Abstract
One of the greatest worldwide problems is environmental pollution, and the elimination of contaminants, such as heavy metals, organic dyes, and aromatic compounds, is vital for human health and ecosystems. Among the various released pollutants into the environment, dyes are classified as highly toxic and hazardous materials, which can cause serious problems due to non-observance of the safety and health laws and regulations in some industries. In this research, the magnetite multi-walled carbon nano-tube (MWCNT) and modified graphene oxide (GO) were successfully modified with sulphonic acid groups as the new carbonic adsorbents. The morphology, surface properties as well as the chemical properties of functional groups of the prepared materials were characterized by scanning electronic microscope (SEM) and Fourier transform infrared spectrometer (FT-IR). The sulphonic acid-functionalized magnetite multi-walled carbon nano-tube and synthetic graphene oxide were applied as an effective adsorbent for cationic dye crystal violet (CV) removal from aqueous solutions using the batch adsorption technique. Several experimental parameters such as pH, adsorbent dosage, initial dye concentration, and contact time for adsorption of the crystal violet on the synthesized sorbents were studied and optimized. The Langmuir, Freundlich, and Temkin adsorption models were investigated to obtain adsorption isotherms. The results exhibited that the MWCNT and GO could be removed more than 80% and 90% of the above-mentioned dye at the first 15 and 20 min of contact time, respectively. The experimental adsorption kinetic was proved to be in accordance with a pseudo-second-order reaction rate. The equilibrium data showed that the adsorption behaviour of the crystal violet dye on the MWCNT and GO well fitted with the Langmuir model. On the Langmuir analysis basis, the maximum adsorption capacity of the MWCNT and GO for crystal violet dye was found to be 384 mg/g and 222 mg/g.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
One of the greatest worldwide problems is environmental pollution [1, 2], and the elimination of contaminants, such as heavy metals [3], organic dyes [4] and aromatic compounds [5], is vital for human health and ecosystems [6].
Among the various released pollutants into the environment, dyes are classified as of the highly toxic and hazardous materials, which can cause serious problems due to non-observance of the safety and health laws and regulations in some industries [7]. Most of dyes are very toxic and mutagenic, therefore we shall protect our rivers, coasts and the naturally occurring water resources with new measures of control over industrial and human effluence of such, for as much as they are recalcitrant, potentially carcinogenic, colourant, stable, and toxic, they can decrease the light influence, while increasing the COD (Chemical Oxygen Demand) in wastewater. Due to their high toxicity, they may be absorbed through the skin causing irritation and are also very harmful by inhalation and ingestion. Dyes presence in our living and or working environments can also lead to kidney failure, severe eye irritation sometimes leading to permanent blindness and even cancer. Therefore, it is to be prioritized to get an organized approach to problem solving and decision-making [8]. Dyes have more complicated structures with some complex compounds, a significant amount of which (0.7 million tons) are produced by the industries annually and eventually, 10–15% of the total amount is discharged into the environment [9], even though there are various techniques such as membrane separation [10], ozonation [11], photocatalysis [12], adsorption [13] and chlorination [14] that have been applied for dye removal [15]. Of the methods mentioned above, the adsorption process has indicated a superior performance within a wide range application as a well-known contributing process in the wastewater treatment industry. Also, the use of adsorbents has been particularly attractive in the recent years due to its high-efficiency, greening, easy-design and cost-effective method [16].
There are many adsorbents for pollutant removal from aqueous solutions, but the “carbonaceous structures” are being more widely used, as one of the most frequently applied methods within low cost—high cycle adsorption rate [17, 18].
In the present study, the carbon structures were classified into four main groups based on the number of their dimensions namely: (i) zero, (ii) one, (iii) two, and (iv) three dimensions. Examples of such grouping include: carbon quantum dots, C60, carbon nano-tubes, graphene, and nano-tube networks, respectively [19].
Carbonaceous adsorbents were synthesized by various methods including: arc discharge [20], laser ablation [21], chemical vapour deposition [22], hydrothermal [23], electrolysis [24], sonochemical [25], carbonization/pyrolysis [26], and diffusion flame [27].
