concluded that herbivore reduction when coupled with nutrient enrichment triggers a rise in the non-calcifying, filamentous algae which surpass the calcifying, encrusting coralline red algae in the reefal environment of Central Red Sea (Jessen et al. 2013).
In case of salinity, the growth of P. calcareum is impaired at salinities <24 units (King and Schramm 1982; Wilson et al. 2004) whereas L. glaciale has shown robust resilience to reduced levels of salinity in comparison to several other red macroalgae (Burdett et al. 2015). However, due to an increase in the overall absorbance of L. glaciale thalli in the low- and very low-salinity treatments, possibility of dynamic photoinhibition cannot be ruled out (Burdett et al. 2015).
Till date coralline red algae have been considered as exclusive marine organisms. However, a recent report of coralline alga Pneophyllum cetinaensis from the Cetina River (Adriatic Sea watershed; Žuljević et al. 2016) defies this long-term paradigm and presents the possibility of discovering more coralline species from freshwater ecosystems. Particular characteristics such as hard water enriched with dissolved calcium carbonate and pH similar to the marine environments favors colonization of the Cetina River by coralline species. To generalize the incidence of coralline algae from such freshwater systems, greater number of experimental studies and long-term monitoring are required.
Fossil Coralline Red Algae from India
India is a country of diverse environments and fossil reserves including numerous carbonate successions spread all across its vast landscape. Several taxa of calcareous algae including coralline red algae have been reported from various sedimentary basins of India (Fig. 1). Among these the Andaman-Nicobar, Assam Shelf, Kachchh and Saurashtra Basins have received substantial attention from the phycologists for evaluating the coralline assemblages, especially for taxonomic and basic palaeoenvironmental studies.
Andaman-Nicobar Basin
The Andaman and Nicobar Islands represent a junction of the Indian and Pacific lithostratigraphic plates in the Bay of Bengal. The Andaman and Nicobar Islands are divided into the northern Andaman and the southern Nicobar with the Ten Degree Channel separating the two groups. The main ridge of the Andaman-Nicobar is dominated by the lithological units dating Palaeogene whereas the islands situated on either side of the main ridge and those constituting the Ritchie’s Archipelago are characterized by a multitude of Neogene successions (Sharma and Srinivasan 2007).
The first record of fossil coralline algae from the Andaman and Nicobar Islands is by Gee (1927). This includes the report of Lithothamnion nummuliticum and L. suganum (Middle Andaman) and fragments of Lithothamnion (Hut Bay, Little Andaman and Wilson Island) from post-Eocene sediments. In the late 20th century several studies pertaining to coralline red algae have been carried out from the Cretaceous-Palaeogene (Badve and Kundal 1986, 1988; Kundal and Wanjarwadkar 2000) and Neogene (Chatterji and Gururaja 1972; Venkatachalapathy and Gururaja 1984; Chandra et al. 1999) sediments of the Andaman-Nicobar basin. Rich assemblages of coralline red algae have been recovered from the Neogene successions of the Little Andaman (Saxena et al. 2005; Sarkar and Ghosh 2015; Sarkar et al. 2016) and Car Nicobar (Chandra et al. 1999; Ghosh et al. 2004; Ghosh and Sarkar 2013c; Sarkar 2015c). A new record of coralline algae has recently been reported from the Pleistocene sediments of the Neill West Coast Formation, Neil Island in South Andaman (Kishore et al. 2015). Lithophylloid corallines represented by species of Lithophyllum and Amphiroa show the maximum abundance in the Neogene sediments of the Andaman-Nicobar Group of Islands, followed by melobesioids (Lithothamnion, Mesophyllum) and mastophoroids (Spongites, Lithoporella, Neogoniolithon). Benthic foraminifera, corals, bryozoans, echinoderms, gastropods and barnacles are the other major biotic components reported from these sediments in association with coralline algae. Based on the coralline red algal assemblages reported, intertidal to mildly deeper water depths featuring moderate to high hydrodynamic energy conditions in tropical depositional environments have been envisaged for the Andaman-Nicobar carbonates. Some examples of coralline red algae from the Neogene sediments of Hut Bay, Little Andaman have been illustrated in Fig. 2 (microphotographs a–e).
Examples of coralline red algae from India: (a–e) Neogene (Middle Miocene) of Hut Bay, Little Andaman Island: a Non-geniculate coralline protuberances, BSIP Slide No. 14860; b Melobesiodeae gen. et spec. indet., BSIP Slide No. 14798; c Lithothamnion libanum, BSIP Slide No. 14801; d Spongites sp., BSIP Slide No. 14811; e Geniculate coralline red alga Amphiroa sp. (Am), BSIP Slide No. 14860; (f–h) Palaeogene (Late Palaeocene-Early Eocene) of Meghalaya, Assam Shelf, N-E India: f Encrusting coralline algae from Early Eocene, BSIP Slide No. 14661; g Sporolithon sp. from Late Palaeocene, BSIP Slide No. 15956; h Corallina sp. (Co) and Distichoplax biserialis (Db) from Late Palaeocene, BSIP Slide No. 15847. Scale bars: (a) 1 mm, (b–c) 150 μm, (d–h) 300 μm
Assam Shelf
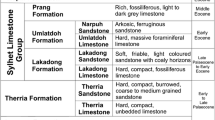
Assam Shelf in north-east India is one of the best studied sedimentary basins of India with respect to fossil calcareous algae. It is the north-eastern extension of the Indian Craton and includes Jaintia, Khasi, Garo, Mikir Hills, Shillong Plateau and the upper Assam Valley. The Sylhet Limestone Group in the Assam Shelf encompasses three carbonate units – Lakadong Limestone, Umlatdoh Limestone and Prang Formation in the ascending order intercalated with Lakadong Sandstone and Narpuh Sandstone (Jauhri and Agarwal 2001; Misra et al. 2011). Based on biostratigraphy pertinent to larger benthic foraminiferal studies, the carbonate lithounits of the Sylhet Limestone Group are assigned to various time slices of Palaeogene ranging from Late Palaeocene to Middle Eocene (Jauhri and Agarwal 2001; Jauhri et al. 2006; Matsumaru and Sarma 2010; Sarkar 2015a). The Sylhet Limestone Group is a carbonate ramp/tidal flat setting (Tewari et al. 2010a, b). It is underlain by the late Early to Middle Palaeocene Therria