Abstract
Wheat plays a major role in feeding the global population. Therefore, given its potential impact on human health, it is very important to determine toxic metal accumulation in the various varieties of wheat. This study was carried out to investigate potentially toxic metal accumulation in the wheat variety Galaxy-2013 following various industrial wastewater irrigation treatments, and to examine various indices such as the bioconcentration factor, enrichment factor, and health risk index in order to better understand how metals are transported and accumulate throughout the food chain. Atomic absorption spectrophotometry was used to evaluate the concentrations of iron (Fe), copper (Cu), chromium (Cr), cadmium (Cd), and cobalt (Co) in the wheat. A multipurpose pot experiment was conducted to determine metal accumulation in wheat samples irrigated with industrial wastewater in the Botanical Garden of the Botany Department at the University of Sargodha. The concentrations of the potentially toxic metals Co, Cd, Cr, Cu, and Fe in the harvested wheat samples ranged from 0.720 to 1.075, from 0.316 to 0.526, from 0.111 to 0.950, from 0.603 to 0.665, and from 1.617 to 1.884 mg/kg, respectively. These concentrations were higher than the safe levels specified by WHO, FAO, and US EPA, except for Cd. However, the health risk index for Cd was found to be above the permissible limit. Pearson correlation coefficients indicated that there were correlations between the potentially toxic metal contents in the soil and those in the plants: a significant positive correlation was observed for Cd, while the correlations for Cr, Cu, and Fe were positive and nonsignificant.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Uncontrolled industrialization and urbanization by nations around the globe have dangerous effects on the environment (including the overexploitation of natural resources) as well as human health (Ugulu 2015a, b; Yorek et al. 2016; Khan et al. 2019a, b). The increasing rate of urbanization has driven industrial development, and both urban areas and industry are major producers of waste materials that disturb the environment (Ahmad et al. 2019; Munir et al. 2019). The volume of urban and industrial wastewaters has increased in parallel with urbanization and industrialization, and these wastewaters pose a major threat to the environment. In many regions of the world, they are discharged untreated into water bodies in neighboring regions, causing serious environmental problems. On the other hand, industrial and domestic wastewaters are used for agricultural irrigation in many countries in which there are shortages of freshwater (Ahmad et al. 2018).
Agriculture is one of the most important drivers of Pakistan's economy (Nadeem et al. 2019). Although agriculture is important in Pakistan, water shortages mean that farmers frequently use industrial wastewater for irrigation purposes (Khan et al. 2019a, b; Munir et al. 2019). While industrial wastewater contains nutrients, it also includes various types of contaminants, such as inorganic and organic molecules, microorganisms, and radioactive elements (Khan et al. 2018a, b). When industrial and urban effluents are discharged directly into the environment, they have serious negative effects on ecosystem elements and agricultural products (Ugulu et al. 2009; Sahin et al. 2016). Industrial wastewater and potentially toxic metals damage fertile land by adding chemical compounds to the soil, which leads to greater yield losses (Khan et al. 2018c, d).
Various studies on the subject have shown that the use of industrial and sewage wastewaters in agricultural irrigation can result in potentially toxic metal accumulation in soil and plants (Siddique et al. 2019). High levels of organic matter are present in the upper layer of the soil. However, in agricultural areas close to cities or industrial facilities, which tend to be the agricultural areas in which wastewater is used for irrigation, there are high trace metal concentrations in the upper layer of the soil that originate from wastewater (Khan et al. 2019c, d). Since the roots of many crops grow in this zone, the upper soil layer is an important influence on the substances that are taken up by plants from the soil (Wajid et al. 2020).
Plants are one of the most important components of both human and animal nutrition. Wheat (Triticum aestivum L.) has more nutritional value than other cereals and helps to fulfill the dietary requirements of one-third of the global population. It is a major source of the protein, vitamins, and minerals that are necessary for human health. However, wheat crops that are irrigated with wastewater show significant accumulation of toxic metals, so it is potentially hazardous for humans to consume these crops (Perveen et al. 2011; Ugulu et al. 2016). Toxic metals can degrade human mental and central nervous system function, negatively affect blood composition, damage vital internal organs such as the liver, kidneys, and lungs, and disrupt metabolic reactions (Khan et al. 2021).
Healthy human nutrition is one of the most popular topics of recent years (Ugulu et al. 2020). Thus, given their potential impact on human health, it is very important to determine the levels of toxic metals in the various varieties of wheat that play a major role in feeding the global population. Literature reviews on this subject indicate that studies on the accumulation of potentially toxic metals in wheat variety Galaxy-2013 and the effect of this accumulation on human health are scarce. Therefore, the present study was performed to determine the levels of the potentially toxic metals Cd, Cr, Fe, Co, and Cu in Galaxy-2013 wheat irrigated with industrial wastewater, and to examine various indices such as the bioconcentration factor, enrichment factor, and health risk index in order to improve our understanding of how metals are transported and accumulate throughout the food chain.