Crystal violet is an important synthetic cationic dye in biological applications and transmits violet colour in aqueous solution. Decomposition of the compound is extremely difficult due to its complex aromatic structure, which causes physical, chemical, thermal, and optical stability in this type of material and has a low biodegradability [28,29,30]. Crystal violet is used extensively in the textile factories for dying different types of silk, wool, cotton, nylon, fabricating the printing inks, etc. Different separation, purification and recovery methods have been proposed as an option for the removal of crystal violet molecules from wastewater in the industries [31]. Several adsorbents were applied to crystal violet removal from aqueous media such as activated carbon [32], chitosan nanocomposite [33], cellulose nanocrystals [34], graphene family materials [35], and organic polymeric material [36]. The contact surface area being a more effective factor in the removal percentage, and the adsorbents having this advantage can significantly increase the efficiency of the removal processes [37]. For example, the large aspect ratio, significant adsorption capability, unique particular structure, and high strength make the CNTs (Carbon Nano-tubes) a suitable item in nanotechnology fields [38,39,40]. Despite their superior properties, CNTs in pure form have a lower performance in the process of pollution removal compared with their functionalized structure [41,42,43]. In addition, MWCNTs (Multi-Walled Carbon Nano-Tube) in comparison with SWCNTs are of lower operating costs while being highly applicable and abundant [44, 45].
The present research investigated the preparation and characterization of the modified graphene oxide as well as the magnetite multi-walled carbon nano-tube (MWCNT) with sulphonic acid groups and studied their application for crystal violet dye as an organic source of pollution in the aqueous solutions. Also, the absorption isotherms (Langmuir and Freundlich) and adsorption kinetics were investigated for obtaining the most appropriate model and order rate.
Experimental methodology
Material supply and specifications
All of the applied chemicals and solvents in the laboratory works were commercial reagent grades and supplied from “Merck” and “Aldrich” suppliers. The MWCNTs were purchased from “Nanostructured & Amorphous Material”, USA. The purity of the MWCNT materials was above 95% and the outside and inside diameters were about 10–20 nm and 5–15 nm, respectively, and their length was 10–30 µm (the relevant specifications were provided by the manufacturer).
Methods and instrumentation for calibrations
The SEM images of the materials were obtained to study the structures morphology and tacked on a MIRA (TESCAN) microscope. The infrared spectra were recorded on a Rayleigh (WQF 510A) spectrophotometer. The Ultraviolet–Visible Spectrophotometry was applied and the UV–Vis spectra were acquired on a Shimadzu UV–Vis (double beam) spectrophotometer.
Preparation and synthesis of the adsorbents
Synthesis of graphene oxide (GO)
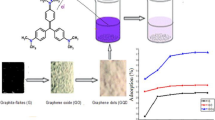
The “Modified Hummers Method” was applied in preparation of the graphene oxide from graphite [46]. For this purpose, a small amount of graphite powder (3 gr) was intensively and homogeneously mixed in the sulphuric acid (46 mL) and nitric acid (18 mL)-as they were both concentrated—and the whole solution placed in the Ice-Water bath below 5 °C temperature and for a period of 15 min time. Then, the Potassium Permanganate (6 gr) was gradually added to the prepared mixture during 15 min, and then, it was warmed up to 15 °C and was stirred for about 120 min. In continuation, mixing of the suspension was constantly carried out for another 30 min at 35 °C as an amount of deionized water (138 mL) was gradually added to it within 15 min and the system was heated up for another 30 min at the temperatures of 95 °C up to 98 °C. Finally, in order to dilute the contents of vessel, an amount of warm water (200 mL) in about 40 °C was added to get rid of excess permanganate, and 18 mL of H2O2 (30%) was added to the Manganese ions after which, the synthesized graphene oxide was washed out with deionized water for several times and dried out overnight at 60 °C.
Modification of graphene oxide with sulphonic acid groups (MGO)
The modification process of the prepared graphene oxide with sulphonic acid groups was performed as follows:
The graphene oxide (1 g) was dispersed in dry toluene (100 mL) under an ultrasound system and then, 10 mL of Trimethoxy-silane (also known as 3-Mercaptopropyl)-in the form of 10% in toluene—solution was added to the suspension after which the mixture was refluxed for 24 h. The solid product was extracted by centrifugation and washed several times by toluene and ethanol.
After that, the modified graphene oxide was hydrolysed with 30% H2O2 in methanol and a few drops of sulphuric acid, so that the obtained contents were reacted at the room temperature for 12 h and then at 95 °C for about 36 h.