Sandstone and overlain by shale/sandstone alternations of the Late Eocene Kopili Formation (Jauhri and Agarwal 2001; Jauhri et al. 2006; Sarma et al. 2014). Coralline red algae are among the principal biotic components of the Palaeogene carbonate sediments outcropping in Meghalaya, Assam Shelf (Jauhri et al. 2006; Tewari et al. 2010a; Misra et al. 2011; Ghosh and Sarkar 2013b; Kalita and Gogoi 2015; Sarkar 2015a, b). Some examples of coralline red algae from the Late Palaeocene-Early Eocene sediments of Meghalaya, Assam Shelf have been demonstrated in Fig. 2 (microphotographs f–h). Lithothamnion, Mesophyllum, Lithophyllum, Distichoplax, Lithoporella, Spongites, Sporolithon, Corallina and Jania are the common coralline taxa reported from various carbonate lithounits of the Sylhet Limestone Group. They show various growth-forms from encrusting to free-living (rhodoliths/maerls) in lagoonal to proximal outer shelf facies (Sarkar 2015a, b). The relative abundance of corallines increase gradually from Late Palaeocene to Middle Eocene and is correlated to the evolution of a reefal environment (Sarkar 2015b). High dominance of calcareous green algae, especially genera like Halimeda and Ovulites in the Umlatdoh Limestone is an interesting observation which can be attributed to changes in substrate lithology and competition dynamics in the benthic environment during the course of evolution. Fossil green algae have been reported from all the carbonate lithounits of the Sylhet Limestone but do not show considerable richness in the Lakadong and Prang units, where coralline red algae are the principal algal components. The δ13C and δ18O isotope data has recently been obtained from the Lakadong Limestone sediments and indicate a shallow marine depositional environment (Tewari et al. 2010a). These isotope data suggest palaeotemperatures in the range of 27–30 °C which indicate optimal conditions for the coralline algae and associated benthic foraminifera during the deposition of the Lakadong Limestone. The abundance, diversity and frequency of coralline red algae and other calcareous algal taxa indicates the overall depositional environment for the Sylhet Limestone at shallow depths within the fair-weather wave base.
Kachchh Basin
The Kachchh Basin is a pericratonic rift basin situated in the western margin of India with rich fossiliferous sequences. It is situated at the northwestern fringe of the Indian Peninsula and bounded by the Arabian Sea on the west, Rajasthan on the east, the Great Rann of Kachchh on the north, and the Gulf of Kachchh and the Little Rann of Kachchh on the south. The rock units present in this basin date Middle Jurassic to the Recent. It is one of the most well documented sedimentary basins of India in terms of palaeontological records including coralline red algae and other fossil algal taxa. The Fulra Limestone Formation (middle Eocene), Maniyara Fort Formation (Oligocene), Khari Nadi Formation (early Miocene) and Chhasra Formation (early middle Miocene) are the four lithounits in the onshore sequence of the Kachchh Basin that have yielded rich assemblages of coralline algae (Kundal and Humane 2012; Kundal 2014). Likewise, the Godhra Formation (early Miocene) represents a unit with coralline algal record from the offshore sequence of the Kachchh Basin (Kundal and Humane 2012). The non-geniculate coralline algae include species of Lithophyllum, Lithoporella, Spongites, Neogoniolithon, Lithothamnion, Phymatolithon, Aethesolithon, Mesophyllum and Sporolithon. The geniculate coralline algae show high abundance and diversity from the Kachchh Basin sequences. Geniculate coralline assemblages include species of Corallina, Jania, Arthrocardia, Calliarthron, Amphiroa, Subterraniphyllum and Metagoniolithon (Misra et al. 2006a; Singh et al. 2009, 2011; Humane and Kundal 2010; Kundal and Humane 2006, 2012; Kundal 2014). In addition to the coralline algae, Kachchh Basin carbonates feature diverse and abundant quantities of dasyclads, halimeds and udoteacean fossil algae (Kundal and Humane 2012; Kundal 2014).
Bathymetric analyses of the coralline algal species indicate a moderate-energy shallow marine depositional environment for the carbonate sequences of the Kachchh Basin yielding coralline algae. Most of the coralline species recorded from these sequences have thin crusts with delicate framework (Kundal and Humane 2012). Therefore high-energy conditions denoted by thick crust corallines with robust framework (Bosence 1991) seem a bleak interpretation. Since the geniculate corallines have been recorded in high abundance with dasycladalean, halimedacean and udoteacean algae, greater proportion of the depositional process within the inner shelf seems a strong possibility. In all probabilities, the algal forms thrived in a moderate-energy inner-middle shelf environment.
Saurashtra Basin
The Saurashtra Basin is a pericratonic shelf basin located in the northern part of the western continental margin of India. The basin lies north of the Bombay Offshore Basin and south of the Kachchh Basin. A detailed lithostratigraphic classification of the Cenozoic-Quaternary sediments of the Saurashtra basin has been provided by Pandey et al. (2007). The Saurashtra Basin is geologically significant due to numerous signatures of sea level fluctuations, depositional environments and hydrocarbon reservoirs (Kundal et al. 2014). Abundant coralline algae have been recorded from three horizons of the Saurashtra Basin: Dwarka-Okha (Kundal and Dharashivkar 2005; Kishore et al. 2012), Porbandar (Kundal and Mude 2009a, b; Mude and Kundal 2012) and Diu (Kundal et al. 2011, 2014). Early Miocene to Late Holocene sediments are exposed in Dwarka-Okha area, while the Porbandar sediments date Middle Miocene to Late Holocene. The Diu area has sediments ranging Middle Pleistocene to Late Holocene. The coralline algae include both non-geniculate forms - species of Lithophyllum, Titanoderma, Lithothamnion, Lithoporella, Mesophyllum, Phymatolithon, Porolithon, Spongites, Sporolithon and Neogoniolithon, and geniculate forms - species of Amphiroa, Corallina, Jania, Arthrocardia, Metagoniolithon and Subterraniphyllum. Calcareous green algae like Dissocladella and Halimeda spp. have also been recorded from the Diu sediments (Kundal et al. 2014).