Materials and methods
Study area
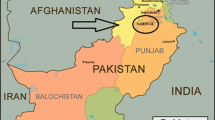
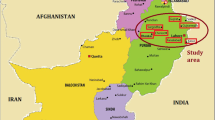
The present research was carried out during the years 2015–2016 in the Botanical Garden of the Botany Department at the University of Sargodha (32°8′0″N, 73°7′0″E), northeastern Punjab, Pakistan (Fig. 1). Sargodha is the fifth most contaminated urban area of Punjab according to Punjab Environment Protection Department officials. While the summer season is very hot in the city of Sargodha, the winter season is quite cold. The Jhelum River flows to the north and west of the city, and the Chenab River passes to the east of the city.
Plant cultivation and sample preparation
Healthy seed samples of the wheat variety Galaxy-2013 were collected in the year 2015. About 6 kg of soil were added to each of 12 plastic pots 30 cm in height and 40 cm in diameter, and 20 seeds were placed in each pot. Pot trials were then conducted in the natural environment (November–April) for 6 months. The seeds were initially watered with tap water until the first leaves appeared, after which they were irrigated with different wastewater treatments. The pots were split into four groups: T-I: control (irrigated with 100% groundwater), T-II: irrigated with 30% industrial wastewater and 70% groundwater, T-III: irrigated with 60% industrial wastewater and 40% groundwater, and T-IV: irrigated with 90% industrial wastewater and 10% groundwater. The industrial wastewater originated from Ramzan Sugar Mill in Jhang. Wheat grains were harvested in April 2016, about 120 days after planting the seeds.
Next, the grain samples were washed with a mixture of distilled water and 5% HCl, placed in an oven at a temperature of 105 °C to dry, and crushed to a fine powder using an electrical grinder before being dried and weighed. Soil samples were collected from the top 15–18 cm of the soil profile. After being dried outdoors, the soil samples were oven dried at 72 °C for 4 days.
The soil and seed samples were digested using a wet digestion method outlined by Allen et al. (1986). One gram of each soil and wheat sample was digested with a mixture of HNO3, H2SO4, and HClO4 (5:1:1) at 80 °C until a transparent solution was obtained. After cooling the solution, it was filtered with Whatman filter paper #42 and the final volume of the solution was made up to 50 mL. It was stored in plastic bottles until further analysis. The concentrations of potentially toxic metals in the samples were measured after digestion with HNO3 and H2O2 followed by atomic absorption spectrophotometry (Allen et al. 1986).
Metal determination
Atomic absorption spectrophotometry (AA-6300, Shimadzu, Japan) was used to assess the concentrations of the potentially toxic metals iron (Fe), copper (Cu), chromium (Cr), and cadmium (Cd). The atomic absorption spectrophotometer was equipped with a graphite furnace and D2 corrector (Perkin–Elmer Model 503) in order to determine the Co content. The iron in the organic layer was analyzed under the standard conditions defined in the manufacturer's instrumentation and application manual. The stock solutions specified in the standard conditions for metals were created and used. The experimental procedures and analytical methods used for AAS were taken from the manufacturer’s guide to instrumentation and applications, with reference to the guidelines of the European Commission (2006), the report of El-Ansary and El-Leboudy (2015), and Liu et al. (2020).
Physicochemical parameters
The physicochemical properties of the soil and water samples, such as the amount of organic matter, the electrical conductivity (EC), the pH, and the Ca, Mg, Cl, and available P and K levels were determined. The pH of a 1:2.5 soil solution (created using deionized water) was tested using a HI 3221 pH meter (Mclean 1982). Titration was used to determine the levels of Ca2+, Mg2+, and Cl−. Soil organic matter was measured by a loss-on-ignition method, ashing at 550 °C for 4 h (Page 1982). Available K and P were determined via the methods of Schollenberger and Simon (1945) and Olsen and Sommers (1982), respectively.
Statistical analysis
Statistical analyses were performed using the Statistical Package of Social Sciences (SPSS), and one-way analysis of variance (ANOVA) was applied to evaluate the concentrations of potentially toxic metals in water, soil, and food grains. Pearson’s correlation coefficient was evaluated to determine the relationships between the concentration of each metal in the soil and its concentration in wheat grains. The correlation analysis applied was a bivariate method that examined the relationships between different parameters using the least significant difference test at probability levels of 0.05, 0.01, and 0.001 (Ugulu 2020).
Bioconcentration factor
The bioconcentration factor (BCF) is a measure of the accumulation of a potentially toxic metal in a plant as a result of the transfer of the metal from the soil to the plant. The following formula is used to calculate the bioconcentration factor:
where Cveg refers to the metal concentration in plant tissues (mg/kg, fresh weight) and Csoil refers to the metal concentration in the soil (mg/kg, dry weight) (Liu et al. 2005).