Functionalized of multi-walled carbon nano-tube (FMWCNT) by carboxylation
For the surface functionalization or carboxylation of the carbon nano-tube, an amount of the MWCNT (2 g) was dispersed into 300 mL of concentrated nitric acid (69%) and then the mixture was refluxed at 120 °C for about 48 h. After cooling it down at the room temperature, the reaction mixture was diluted with 500 mL of deionized water and extracted by using a 3 µm thick filter under vacuum pumping. The washing operation was repeated until the mixture reached a pH similar to that of deionized water so then to be dried out in the oven at 100 °C. The conditions lead to the removal of catalysts from carbon nano-tube and the tube caps opening. Additionally, some holes were formed on lateral sides, and oxidation etching was carried out along the walls by releasing the carbon dioxide. These conditions were poorly performed to minimize the destruction of the structure, while the chemical modification was limited to the opening of the tube caps and the formation of functional groups at the defective points around the walls. The final products were fragments of a nano-tube with decorated walls by different oxygen groups (mostly carboxyl). The percentage of carboxylic groups on the oxidized MWCNT surface did not exceed 4%, which corresponds to the structure.
Modification of the functionalized carbon nano-tube with chlorosulphonic acid
In order to modify the functionalized carbon nano-tube with chlorosulphonic acid having the molecular formula of ClHO3S or SO2 (O–H) Cl, an amount of chlorosulphonic acid (10 mL) was gradually mixed with Pyridine (60 mL) in Ice-bath, then the prepared MMWCNT (1 g) was dispersed into the Pyridine (40 mL) by ultrasonic irradiation, and heated up at 80 °C. The obtained suspension was added to the chlorosulphonic acid solution and was reacted at 80 °C for 6 h. Finally, the mixture was diluted with 200 mL of deionized water and was neutralized with an equivalent molar of NaOH solution. The mixture was filtered and dried out by the vacuum pump.
Results and discussion
Characterization
The Fourier transform infrared spectrometer (FT-IR) graphene oxide
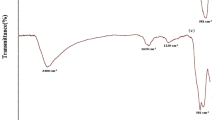
The following figure (Fig. 1a) depicts the infrared spectrum of the MGO. The observed peak in 3437 cm−1 is related to the stretching vibrations of O–H bonds, which could be attributed to the adsorption of water. The absorption peaks of 2855 cm−1 and 2922 cm−1 correspond to the symmetric and asymmetric C–H aliphatic bonds. In addition, this spectrum shows the presence of C–O, C–O–C, C–O–H and C=O in the 1060 cm−1, 1120 cm−1, 1380 cm−1 and 1625 cm−1, respectively (as contained in the carboxylic acid and carbonyl group). The absorption characteristic peak at 1581 cm−1 indicates the vibrations attributed to the non-oxidized graphene area. Finally, it can be concluded that there are carbonyl, epoxy and hydroxyl groups in the surface of graphene oxide [47].
Also, the prepared modified nano-tube was determined with FT-IR (Fig. 1b). Due to the structure of MWCNTs and the strong absorption of the light that can often appear at the background in the spectra, it is necessary to use a very poor concentration of nano-tubes in the KBr pellet. Also, in this case, the related peaks to the boundary sites in the nano-tube surface such as carboxylic acid groups were obtained in 1791, 1203 and 1082 cm−1 of the acquired wavenumbers. According to the explained analysis, both products were successfully modified with sulphonic acid groups [48].
The scanning electronic microscope (SEM)
The scanning electronic microscope images were used to illustrate the morphology and size of the modified graphene oxide and carbon nano-tube, as presented in Fig. 2. According to the image in the left (2-a), the graphene oxide nano-sheets appeared separately. Also, the image showed that the obtained morphology is similar to reported literature for the graphene oxide, which is fermented and associated with a layered rigid structure [49].
Besides, according to the image given for the carbon nano-tube in the right (2-b), the modified carbon nano-tube with almost the same diameter appeared distinctively. The scanning electron microscope images showed that the morphology of the products was similar to reported isomorph intertwined tubes in the previous researches [50].
Removal of crystal violet (CV) with prepared adsorbents
The crystal violet (100 mL) solution (with initial concentrations between 50 and 250 mg/L) was transferred into the vessel. Different pHs of initial solution (from 2 to 10) were adjusted by 0.1 M of NaOH and 0.1 M of HCl. Then, various adsorbent dosages (10–70 mg) were added to the solution of crystal violet solutions with specified concentrations in the different operation times (5–25 min) at a constant temperature. The concentration of crystal violet in the remained solution was determined using UV–Vis spectrophotometer at the suitable wavelength related to the maximum absorption of the residual solution (i.e. 586 nm).
Optimization of the adsorption efficiency
Effect of the adsorbent dosage
The obtained results of the two different modified graphene oxide and carbon nano-tube adsorbents dosage effect on the crystal violet removal (in the initial concentration of analyte 100 mg/L) are shown in Fig. 3. For this purpose, various amount of both adsorbents from 10 to 70 mg were used separately. As a result, the crystal violet concentration decreased with the increase of adsorbents amount. Meanwhile, as the adsorbents increased in the amount, of available active sites increased and then the removal efficiency increased for the tested dye. Depending on the obtained values, after applying 40 mg of modified carbon nano-tube and 60 mg of the MGO, the removal percentage was kept constant at maximum amount with minor changes. Therefore, the amounts of 40 mg and 60 mg were selected as the optimal amount for MMWCNT and MGO, respectively [4].