Based on the coralline algal associations, 25–60 m water depth with a moderate hydrodynamic energy regime has been interpreted for the deposition of the Dwarka-Okha sediments including the Kalyanpur Limestone Member (Early Pliocene) of the Dwarka Formation and the Okha Shell Limestone Member (Middle to Late Pleistocene) of the Chaya Formation (Kundal et al. 2014). Based on a detailed study of ichnofossils from this area, the depositional environment for these sediments has been stated to be littoral to sandy shoreline (Kundal and Dharashivkar 2006). The Aramda Reef Member (Middle Pleistocene) of the Chaya Formation is characterized by thick branched and encrusting coralline algal species including abundant Amphiroa (Kundal and Dharashivkar 2005; Kishore et al. 2012; Kundal et al. 2014). A depositional depth of 0–20 m has been interpreted for the Aramda Reef Member. Kishore et al. (2012) have divided the Aramda Reef Member into two parts: the lower part dominated by warty to layered Mesophyllum, Lithothamnion and Sporolithon is interpreted to have deposited under warm, low-moderate energy environment, while the upper part is dominated by fruticose Lithophyllum and Titanoderma which indicate high-energy conditions.
The Dwarka Formation sediments dating Middle Miocene outcropping in Porbandar area encompasses plentiful coralline algae with robust framework and thick branches (Kundal and Mude 2009a, b; Kundal et al. 2014). Water depth of 0–20 m has been interpreted for the deposition of the Dwarka Formation and the Porbandar Calcarenite Member of the Late Pleistocene to Late Holocene Chaya Formation (Kundal et al. 2014). The Adatiana Member of the Miliolite Formation (Middle Pleistocene) in both Porbandar and Diu areas has been envisaged to have deposited at bathymetry of 25–30 m below the low tide level (Kundal and Mude 2009a, b; Kundal et al. 2014).
Other Basins
Apart from the aforementioned sedimentary basins, varying proportions of coralline red algae have also been reported from the Cauvery Basin (Misra and Kumar 1988; Misra et al. 2006b, 2009; Ghosh and Sarkar 2013a), Narmada Valley Basin (Durge 1965; Kundal and Sanganwar 1998), Bombay Offshore Basin (Kundal et al. 2007, 2013; Kundal 2014) and Kerala-Konkan-Lakshadweep Basin (Reuter et al. 2011; Misra et al. 2016).
Future Prospects
Despite several studies on coralline red algae from the sedimentary basins of India, the work has largely been limited to pure taxonomic descriptions of the taxa with very elementary palaeoenvironmental interpretations. This does not sufficiently justify the high palaeoecological potential of the corallines, as evident from the broad spectrum of multi-dimensional research continuing in several laboratories of Europe, Australia and America. A large proportion of the Indian marine systems are shallower than 100 m, so further large-scale studies on coralline red algae become a necessity.
-
1)
Major emphasis till date has been given to taxonomy but detailed ecological evaluations of the fossil coralline red algae are lacking. Response of the corallines to fluctuations in sea-levels, SSTs and nutrient inputs are important in understanding their evolutionary significance in the Indian subcontinent and make future projections both for climate as well as the marine benthic environment. Discerning the specific effect of individual ecological factors, and distinguishing them from the regional and global impacts is however challenging.
-
2)
The distribution of ecologically important benthic facies such as those dominated by corallines on both hard and soft bottoms, is largely unknown from the Indian subcontinent, thus introducing a bias in predicting the response of the shelves to short- and long-term global changes.
-
3)
Bathymetric data based on the assessment of coralline red algae is very limited. Based on the dominance and abundance of the corallines, depositional water-depths during different Cenozoic time slices need better analyses that will aid in palaeoenvironmental reconstructions.
-
4)
For precise assessment of the ecological status of the coralline red algae, extensive quantitative data pertaining to the assemblage/community composition are essential. Comprehensive quantitative datasets are still relatively unknown for the Indian coralline assemblages.
-
5)
In addition to the general geological setting, description of the coralline red algae both individually and at the community levels should be supplemented with the sedimentological characteristics of the lithounits and mean percentage of the algal assemblages with respect to the characterized bio/microfacies.
-
6)
Regional mapping should include identification and labeling of the regions with algal-dominated lithounits by application of advanced scales and detailed monitoring.
-
7)
Response of the coralline red algae to the extinction episodes are poorly understood and need better case studies to develop improved qualitative and quantitative datasets. Recovery dynamics of the coralline algae have huge potential in deciphering the past ecosystems.
-
8)
Discipline of algochronology is severely under-developed in India and needs attention of phycologists and palaeoclimate workers to enable reconstruction of marine cloud cover, temperature gradients, ocean productivities and ecosystem models.
-
9)
Role of coralline red algae in the carbonate budget and ocean dynamics of the tropical Indian sedimentary basins are very poorly understood and demands polished evaluations to better utilize their ecosystem functions. Locating and describing the algal carbonate factories, and the evaluation of their extent and production are a priority for future research as a tool for the environmental management of the tropical biodiversity hot-spots and to contribute well to a reliable carbonate budget for the world shelves.