Daily intake of metals
One of the methods that is used to find consumption-related health risks is the daily intake of metals (DIM). This was calculated using the following equation described by Wang et al. (2005):
where Cmetal represents the metal concentration in grains, Cfood intake represents the daily food intake, and Baverage weight represents the average body weight. An average daily vegetable intake rate of 0.345 kg/person/day and an average body mass of 55.9 kg for adults were used, based on data in the literature (Wang et al. 2005).
Health risk index
The health risk index (HRI) indicates the threat to the health of humans who consume contaminated food. In this study, it was used to calculate human exposure to potentially toxic metals due to the consumption of metal-contaminated wheat. The HRI is calculated as the ratio of the DIM from food crops to the oral reference dose (Stephens et al. 2001):
Results and discussion
Physicochemical parameters of the irrigation water
The electrical conductivity (EC) is an important indicator as it can be used to check the ionic content of water. In this study, EC values of irrigation water samples across all wastewater treatments varied from 19.3 to 50 dS/m, with mean values per treatment of: T-I, 0.79; T-II, 19.3; T-III, 35; T-IV, 50 dS/m. The values for the wastewater treatments were higher than 7.8 dS/m, the value reported by Pandey and Singh (2015), and much higher than the EC value (1.032 dS/m) determined by Alghobar and Suresha (2015) for wastewater used for irrigation purposes. US regulations state that high-quality irrigation water should have EC < 0.7 dS/m, while acceptable irrigation water should have EC < 3 dS/m. Thus, the average EC values for the irrigation water used in treatments T-II, T-III, and T-IV were above the permissible limit.
Combined Ca2+ and Mg2+ concentrations in the irrigation water samples ranged from 7.8 to 620 mg/L, with mean concentrations per treatment of: T-I, 7.8; T-II, 45; T-III, 540; T-IV, 620 mg/L. Na+ concentrations ranged from 0.1 to 1400 mg/L, with mean concentrations per treatment of: T-I, 0.1; T-II, 27; T-III, 1120; T-IV, 1400 mg/L. Bicarbonate concentrations were 6.2–894 mg/L, with mean concentrations per treatment of: T-I, 6.2; T-II, 38.2; T-III, 540; T-IV, 894 mg/L. Chloride concentrations ranged from 1.1 to 194 mg/L, with mean concentrations of: T-I, 1.1; T-II, 45; T-III, 95.5; T-IV, 194 mg/L (Table 1). These concentrations of the major cations Ca2+, Mg2+, and Na+ in the wastewater treatments are higher than the corresponding concentrations in sugarcane (48, 43, and 27 mg/L, respectively) reported by Alghobar and Suresha (2015). On the other hand, the concentrations of major anions such as HCO3– and Cl– in the wastewater treatments in the present study were found to be higher than those determined by Alghobar and Suresha (2015): 296 and 93 mg/L, respectively.
Physicochemical parameters of soil samples taken after wheat cultivation
The soils collected from the four treatments after wheat cultivation had a clay loam texture. The soil pH ranged between 8.1 and 8.3, with average values per treatment of: T-I, 8.1; T-II, 8.2; T-III, 8.3; T-IV, 8.2. The soil EC ranged between 1.58 and 1.80 dS/m, with average EC values per treatment of: T-I, 1.80; T-II, 1.58; T-III, 1.58; T-IV, 1.80 dS/m. The organic matter (OM) contents of the soil samples ranged between 1.11 and 1.25%, with mean percentages per treatment of: T-I, 1.25; T-II, 1.11; T-III, 1.18; T-IV, 1.24%. Soil phosphorus (P) values ranged between 1.71 and 2.91 mg/kg, with mean values per treatment of: T-I, 2.91; T-II, 1.72; T-III, 1.71; T-IV, 1.90 mg/kg. Soil potassium (K) ranged between 3.40 and 5.90 mg/kg, with mean values per treatment of: T-I, 5.22; T-II, 4.90; T-III, 3.40; T-IV, 5.90 mg/kg (Table 2). Numerous studies have reported that the soil pH decreases significantly after irrigation with wastewater (Li et al. 1987), possibly due to organic acid production and organic matter decomposition (Khan et al. 2020c).
Potentially toxic metal concentrations in irrigation water
In the present study, the concentrations of the potentially toxic metals Co, Cd, Cu, Cr and Fe in irrigation water samples ranged from 1.823 to 2.463, from 0.333 to 1.036, from 0.354 to 1.440, from 0.523 to 1.183, and from 0.533 to 0.926 mg/L, respectively (Table 3). When the metals were ranked based on their abundances in the water samples, we found that Co < Cr < Cu < Fe < Cd for T-I, Co < Cr < Fe < Cu < Cd for T-II, Co < Cr < Fe < Cu < Cd for T-III, and Fe < Co < Cu < Cr < Cd for T-IV (Fig. 2). While the Cd concentration was high in water samples from all the treatments, the Cu, Cr, and Fe concentrations increased as the percentage of wastewater in the irrigation water increased. Metal accumulation was highest in the irrigation water used in the T-IV treatment, which included the most industrial wastewater. However, the results of one-way ANOVA indicated that treatment had a nonsignificant effect (p > 0.05) on the concentrations of the metals (Cd, Cr, Cu, Co, and Fe) in the irrigation water (Table 4).