References
Adey WH (1979) Crustose coralline algae as microenvironmental indicators in the Tertiary. In: Gray J, Boucot AJ (eds) Historical biogeography, plate tectonics and the changing environment. Oregon University Press, Corvallis, pp 459–464
Adey WH (1986) Coralline algae as indicators of sea-level. In: van de Plassche O (ed) Sea-level research: a manual for the collection and evaluation of data. Geo Books, Norwich, pp 229–280
Adey WH, Adey PJ (1973) Studies of the biosystematics and ecology of the epilithic crustose Corallinaceae of the British Isles. Brit Phycol J 8:343–407
Adey WH, Mckibbin DL (1970) Studies on the maerl species Phymatolithon calcareum (Pallas) nov. comb. and Lithothamnion corallioides Crouan in the Ria de Vigo. Bot Mar 13:100–106
Adey WH, Steneck RS (2001) Thermogeography over time creates biogeographic regions: a temperature/space/time-integrated model and an abundance-weighted test for benthic marine algae. J Phycol 37:677–698
Adey WH, Townsend RA, Boykins WT (1982) The crustose coralline algae (Rhodophyta: Corallinaceae) of the Hawaiian Islands. Smithson Cont Mar Sci 15:1–74
Aguirre J, Riding R, Braga JC (2000a) Diversity of coralline red algae: origination and extinction patterns from the early Cretaceous to the Pleistocene. Paleobiology 26:651–667
Aguirre J, Riding R, Braga JC (2000b) Late Cretaceous incident light reduction: evidence from benthic algae. Lethaia 33:205–213
Aguirre J, Baceta JI, Braga JC (2007) Recovery of primary producers after the Cretaceous-Tertiary mass extinction: Paleocene calcareous red algae from the Iberian Peninsula. Palaeogeog Palaeoclimatol Palaeoecol 249:393–411
Aguirre J, Perfectti F, Braga JC (2010) Integrating phylogeny, molecular clocks, and the fossil record in the evolution of coralline algae (Corallinales and Sporolithales, Rhodophyta). Paleobiology 36:519–533
Andersson AJ, Mackenzie FT, Lerman A (2005) Coastal ocean carbonate ecosystems in the high CO2 world of the Anthropocene. Amer J Sci 305:875–918
Anthony KRN, Kline DI, Diaz-Pulido G, Dove S, Hoegh-Guldberg O (2008) Ocean acidification causes bleaching and productivity loss in coral reef builders. PNAS USA 105:17442–17446
Badve RM, Kundal P (1986) Marine Cretaceous algae from the Baratang Formation, Andaman, India. Bull Geol Min Metal Soc India 54:149–158
Badve RM, Kundal P (1988) Distichoplax Pia from Baratang Island, Andaman, India. Biovigyanam 14:95–102
Bassi D (1998) Coralline algal facies and their palaeoenvironments in the Late Eocene of northern Italy (Calcare di Nago, Trento). Facies 39:179–202
Bassi D (2005) Larger foraminiferal and coralline algal facies in an Upper Eocene storm influenced, shallow-water carbonate platform (Colli Berici, north-eastern Italy). Palaeogeog Palaeoclimatol Palaeoecol 226:17–35
Bassi D, Woelkerling WJ, Nebelsick JH (2000) Taxonomic and biostratigraphical re-assessments of Subterraniphyllum Elliott (Corallinales, Rhodophyta). Palaeontology 43:405–425
Basso D (1998) Deep rhodolith distribution in the Pontian Islands, Italy: a model for the paleoecology of a temperate sea. Palaeogeog Palaeoclimatol Palaeoecol 137:173–187
Basso D (2012) Carbonate production by calcareous red algae and global change. Geodiversitas 34:13–33
Blake C, Maggs CA (2003) Comparative growth rates and internal banding periodicity of maerl species (Corallinales, Rhodophyta) from northern Europe. Phycologia 42:606–612
Bosence DWJ (1983) The occurrence and ecology of recent rhodoliths – a review. In: Peryt TM (ed) Coated grains. Springer, Berlin, pp 217–224
Bosence DWJ (1984) Construction and preservation of two Recent coralline algal reefs, St. Croix, Caribbean. Palaeontology 27:549–574
Bosence DWJ (1991) Coralline algae: mineralization, taxonomy and paleoecology. In: Riding R (ed) Calcareous algae and stromatolites. Springer, Berlin, pp 98–113
Braga JC, Aguirre J (2001) Coralline algal assemblages in upper Neogene reef and temperate carbonates in Southern Spain. Palaeogeog Palaeoclimatol Palaeoecol 175:27–41
Braga JC, Aguirre J (2004) Coralline algae indicate Pleistocene evolution from deep, open platform to outer barrier reef environments in the northern Great Barrier Reef margin. Coral Reefs 23:547–558
Braga JC, Davies PJ (1993) Coralline algal distribution in One Tree Reef (Southern Great Barrier Reef, NE Australia). In: International Society for Reef Studies: 1st European Regional Meet, Vienna, Abstract 9
Braga JC, Martín JM (1988) Neogene coralline-algal growth forms and their palaeoenvironments in the Almanzora River Valley (Almeria, S.E. Spain). Palaeogeog Palaeoclimatol Palaeoecol 67:285–303
Braga JC, Vescogni A, Bosellini FR, Aguirre J (2009) Coralline algae (Corallinales, Rhodophyta) in western and central Mediterranean Messinian reefs. Palaeogeog Palaeoclimatol Palaeoecol 275:113–128
Burdett HL, Kamenos NA, Law A (2011) Using coralline algae to understand historic marine cloud cover. Palaeogeog Palaeoclimatol Palaeoecol 302:65–70
Burdett HL, Aloisio E, Calosi P, Findlay HS, Widdicombe S, Hatton A, Kamenos NA (2012) The effect of chronic and acute low pH on the intracellular DMSP production and epithelial cell morphology of red coralline algae. Mar Biol Res 8:756–763
Burdett HL, Hatton AD, Kamenos NA (2015) Effects of reduced salinity on the photosynthetic characteristics and intracellular DMSP concentrations of the red coralline alga, Lithothamnion glaciale. Mar Biol 162:1077–1085
Cabioch GM, Montaggioni LF, Faure G, Ribaud-Leurenti A (1999) Reef coral-algal assemblages as recorders of paleobathymetry and sea level changes in the Indo-Pacific province. Quat Sci Rev 18:1681–1695
Caldeira K, Wickett ME (2005) Ocean model predictions of chemistry changes from carbon dioxide emissions to the atmosphere and ocean. J Geophys Res 110(C09S04):1–12
Camoin G, Cabioch G, Eisenhauer A, Braga JC, Hamelin B, Lericolais G (2006) Environmental significance of microbialites in reef environments during the last deglaciation. Sed Geol 185:277–295
Canals M, Ballesteros E (1997) Production of carbonate benthic particles by phytobenthic communities on the Mallorca-Menorca shelf, northwestern Mediterranean Sea. Deep Sea Res II 44:611–629
Carannante G, Esteban M, Milliman JD, Simone L (1988) Carbonate lithofacies as paleolatitude indicators: problems and limitations. Sed Geol 60:333–346
Carannante G, Cherchi A, Simone L (1995) Chlorozoan versus foramol lithofacies in Upper Cretaceous rudist limestones. Palaeogeog Palaeoclimatol Palaeoecol 119:137–154
Chamberlain YM (1996) Lithophylloid Corallinaceae (Rhodophyta) of the genera Lithophyllum and Titanoderma from southern Africa. Phycologia 35:204–221
Chandra A, Saxena RK, Ghosh AK (1999) Coralline algae from the Kakana Formation (Middle Pliocene) of Car Nicobar Island, India and their implications in biostratigraphy, palaeoenvironment and palaeobathymetry. Curr Sci 76:1498–1502
Chatterji AK, Gururaja MN (1972) Coralline algae from Andaman Islands, India. Rec Geol Surv India 99:133–144
Dethier MN (1994) The ecology of intertidal algal crusts: variation within a functional group. J Exp Mar Biol Ecol 177:37–71
Dethier MN, Steneck RS (2001) Growth and persistence of diverse intertidal crusts: survival of the slow in a fast-paced world. Mar Ecol Prog Ser 223:89–100
Di Geronimo R, Alongi G, Giaccone G (1993) Formazione organogene a Lithophyllum lichenoides Philippi (Rhodophyta, Corallinales) nel Mesolitorale di Capo S. Alessio (Sicilia orientale). Boll Accad Gioenia Sci Nat 26:145–172
Durge MV (1965) Archaeolithothamnium from the Bagh Beds of Madhya Pradesh. J Geol Soc Saugar 1:34–37
Foster MS (2001) Rhodoliths: between rocks and soft places. J Phycol 37:659–667
Freiwald A, Henrich R (1994) Reefal coralline algal build-ups within the Arctic Circle: morphology and sedimentary dynamics under extreme environmental seasonality. Sedimentology 41:963–984
Gee ER (1927) The geology of the Andaman and Nicobar Islands with special reference to Middle Andaman. Rec Geol Surv India 59:208–232
Gherardi DFM, Bosence DWJ (2005) Late Holocene reef growth and relative sea-level changes in Atol das Rocas, equatorial South Atlantic. Coral Reefs 24:264–272
Ghosh AK, Sarkar S (2013a) Diversification of the Family Sporolithaceae: a case of successful survival in the perspective of Cretaceous-Tertiary mass extinctions in India. Nat Acad Sci Lett 36:215–224
Ghosh AK, Sarkar S (2013b) Palaeoecological implications of corallinacean red algae and halimedacean green algae from the Prang Formation of South Shillong Plateau, Meghalaya. J Geol Soc India 81:531–542
Ghosh AK, Sarkar S (2013c) Facies analysis and paleoenvironmental interpretation of Piacenzian carbonate deposits from the Guitar Formation of Car Nicobar Island, India. Geosci Front 4:755–764
Ghosh AK, Chandra A, Saxena RK (2004) Middle Pliocene non-geniculate and geniculate coralline algae from the Car Nicobar Island, India. In: Srivastava PC (ed) Vistas in palaeobotany and plant morphology: evolutionary and environmental perspectives. Prof. D.D. Pant Memorial Volume, pp 249–62
Guenther RJ, Martone PT (2014) Physiological performance of intertidal coralline algae during a simulated tidal cycle. J Phycol 50:310–321
Halfar J, Mutti M (2005) Global dominance of coralline red-algal facies: a response to Miocene oceanographic events. Geology 33:481–484
Halfar J, Zack T, Kronz A, Zachos JC (2000) Growth and high-resolution paleoenvironmental signals of rhodoliths (coralline red algae): a new biogenic archive. J Geophys Res 105:22107–22116
Halfar J, Godinez-Orta L, Mutti M, Valdez-Holguín JE, Borges JM (2004) Nutrient and temperature controls on modern carbonate production: an example from the Gulf of California, Mexico. Geology 32:213–216
Halfar J, Steneck RS, Joachimski M, Kronz A, Wanamaker AD (2008) Coralline red algae as high-resolution climate recorders. Geology 36:463–466
Halfar J, Hetzinger S, Adey W, Zack T, Gamboa G, Kunz B, Williams B, Jacob DE (2010) Coralline algal growth-increment widths archive North Atlantic climate variability. Palaeogeog Palaeoclimatol Palaeoecol 302:71–80
Halfar J, Williams B, Hetzinger S, Steneck RS, Lebednik P, Winsborough C, Omar A, Chan P, Wanamaker A (2011) 225 Years of Bering Sea climate and ecosystem dynamics revealed by coralline algal growth-increment widths. Geology 39:579–582
Hallock P, Schlager W (1986) Nutrient excess and the demise of coral reefs and carbonate platform. Palaios 1:389–398
Hallock P, Hine AC, Vargo GA, Elrod JA, Jaap WC (1988) Platforms of the Nicaraguan Rise: examples of the sensitivity of carbonate sedimentation to excess trophic resources. Geology 16:1104–1107
Hall-Spencer JM, Rodolfo-Metalpa R, Martin S, Ransome E, Fine M, Turner SM, Rowley SJ, Tedesco D, Buia M (2008) Volcanic carbon dioxide vents show ecosystem effects of ocean acidification. Nature 454:96–99
Harvey AS, Woelkerling WJ (2007) A guide to nongeniculate coralline red algal (Corallinales, Rhodophyta) rhodolith identification. Cien Mar 33:411–426
Humane SK, Kundal P (2010) Nongeniculate coralline algae from Middle Eocene to late Lower Miocene of southwestern part of Kachchh, India. ONGC Bulletin 45:30–45
Iryu Y, Nakamori T, Matsuda S, Abe O (1995) Distribution of marine organisms and its ecological significance in the modern reef complex of the Ryukyu Islands. Sed Geol 99:243–258