The maximum allowable limits for Co, Cd, Cu, Cr, and Fe in water are 0.05, 0.01, 0.01, 0.55, and 0.50 mg/L, respectively, as defined by WHO, FAO, Standard Guidelines in Europe (Chiroma et al. 2014), and US EPA (2002). The accumulated levels of potential toxic metals in the irrigation water samples in the present study were higher than those maximum limits except in the case of Fe. Studies investigating this issue have revealed that increased acidity of the irrigation water increases potentially toxic metal accumulation (Wajid et al. 2020). Therefore, the high potentially toxic metal concentrations measured in the irrigation water samples in the present study may be due to the low pH of the wastewater. However, the Fe and Cr concentrations were lower whereas the Cd and Cu levels were higher than the corresponding values recorded by Antil (2012): 5.6, 3.5, 0.97, and 0.46 mg/L, respectively. The concentrations of Cu, Cd, Co, and Cr (but not Fe) were also higher than the corresponding values measured by Alghobar and Suresha (2015) when sugarcane crop samples were irrigated with wastewater. In an experiment to investigate the effect of wastewater on soil and crops, Alghobar and Suresha (2015) observed concentrations of 2.93, 0.05, 0.047, 0.055, and 0.32 mg/L for Cu, Cd, Co, Cr, and Fe, respectively, in the wastewater.
Mean concentrations of potentially toxic metals in soil samples taken after wheat cultivation
In the present study, the Cd, Co, Cr, Cu, and Fe concentrations in the soil samples ranged from 1.815 to 1.908, from 0.561 to 0.730, from 0.740 to 1.260, from 1.193 to 1.931, and from 2.101 to 2.836 mg/kg, respectively (Table 3). When the metals were ranked based on their abundances in the soil samples, we found that Co < Cr < Cu < Cd < Fe for T-I, Co < Cr < Cu < Cd < Fe for T-II, Co < Cr < Cu < Cd < Fe for T-III, and Co < Cr < Cu < Cd < Fe for T-IV (Fig. 3). When the treatments were compared in terms of the overall potentially toxic metal concentration in samples, none of the treatments were found to result in a much higher potentially toxic metal concentration than the other treatments. However, different treatments led to the accumulation of different metals. The mean Co concentrations in the soil samples were low whereas the Fe, Cd, Cu, and Cr concentrations were high regardless of the treatment applied. The results from ANOVA showed that treatment had a nonsignificant effect (p > 0.05) on the concentrations of Cd, Cr, Co, and Fe in the soil samples, whereas treatment exerted a significant effect on the Cu concentration (p < 0.05) (Table 4).
The maximum allowable concentrations of Co, Cd, Cu, Cr, and Fe in soil are defined as 50, 3, 100, 100, and 50,000 mg/kg, respectively, by US EPA (1997). The Co, Cd, Cu, Cr, and Fe concentrations in the soil samples examined in this work were below the allowable limits defined by US EPA (1997), regardless of the treatment applied. Also, the mean Cd, Cr, Cu, and Fe concentrations in the soil samples were lower than the corresponding values measured by Asdeo (2014) and Rattan et al. (2005). These findings indicate that there is low absorption of potentially toxic metals from the soil, so the metal concentrations in the soil samples were in the safe range (Ugulu et al. 2019a, b).
Mean concentrations of potentially toxic metals in wheat grains
In the present study, the concentrations of the potentially toxic metals Co, Cd, Cr, Cu, and Fe in wheat samples ranged from 0.720 to 1.075, from 0.316 to 0.526, from 0.111 to 0.950, from 0.603 to 0.665, and from 1.617 to 1.884 mg/kg, respectively (Table 3). When the metals were ranked based on their abundances in the wheat grains, we found that Co < Cr < Cu < Cd < Fe for T-I, Cr < Co < Cu < Cd < Fe for T-II, Cr < Co < Cu < Cd < Fe for T-III, and Cr < Co < Cu < Cd < Fe for T-IV (Fig. 4). The Cr concentrations in grains obtained with the T-II, T-III, and T-IV treatments were lower than those in grains obtained with the T-I treatment, while the Fe, Cd, and Cu concentrations in the grains were high with all treatments. Treatment had a nonsignificant effect (p > 0.05) on the concentrations of Cd, Cu, and Co in the grains, whereas treatment had a significant effect on Cr and Fe concentrations (Table 4).
The maximum allowable concentrations of Co, Cd, Cu, Cr, and Fe in vegetables are defined as 50, 0.1, 73, 50, and 425 mg/L, respectively, by WHO, FAO, Standard Guidelines in Europe (Chiroma et al. 2014), and US EPA (2002). The concentrations of potentially toxic metals in the grains studied here were higher than the maximum permissible levels in vegetables, except for Cd. The main factors that affect the levels of potentially toxic metals taken up by plants from the soil are the pH, temperature, and the cation exchange capacity of the soil, the levels of other metals in the soil, chemical selectivity, and the plant species (Khan et al. 2020a, b, c).