Jauhri AK, Agarwal KK (2001) Early Palaeogene in the south Shillong Plateau, NE India: local biostratigraphic signals of global tectonic and ocean changes. Palaeogeog Palaeoclimatol Palaeoecol 168:187–203
Jauhri AK, Misra PK, Kishore S, Singh SK (2006) Larger foraminiferal and calcareous algal facies in the Lakadong Formation of the South Shillong Plateau, NE India. J Palaeontol Soc India 51:51–61
Jessen C, Roder C, Villa Lizcano JF, Voolstra CR, Wild C (2013) In-Situ effects of simulated overfishing and eutrophication on benthic coral reef algae growth, succession and composition in the Central Red Sea. PLoS ONE 8:e66992
Johansen HW (1981) Coralline algae, a first synthesis. CRC, Boca Baton, 239 pp
Jokiel PL, Rodgers KS, Kuffner IB, Andersson AJ, Cox EF, Mackenzie FT (2008) Ocean acidification and calcifying reef organisms: a mesocosm investigation. Coral Reefs 27:473–483
Kalita KD, Gogoi H (2015) Microfacies types (MFT) and palaeoenvironment of the Umlatdoh carbonates in the Shillong Plateau of Meghalaya, NE India. J Geol Soc India 85:686–696
Kamenos NA (2010) North Atlantic summers have warmed more than winters since 1353, and the response of marine zooplankton. Proc Natl Acad Sci U S A 107:22442–22447
Kamenos NA, Law A (2010) Temperature controls on coralline algal skeletal growth. J Phycol 46:331–335
Kamenos NA, Moore PG, Hall-Spencer JM (2004) Nursery-area function of maerl grounds for juvenile queen scallops Aequipecten opercularis and other invertebrates. Mar Ecol Prog Ser 274:183–189
Kamenos NA, Burdett HL, Alioso E, Findlay HS, Martin S, Longbone C, Dunn J, Widdicombe S, Calosi P (2013) Coralline algal structure is more sensitive to rate, rather than magnitude, of ocean acidification. Glob Change Biol 19:3621–3628
Keats DW, Maneveldt G, Chamberlain YM (2000) Lithothamnion superpositum Foslie: a common crustose red alga (Corallinaceae) in South Africa. Crypt Algol 21:381–400
King RJ, Schramm W (1982) Calcification in the maerl coralline alga Phymatolithon calcareum, effects of salinity and temperature. Mar Biol 70:197–204
Kishore S, Singh SK, Misra PK, Jauhri AK (2012) Species of Amphiroa Lamourox (Corallinaceae, Rhodophyta) from the Chaya Formation (Quaternary) of the Dwarka Area, Gujarat and their significance. J Palaeontol Soc India 57:153–158
Kishore S, Jauhri AK, Singh SK, Malakar B, Misra PK (2015) Coralline algae from the Neill West Coast Formation (Pleistocene), Neil Island, South Andaman, India. J Palaeontol Soc India 60:57–69
Kremer BP (1981) Aspects of carbon metabolism in marine microalgae. Oceanogr Mar Biol: An Ann Rev 19:41–95
Kroeger KF, Reuter M, Brachert TC (2006) Palaeoenvironmental reconstruction based on non-geniculate coralline red algal assemblages in Miocene limestone of central Crete. Facies 52:381–409
Kuffner IB, Andersson AJ, Jokiel PL, Rodgers KS, Mackenzie FT (2008) Decreased abundance of crustose coralline algae due to ocean acidification. Nat Geosci 1:114–117
Kundal P (2011) Generic distinguishing characteristics and stratigraphic ranges of fossil corallines: an update. J Geol Soc India 78:571–586
Kundal P (2014) Miocene calcareous algae from India: retrospect and prospect. Spec Publ Palaeontol Soc India 5:135–143
Kundal P, Dharashivkar AP (2005) Record of rhodoliths from Aramda Reef Member (Late Pleistocene to Holocene) of Chaya Formation, Dwarka-Okha area, Gujarat and their paleonevironmental significance. Curr Sci 88:1684–1689
Kundal P, Dharashivkar AP (2006) Ichnofossils from Neogene and Quaternary deposits of Dwarka Okha area, Jamnagar District, Gujarat, India. J Geol Soc India 68:299–315
Kundal P, Humane SK (2006) Record of Metagoniolithon (Corallinales, Rhodophyta) from the Burdigalian of western India. Curr Sci 91:221–224
Kundal MP, Humane SK (2012) Geniculate coralline algae from the Early Miocene Godhra Formation of the Kachchh offshore basin, western India. J Palaeontol Soc India 57:143–151
Kundal P, Mude SN (2009a) Nongeniculate coralline algae from the Early Miocene to Late Holocene sequence of the Porbandar area, Saurashtra, Gujarat, India. J Palaeontol Soc India 54:73–80
Kundal P, Mude SN (2009b) Geniculate coralline algae from the Neogene-Quaternary sediments in and around Porbandar area, Saurashtra, Gujarat, India. J Geol Soc India 74:267–274
Kundal P, Sanganwar BN (1998) Stratigraphical, Palaeogeographical and Palaeoenvironmental significance of fossil calcareous algae from Nimar Sandstone Formation, Bagh-Group (Cenomanian-Turonian) of Pipaldehla, Jhabua Dt, MP. Curr Sci 75:702–708
Kundal P, Wanjarwadkar KM (2000) Jania Lamouroux from Late Paleocene limestone of Middle Andaman, India. ONGC Bulletin 37:227–237
Kundal P, Bhagat MB, Humane SK (2007) Paleoenvironmental significance of coralline algae from Early Miocene Bombay Formation, Bombay Offshore Basin. J Geol Soc India 69:274–278
Kundal P, Humane SS, Humane SK (2011) Calcareous algae from the Miliolite Formation (Middle Pleistocene) of Diu, Saurashtra. J Palaeontol Soc India 56:181–194
Kundal P, Bhagat MB, Kundal MP (2013) Coralline algae from the Bassein Formation (Middle to Late Eocene) of Mumbai Offshore Basin and its paleoenvironmental significance. ONGC Bulletin 48:15–34
Kundal P, Kundal MP, Mude SN (2014) Neogene-Quaternary calcareous algae from Saurashtra Basin, western India: implications on paleoenvironments and hydrocarbon exploration. J Geol Soc India 83:183–190
Laborel J (1986) Vermetid gastropods as sea-level indicators. In: van de Plassche O (ed) Sea-level research: a manual for the collection and evaluation of data. Geo Books, Norwich, pp 281–310