Ahmad et al. (2019) studied potentially toxic metal accumulation in wheat variety Chagi-4 caused by irrigation with sewage water in Sargodha, Pakistan. They observed that the mean concentrations of metals in Chagi-4 wheat ranged from 0.03 to 0.2 for Co, from 1.2 to 1.6 for Cd, from 0.3 to 0.5 for Cr, from 0.7 to 1.4 for Cu, and from 0.8 to 1.6 for Fe (mg/kg), respectively. All metal concentrations in the wheat (Galaxy-2013) grown in the present study were higher than those observed in the Chagi-4 variety, except for Cu. The metal concentrations measured in wheat grains in the present study were also higher than those in the wheat grains analyzed by Asdeo (2014). The differences in metal concentrations between these studies may be due to the different wastewaters used to irrigate the wheat varieties, or differences in the metal intake characteristics of these varieties.
Bioconcentration factor (BCF)
BCF values were calculated for all the metals of interest in each treatment. The highest value was observed for Cr, and the smallest for Cu. After ranking the BCF values, we found that Cr > Co > Fe > Cd > Cu for T-I, Cr > Fe > Co > Cu > Cd for T-II, Cr > Co > Fe > Cd > Cu for T-III, and Cr > Co > Fe > Cd > Cu for T-IV (Table 5). The BCF is the most accurate indicator of the tendency of a metal to be transferred from the soil to a vegetable. All of the BCF values for Cr were > 1, meaning that this metal is readily available to and relatively likely accumulate in vegetables (Khan et al. 2020c). The results therefore suggest that the wheat variety Galaxy-2013 accumulates Cr. In the present study, the BCF values for Cr and Cu were higher than the corresponding values reported by Verma et al. (2015). The BCF values of Cr, Co, Cu, and Fe were also higher than those reported by Alghobar and Suresha (2015). Thus, it appears that Cr, Co, Cu, and Fe have a high tendency to move from the soil to the wheat variety Galaxy-2013. Higher BCF values were also observed for Cd, Cr, and Cu in the present study than in the studies performed by Asdeo (2014) and Jia et al. (2010).
Daily intake of metals (DIM)
The possible exposure of humans to each potentially toxic metal due to the consumption of wheat variety Galaxy-2013 was estimated in order to evaluate the health risk from that metal. In the present study, the sole pathway for the studied metals into humans was assumed to be the consumption of wheat grains. The DIM values for the metals of interest were estimated based on the average wheat grain consumption of an adult. High DIM values were observed in all the treatments for Fe, Cr, Cd, and Cu but not for Co. When the metals were ranked based on their DIM values, we found that Fe > Cr > Cd > Cu > Co for T-I, Fe > Cu > Cd > Co > Cr for T-II, Fe > Cd > Cu > Co > Cr for T-III, and Fe > Cd > Cu > Co > Cr for T-IV (Table 6). The DIM values for these metals were lower than the corresponding values reported by Antil (2012), indicating that all of the wheat grain samples were safe for consumption.
Health risk index (HRI)
Health risk index values for the potentially toxic metals in Galaxy-2013 wheat grains were estimated by performing health risk assessments (US EPA 2002; Cui et al. 2004; Wang et al. 2005). If the HRI of a metal in a specific food crop is < 1, the concentration of the metal in the food is low enough for the food to be safe for human consumption, whereas a HRI of > 1 is harmful and dangerous (US EPA 2002). For the wheat grains grown in treatment T-I, the health risk index values of the metals were ranked as follows: Cd > Cr > Fe > Cu > Co. The corresponding rankings for the wastewater treatments were as follows: Cd > Cr > Fe > Cu > Co (T-II); Cd > Cr > Fe > Cu > Co (T-III); and Cd > Cr > Fe > Cu > Co (T-IV) (Table 6). The HRI values for Cr, Fe, Cu, and Co were < 1 in all treatments, indicating that the accumulated concentrations of these metals in Galaxy-2013 wheat grains were below their maximum permissible limits (i.e., not hazardous to human health). However, it appears that the consumption of crops that have been irrigated with wastewater and or grown near wastewater sources could be dangerous to human health as it could result in exposure to high levels of Cd.
Correlation
Pearson’s bivariate correlation coefficient was used to determine the association between the concentration of a particular potentially toxic metal in soil post-cultivation and its concentration in Galaxy-2013 wheat grains. In the present study, the Cd concentrations in soil and grains were found to be significantly positively correlated, whereas the corresponding correlations for Cr, Cu, and Fe concentrations in soil and grains were weak (Table 7). The significant correlations of potentially toxic metals indicate that they may have originated from irrigation with wastewater. The correlation between Fe and Cr concentrations showed that they both originated from the tannery industry. However, the strong association of Cd, Zn, and Cu concentrations also indicates that these metals originated from the same anthropogenic sources, such as the paint industry and the municipal sewage system.
Conclusion
Wastewater irrigation is common practice when insufficient freshwater resources are available. Wastewaters may contain nutrients that are essential for the growth of plants, but they can also contain toxic metals.
In the present study, the concentrations of potentially toxic metals in irrigation water samples were higher than their permissible limits reported by WHO, FAO, US EPA, except for Fe. While the Co, Cd, Cu, Cr, and Fe concentrations in soil samples taken after wheat cultivation remained below their permissible limits under all treatment conditions, the concentrations of all the metals in the wheat grains were higher than the permissible limits, except for Cd. However, the health risk index value of Cd was found to be > 1. This value indicates that irrigating Galaxy-2013 wheat with wastewater from the Jhang region of Pakistan may lead to the accumulation of dangerous levels of Cd in the resulting wheat grain. Therefore, precautionary measurements should be taken to check that the wastewater in this region is treated properly before it is applied to agricultural lands.
References
Ahmad K, Nawaz K, Khan ZI, Nadeem M, Wajid K et al (2018) Effect of diverse regimes of irrigation on metals accumulation in wheat crop: an assessment-dire need of the day. Fresen Environ Bull 27(2):846–855
Ahmad K, Wajid K, Khan ZI, Ugulu I, Memoona H, Sana M, Nawaz K, Malik IS, Bashir H, Sher M (2019) Evaluation of potential toxic metals accumulation in wheat irrigated with wastewater. Bull Environ Contam Toxicol 102:822–828. https://doi.org/10.1007/s00128-019-02605-1
Alghobar MA, Suresha S (2015) Evaluation of nutrients and trace metals and their enrichment factors in soil and sugarcane crop irrigated with wastewater. J Geosci Environ Prot 3(1):46–56
Allen S, Grimshaw H, Rowland A (1986) Chemical analysis. In: Moore PD, Chapman SB (eds) Methods in plant ecology. Blackwell Scientific, Oxford, pp 285–344
Antil RS (2012) Impact of sewage and industrial effluents on soil-plant health. https://www.intechopen.com/books/industrial-waste/impact-of-sewer-water-and-industrial-wastewaters-on-soil-plant-health. Accessed 15 Mar 2020
Asdeo A (2014) Toxic metal contamination of staple crops (wheat and millet) in periurban area of western Rajasthan. Int Ref J Eng Sci 3(4):8–18
Chiroma TM, Ebewele RO, Hymore FK (2014) Comparative assessment of heavy metal levels in soil, vegetables and urban grey wastewater used for irrigation in Yola and Kano. Int Ref J Eng Sci 3:1–9
Cui YG, Zhu YG, Zhai RH et al (2004) Transfer of metals from soil to vegetables in an area near a smelter in Nanning. China Environ Interact 30(1):785–791
El-Ansary M, El-Leboudy A (2015) Levels of cadmium and lead in raw cow and buffalo’s milk samples collected from local markets of El-Behera governorate. Alex J Vet Sci 47:129–133
European Commission (2006) Commission Regulation (EC) no. 1181/2006: Setting maximum levels for certain contaminants in food stuffs. http://data.europa.eu/eli/reg/2006/1881/oj. Accessed 15 Mar 2020
FAO (1985) Guidelines: land evaluation for irrigated agriculture. Soils Bulletin 55. Food and Agriculture Organization of the United Nations, Rome
Jia L, Wang W, Li Y, Yang L (2010) Heavy metals in soil and crops of an intensively farmed area: a case study in Yucheng city, Shandong province, China. Int J Environ Res Publ Health 7(1):395–412
Khan ZI, Ugulu I, Ahmad K, Yasmeen S, Noorka IR, Mehmood N, Sher M (2018a) Assessment of trace metal and metalloid accumulation and human health risk from vegetables consumption through spinach and coriander specimens irrigated with wastewater. Bull Environ Contam Toxicol 101:787–795. https://doi.org/10.1007/s00128-018-2448-8
Khan ZI, Ugulu I, Sahira S, Ahmad K, Ashfaq A, Mehmood N, Dogan Y (2018b) Determination of toxic metals in fruits of Abelmoschus esculentus grown in contaminated soils with different irrigation sources by spectroscopic method. Int J Environ Res 12:503–511. https://doi.org/10.1007/s41742-018-0110-2
Khan ZI, Ugulu I, Umar S, Ahmad K, Mehmood N, Ashfaq A, Bashir H, Sohail M (2018c) Potential toxic metal accumulation in soil, forage and blood plasma of buffaloes sampled from Jhang, Pakistan. Bull Environ Contam Toxicol 101:235–242. https://doi.org/10.1007/s00128-018-2353-1