Lund MJ, Davies PJ, Braga JC (2000) Coralline algal nodules off Fraser Island, eastern Australia. Facies 42:25–34
Macintyre IG, Glynn PW, Steneck RS (2001) A classic Caribbean algal ridge, Holandés Cays, Panamá: an algal coated storm deposit. Coral Reefs 20:95–105
Martin S, Gattuso JP (2009) Response of Mediterranean coralline algae to ocean acidification and elevated temperature. Glob Change Biol 15:2089–2100
Martin S, Cohu S, Vignot C, Zimmerman G, Gattuso JP (2013) One-year experiment on the physiological response of the Mediterranean crustose coralline alga, Lithophyllum cabiochae, to elevated pCO2 and temperature. Ecol Evol 3:676–693
Martone PT (2010) Quantifying growth and calcium carbonate deposition of Calliarthron cheilosporioides (Corallinales, Rhodophyta) in the field using a persistent vital stain. J Phycol 46:13–17
Matsumaru K, Sarma A (2010) Larger foraminiferal biostratigraphy of the lower Tertiary of Jaintia Hills, Meghalaya, NE India. Micropaleontology 56:539–565
McCoy SJ, Kamenos NA (2015) Coralline algae (Rhodophyta) in a changing world: integrating ecological, physiological and geochemical responses to global change. J Phycol 51:6–24
Milliman JD (1974) Marine carbonates. Springer, New York
Misra PK, Kumar P (1988) Fossil algae from the Cretaceous of Varagur, Tiruchirapalli district, Tamil Nadu. Palaeobotanist 37:36–51
Misra PK, Jauhri AK, Singh SK, Kishore S (2006a) Coralline algae from Fulra Limestone (Middle Eocene) of Kachchh, Gujarat, western India. J Geol Soc India 67:495–502
Misra PK, Jauhri AK, Singh SK, Kishore S, Rajanikanth A (2006b) Non-geniculate coralline algae from the Uttatur Group (early Cretaceous), South India. Palaeobotanist 55:29–40
Misra PK, Kishore S, Singh SK, Jauhri AK (2009) Rhodophycean algae from the lower Cretaceous of the Cauvery Basin, South India. J Geol Soc India 73:325–334
Misra PK, Jauhri AK, Tiwari RP, Kishore S, Singh AP, Singh SK (2011) Coralline algae from the Prang Formation (middle-late Eocene) of the Lumshnong area, Jaintia Hills, Meghalaya. J Geol Soc India 78:355–364
Misra U, Kishore S, Singh SK, Misra PK, Jauhri AK (2016) New record of coralline algae from the Holocene sediments of Agatti Island, Lakshadweep, India. J Geol Soc India 87:308–316
Montaggioni LF, Camoin GF (1993) Stromatolites associated with coralgal communities in Holocene high-energy reefs. Geology 21:149–152
Montaggioni LF, Cabioch G, Camoin GF, Bard E, Ribaud-Laurenti A, Faure G, Déjardin P, Récy J (1997) Continuous record of reef growth over the past 14 k.y. on the mid-Pacific island of Tahiti. Geology 25:555–558
Mude SN, Kundal P (2012) Additional coralline algae from the Lower Miocene to Late Holocene sediments of the Porbandar Group, Gujarat. J Geol Soc India 79:69–76
Nebelsick JH, Bassi D, Lempp J (2013) Tracking paleoenvironmental changes in coralline algal-dominated carbonates of the Lower Oligocene Calcareniti di Castelgomberto formation (Monti Berici, Italy). Facies 59:133–148
Pandey DK, Bahadur T, Mathur UB (2007) Stratigraphic distribution and depositional environment of the Chaya Formation along the Northwestern coast of Saurasthra peninsula, Western India. J Geol Soc India 69:1215–1230
Payri CE, Cabioch G (2004) The systematics and significance of coralline red algae in the rhodolith sequence of the Amédée 4 drill core (Southwest New Caledonia). Palaeogeog Palaeoclimatol Palaeoecol 204:187–208
Perrin C, Bosence D, Rosen B (1995) Quantitative approaches to palaeozonation and palaeobathymetry of corals and coralline algae in Cenozoic reefs. In: Bosence DWJ, Allison PA (eds) Marine palaeoenvironmental analysis from fossils. Geol Soc London Spec Publ 83:181–229
Perry CT, Hepburn LJ (2008) Syn-depositional alteration of coral reef framework through bioerosion, encrustation and cementation: taphonomic signatures of reef accretion and reef depositional events. Earth Sci Rev 86:106–144
Piller WE, Rasser M (1996) Rhodolith formation induced by reef erosion in the Red Sea, Egypt. Coral Reefs 15:191–198
Pomar L, Ward WC (1999) Reservoir-scale heterogeneity in depositional packages and diagenetic patterns on a reef-rimmed platform, Upper Miocene, Mallorca, Spain. Bull AAPG 83:1759–1773
Pomar L, Brandano M, Westphal H (2004) Environmental factors influencing skeletal grain associations: a critical review of Miocene examples from the western Mediterranean. Sedimentology 51:627–651
Quaranta F, Tomassetti L, Vannucci G, Brandano M (2012) Coralline algae as environmental indicators: a case study from the Attard member (Chattian, Malta). Geodiversitas 34:151–166
Ragazzola F, Foster LC, Form A, Anderson PSL, Hansteen TH, Fietzke J (2012) Ocean acidification weakens the structural integrity of coralline algae. Glob Change Biol 18:2804–2812
Rasser M, Piller WE (1997) Depth distribution of calcareous encrusting associations in the northern Red Sea (Safarga, Egypt) and their geological implications. Proc 8th Int Coral Reef Symp 1:743–748
Reuter M, Piller WE, Harzhauser M, Kroh A, Rögl F, Ćorić S (2011) The Quilon Limestone, Kerala Basin, India: an archive for Miocene Indo-Pacific seagrass beds. Lethaia 44:76–86
Ringeltaube P, Harvey A (2000) Non-geniculate coralline algae (Corallinales, Rhodophyta) on Heron Reef, Great Barrier Reef (Australia). Bot Mar 43:431–454
Sarkar S (2015a) Thanetian-Ilerdian coralline algae and benthic foraminifera from northeast India: microfacies analysis and new insights into the Tethyan perspective. Lethaia 48:13–28