Khan ZI, Ahmad K, Safdar H, Ugulu I, Wajid K, Bashir H, Dogan Y (2018d) Manganese bioaccumulation and translocation of in forages grown in soil irrigated with city effluent: an evaluation on health risk. Res J Pharm Biol Chem Sci 9(5):759–770
Khan ZI, Safdar H, Ahmad K, Wajid K, Bashir H, Ugulu I, Dogan Y (2019a) Health risk assessment through determining bioaccumulation of iron in forages grown in soil irrigated with city effluent. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-019-04721-1
Khan ZI, Arshad N, Ahmad K, Nadeem M, Ashfaq A et al (2019b) Toxicological potential of cobalt in forage for ruminants grown in polluted soil: a health risk assessment from trace metal pollution for livestock. Environ Sci Pollut Res 26:15381–15389
Khan ZI, Nisar A, Ugulu I, Ahmad K, Wajid K, Bashir H, Dogan Y (2019c) Determination of cadmium concentrations of vegetables grown in soil irrigated with wastewater: evaluation of health risk to the public. Egypt J Bot 59(3):753–762. https://doi.org/10.21608/ejbo.2019.9969.1296
Khan ZI, Ahmad K, Rehman S, Ashfaq A, Mehmood N, Ugulu I, Dogan Y (2019d) Effect of sewage water irrigation on accumulation of metals in soil and wheat in Punjab, Pakistan. Pak J Anal Environ Chem 20(1):60–66. https://doi.org/10.21743/pjaec/2019.06.08
Khan ZI, Safdar H, Ahmad K, Wajid K, Bashir H, Ugulu I, Dogan Y (2020a) Copper bioaccumulation and translocation in forages grown in soil irrigated with sewage water. Pak J Bot 52(1):111–119. https://doi.org/10.30848/PJB2020-1(12)
Khan ZI, Ugulu I, Sahira S, Mehmood N, Ahmad K, Bashir H, Dogan Y (2020b) Human health risk assessment through the comparative analysis of diverse irrigation regimes for Luffa (Luffa cylindrica (L.) Roem.). J Water Sanit Hyg Dev 10(2):249–261. https://doi.org/10.2166/washdev.2020.132
Khan ZI, Ahmad K, Siddique S, Ahmed T, Bashir H, Munir M, Mahpara S, Malik IS, Wajid K, Ugulu I, Nadeem M, Noorka IR, Chen F (2020c) A study on the transfer of chromium from meadows to grazing livestock: an assessment of health risk. Environ Sci Pollut Res 27:26694–26701. https://doi.org/10.1007/s11356-020-09062-y
Khan ZI, Ugulu I, Zafar A, Mehmood N, Bashir H, Ahmad K, Sana M (2021) Biomonitoring of heavy metals accumulation in wild plants growing at Soon Valley, Khushab, Pakistan. Pak J Bot. https://doi.org/10.30848/PJB2021-1(14)
Li YJ, Ning RA, Wang DZ (1987) Mapping of soil background content map in 11 elements in aquic soils of the Yellow River downstream. Agro-Environ Prot 6(1):18–21
Liu W, Zhao JZ, Ouyang ZY, Soderlund L, Liu GH (2005) Impacts of sewage irrigation on heavy metal distribution and contamination in Beijing, China. Environ Int 31(1):805–812
Liu W, Mehmood N, Saeed H, Arshad M, Khan ZI, Muqaddas H (2020) Quantitative analysis of lead in cows and buffaloes for health assessment. Environ Sci Pollut Res 27:8621–8627. https://doi.org/10.1007/s11356-019-07556-y
Mclean EO (1982) Soil pH and lime requirement. In: Page AL, Miller RH, Keeney DR (eds) Methods of soil analysis. Part 2. Chemical and microbiological properties. American Society of Agronomy, Madison, pp 199–223
Munir M, Khan ZI, Ahmad K, Wajid K, Bashir H et al (2019) Transfer of heavy metals from different sources of fertilizers in wheat variety (Galaxy-13). Asian J Biol Sci 12(4):832–841. https://doi.org/10.3923/ajbs.2019.832.841
Nadeem M, Qureshi TM, Ugulu I, Riaz MN, An QU et al (2019) Mineral, vitamin and phenolic contents and sugar profiles of some prominent date palm (Phoenix dactylifera) varieties of Pakistan. Pak J Bot 51(1):171–178
Olsen SR, Sommers LE (1982) Phosphorous. In: Page AL, Miller RH, Keeney DR (eds) Methods of soil analysis. Part 2. Chemical and microbiological properties. American Society of Agronomy, Madison, pp 403–430
Page AL (ed)(1982) Methods of soil analysis. Part 2. Chemical and microbiological properties. Agronomy monographs 9.2. American Society of Agronomy, Madison
Pandey R, Singh J (2015) Effect of textile factory effluent irrigation on productivity of wheat crop. Int J Sci Environ 4(3):727–736
Perveen S, Ihsanullah I, Shah Z, Nazif W, Shah SS, Shah H (2011) Study on accumulation of heavy metals in vegetables receiving sewage water. J Chem Soc Pak 33(2):220–227
Rattan RK, Datta SP, Chhonkar PK, Suribabu K, Singh AK (2005) Long term impact of irrigation with sewage effluents on heavy metal content in soils, crops and groundwater. Agric Ecosyst Environ 109(1):310–322