Sarkar S (2015b) Calcareous algal-rich carbonate sediments from Assam Shelf, N-E India: an overview of the palaeoenvironmental implications. In: Mukherjee S (ed) Petroleum geosciences: Indian contexts. Springer Geology, Switzerland, pp 175–189
Sarkar S (2015c) Upper Pliocene heterozoan assemblage from the Guitar Formation of Car Nicobar Island, India: palaeoecological implications and taphonomic signatures. Palaeobiodiv Palaeoenv 96:221–237
Sarkar S, Ghosh AK (2015) Evaluation of coralline algal diversity from the Serravallian carbonate sediments of Little Andaman Island (Hut Bay), India. Carbonate Evaporite 30:13–24
Sarkar S, Sarkar S (2015) Diversity of corals and benthic algae across the shallow-water reefs of Andaman Islands: managing the valuable ecosystems. Env Dev Sust. doi:10.1007/s10668-015-9709-z
Sarkar S, Ghosh AK, Rao GMN (2016) Coralline algae and benthic foraminifera from the Long Formation (middle Miocene) of the Little Andaman Island, India: biofacies analysis, systematic and palaeoenvironmental implications. J Geol Soc India 87:69–84
Sarma A, Ghosh AK, Sarkar S (2014) First record of coralline red algae from the Kopili Formation (late Eocene) of Meghalaya, N-E India. Nat Acad Sci Lett 37:503–507
Savini A, Basso D, Bracchi VA, Corselli C, Pennetta M (2012) Maerl-bed mapping and carbonate quantification on submerged terraces offshore the Cilento peninsula (Tyrrhenian Sea). Geodiversitas 34:77–98
Saxena RK, Ghosh AK, Chandra A (2005) Calcareous algae from the limestone unit of Hut Bay Formation (Late Middle Miocene) of Little Andaman Island, India. In: Keshri JP, Kargupta AN (eds) Glimpses of Indian phycology. Bishen Singh Mahendra Pal Singh Press, Dehradun, pp 275–301
Sharma V, Srinivasan MS (2007) Geology of Andaman-Nicobar: the neogene. Capital Publishing Company, New Delhi, 162 pp
Singh SK, Kishore S, Singh AP, Misra PK, Jauhri AK (2009) Coralline algae from the Maniyara Fort Formation (Lower Oligocene) of Kachchh, Gujarat, India. Rev de Paleobiol 28:19–32
Singh SK, Kishore S, Jauhri AK, Misra PK (2011) Coralline algae from the Bermoti Member (Upper Oligocene) of the Maniyara Fort Formation of Kachchh, Gujarat, India. Rev de Paleobiol 30:177–190
Stanley SM, Hardie LA (1998) Secular oscillations in the carbonate mineralogy of reef-building and sediment-producing organisms driven by tectonically forced shifts in seawater chemistry. Palaeogeog Palaeoclimatol Palaeoecol 144:3–19
Steneck RS (1986) The ecology of coralline algal crusts: convergent patterns and adaptive strategies. Ann Rev Ecol Evol Syst 17:273–303
Steneck RS, Adey WH (1976) The role of environment in control of morphology in Lithophyllum congestum, a Caribbean algal ridge builder. Bot Mar 59:197–215
Steneck RS, Dethier MN (1994) A functional group approach to the structure of algal-dominated communities. Oikos 69:476–498
Tewari VC, Kumar K, Lokho K, Siddaiah NS (2010a) Lakadong limestone: Paleocene-Eocene boundary carbonate sedimentation in Meghalaya, northeastern India. Curr Sci 98:88–95
Tewari VC, Lokho K, Kumar K, Siddaiah NS (2010b) Late Cretaceous-Paleogene basin architecture and evolution of the Shillong Shelf sedimentation, Meghalaya, Northeast India. J Ind Geol Cong 2:61–73
Tierney PW, Johnson ME (2012) Stabilization role of crustose coralline algae during Late Pleistocene reef development on Isla Cerralvo, Baja California Sur (Mexico). J Coastal Res 279:244–254
Toth LT, Aronson RB, Vollmer SB, Hobbs JW, Urrego DH, Cheng H, Enochs IC, Combosch DJ, Van Woesik R, Macintyre IG (2012) ENSO drove 2500-year collapse of eastern Pacific coral reefs. Science 337:81–84
Vásquez-Elizondo RM, Enriquez S (2016) Coralline algal physiology is more adversely affected by elevated temperature than reduced pH. Sci Rep 6. doi:10.1038/srep19030
Venkatachalapathy V, Gururaja MN (1984) Algal genus Aetesolithon from Neogene of Little Andaman Island. J Geol Soc India 25:63–66
Verheij E, Erftemeijer PLA (1993) Distribution of seagrasses and associated macroalgae in South Sulawesi, Indonesia. Blumea 38:45–64
Williams B, Halfar J, Steneck RS, Wortmann UG, Hetzinger S, Adey WH, Lebednik P, Joachimski M (2011) Twentieth century δC13 variability in surface water dissolved inorganic carbon recorded by coralline algae in the northern North Pacific Ocean and the Bering Sea. Biogeosciences 8:165–174
Wilson S, Blake C, Berges JA, Maggs CA (2004) Environmental tolerances of free-living coralline algae (maerl): implications for European maine conservation. Biol Conserv 120:283–293
Yamano H, Kayanne H, Yonekura N (2001) Anatomy of a modern coral reef flat: a recorder of storms and uplift in the late Holocene. J Sed Res 71:295–304
Žuljević A, Kaleb S, Peña V, Despalatović M, Cvitković I, De Clerck O, Le Gall L, Falace A, Vita F, Braga JC, Antolić B (2016) First freshwater coralline alga and the role of local features in a major biome transition. Sci Rep 6. doi:10.1038/srep19642
Acknowledgments
I extend my heartiest gratitude to Prof. Sunil Bajpai, Director, Birbal Sahni Institute of Palaeosciences for constant encouragement and providing the necessary infrastructure facilities. Sincere thanks are extended to Dr. Gavin W. Maneveldt and the anonymous reviewers for their valuable suggestions that helped a lot in improving the manuscript. The financial support from the Science and Engineering Research Board, Department of Science and Technology, New Delhi, India is gratefully acknowledged (Grant No. SR/FTP/ES-143/2014).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sarkar, S. Ecology of Coralline Red Algae and Their Fossil Evidences from India. Thalassas 33, 15–28 (2017). https://doi.org/10.1007/s41208-016-0017-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41208-016-0017-7