Sahin I, Akcicek E, Guner O, Dogan Y, Ugulu I (2016) An investigation on determining heavy metal accumulation in plants growing at Kumalar Mountain in Turkey. Eur Asian J BioSci 10:22–29. https://doi.org/10.5053/ejobios.2016.10.0.3
Schollenberger CJ, Simon RH (1945) Determination of exchange capacity and exchangeable bases in soil ammonium acetate method. Soil Sci 59:13–24
Siddique S, Ahmad K, Khan ZI, Wajid K, Bashir H et al (2019) Sodium status of soil, forages, and small ruminants of Punjab Pakistan. Pure Appl Biol 8(3):1950–1961. https://doi.org/10.19045/bspab.2019.80139
Stephens SR, Alloway BJ, Carter JE et al (2001) Towards the characterization of heavy metals in dredged canal sediments and an appreciation of availability: two examples from the UK. Environ Pollut 113(3):395–401
Ugulu I (2015a) Development and validation of an instrument for assessing attitudes of high school students about recycling. Environ Educ Res 21(6):916–942. https://doi.org/10.1080/13504622.2014.923381
Ugulu I (2015b) A quantitative investigation on recycling attitudes of gifted/talented students. Biotechnol Biotechnol Equip 29:20–26. https://doi.org/10.1080/13102818.2015.1047168
Ugulu I (2020) Gifted students’ attitudes towards science. Int J Edu Sci 28(1–3):7–14. https://doi.org/10.31901/24566322.2020/28.1-3.1088
Ugulu I, Baslar S, Dogan Y, Aydin H (2009) The determination of colour intensity of Rubia tinctorum and Chrozophora tinctoria distributed in Western Anatolia. Biotechnol Biotechnol Equip 23(SE):410–413
Ugulu I, Unver MC, Dogan Y (2016) Determination and comparison of heavy metal accumulation level of Ficus carica bark and leaf samples in Artvin, Turkey. Oxid Commun 39(1):765–775
Ugulu I, Unver MC, Dogan Y (2019a) Potentially toxic metal accumulation and human health risk from consuming wild Urtica urens sold on the open markets of Izmir. Euro-Mediterr J Environ Integr 4:36. https://doi.org/10.1007/s41207-019-0128-7
Ugulu I, Khan ZI, Rehman S, Ahmad K, Munir M, Bashir H, Nawaz K (2019b) Trace metal accumulation in Trigonella foenum-graecum irrigated with wastewater and human health risk of metal access through the consumption. Bull Environ Contam Toxicol 103:468–475. https://doi.org/10.1007/s00128-019-02673-3
Ugulu I, Khan ZI, Rehman S, Ahmad K, Munir M, Bashir H (2020) Effect of wastewater irrigation on trace metal accumulation in spinach (Spinacia oleracea L.) and human health risk. Pak J Anal Environ Chem 21(1):92–101. https://doi.org/10.21743/pjaec/2020.06.11
US EPA (1997) Calculating upper confidence limits for exposure point concentrations at hazardous waste sites. Office of emergency and remedial response, Washington, DC
US EPA (2002) Region 9, preliminary remediation goals. http://www.epa.gov/region09/waste/sfund/prg
Verma S, Yadav S, Yadav S, Kadyan PS, Singh I, Singh D (2015) Heavy metals in wheat grains of Haryana (India) and their health implications. J Chem Pharm Res 10:342–357
Wajid K, Ahmad K, Khan ZI, Nadeem M, Bashir H, Chen F, Ugulu I (2020) Effect of organic manure and mineral fertilizers on bioaccumulation and translocation of trace metals in maize. Bull Environ Contam Toxicol 104:649–657. https://doi.org/10.1007/s00128-020-02841-w
Wang X, Sato T, Xing TB, Tao S (2005) Health risks of heavy metals to the general public in Tianjin, China via consumption of vegetables and fish. Sci Total Environ 350:28–37
WHO/FAO (2007) Joint WHO/FAO food standards programme Codex Alimentarius Commission 13th Session. Report of the Thirty-Eighth Session of the Codex Committee on Food Hygiene, Houston, Texas, USA. Codex Alimentarius Commission, Rome
Yorek N, Ugulu I, Aydin H (2016) Using self-organizing neural network map combined with Ward’s clustering algorithm for visualization of students’ cognitive structural models about aliveness concept. Comput Intell Neurosci. https://doi.org/10.1155/2016/2476256
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest regarding the publication of this paper.
Additional information
Communicated by Eric van Hullebusch, Chief Editor.
Rights and permissions
About this article
Cite this article
Ugulu, I., Khan, Z.I., Aslam, Z. et al. Potentially toxic metal accumulation in grains of wheat variety Galaxy-2013 irrigated with sugar industry wastewater and human health risk assessment. Euro-Mediterr J Environ Integr 6, 38 (2021). https://doi.org/10.1007/s41207-020-00203-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41207-020-00203-w