Effect of the pH of the solution
Figure 4 shows the effect of solution’s pH on the crystal violet removal in the range of 2–10. It was shown that the removal rate of the crystal violet dye by changes in pH on both types of adsorbents (MMWCNT and MGO) indicated a similar behaviour. Therefore, the removal efficiency was increased with the increase of pH. At low pH(s), due to the presence of H+ ions, and the adsorbents’ surface being covered by the same ions that are positively charged, adsorption of these inactive surfaces for studied analyte decreased. Also, the adsorbing competition of H+ ions with crystal violet cationic molecules occurred on active sites and hence, reduced the adsorption capacity.
At higher pH(s), the adsorption rate showed an increase, which could be attributed to the electrostatic attraction between the negatively charged surface of adsorbents and the cationic dye. It is also observed that at pH(s) higher than 7, the trend of the adsorption capacity increasing for dye removal, is slowed down. This phenomenon can be justified by the fact that, at higher pH(s), other mechanisms of adsorption such as hydrogen bond, ion-exchange absorption and π-π interactions are likely to overcome the electrostatic mechanism and therefore, the pH of the solution has less effect on the adsorption capacity. So, the pH of 6 was selected as optimal point for crystal violet removal with both prepared adsorbents [51].
Effect of the contact time
The effect of the contact time for the removal of the crystal violet with the initial concentration was shown as removal percentage in Fig. 5. The obtained results proved that with the increase of time, the removal percentage increased and a higher amount of adsorption was carried out of the initial solution. This process was continued until balancing and then was stopped. Clearly, during the first 15 min, the adsorption rate was rapid and about 80% of the total analyte molecules were removed at the initial concentration of 100 mg/L. This period of time in the reported adsorbents was acceptable for crystal violet adsorptions. After that, the amount of the adsorption percentage was remained constant and gradually returned to the equilibrium. The obtained time for the high removal efficiency of the two prepared adsorbents was somewhat different and depended on the structure. The balancing time for graphene-based adsorbent was about 20 min versus 15 min for nano-tube-based material. To ensure the more accurate values, the adsorption process was tested for a longer time and no significant changes occurred. Therefore, the times of 15 min and 20 min were selected as optimal times for MMWCNT and MGO, respectively [31].
Modelling the adsorption isotherm and the experimental results
The adsorption isotherms and parameters derived from Freundlich’s or Langmuir’s models which provide significant information on the mechanism of adsorption, the surface properties and the sorbent affinities. Langmuir’s isotherm is based on adsorption active site at homogeneous adsorbent place in a few monolayers processes, while Freundlich’s model is applied to description of heterogeneous multilayer adsorption systems [52].
Hence, in order to measure the adsorption behaviour of the solid–liquid system, in particular estimation of the dye molecules distribution at the sorption sites, the adsorption isotherms study was done according to Freundlich’s (heterogeneous multilayers), Langmuir’s (homogeneous sites) and \(\mathrm{Temkin}\)’s formula:
where in the Freundlich’s equation, the constant KF (intercept) is the adsorption capacity (L/mg) and 1/n (slope) is the adsorption intensity in which it does indicate the relative distribution of the energy and the heterogeneity of the adsorbate sites at a particular temperature and for a particular adsorbent and adsorbate (gas), n is always greater than one. In the Langmuir’s equation, KL is Langmuir’s constant related to adsorption capacity (L/mg), Ce is the equilibrium concentration of dye (mg/L), as the initial concentration is defined by C0. Meanwhile, qe is the adsorbed crystal violet amount in equilibrium on the prepared adsorbents (mg/g), and qmax is the maximal capacity for the adsorbent. In the Temkin’s equation, KT is the equilibrium binding constant (L/mol) that corresponds to the maximum binding energy, and b is denoting the adsorption heat, R is the universal gas constant (8.314 J/K.mol) and T is the temperature (K). Plotted qe versus ln (Ce) illustrates a straight line of slope RT/b and intercept (RT ln KT)/b. The quantification and identification of the concentrations were done by applying UV–Vis spectroscopy [1, 53].
The results revealed that the adsorption of crystal violet dye was in accordance with the Langmuir’s isotherm for both synthesized adsorbents. Although, in the Freundlich’s model, the plots of ln Ce versus ln qe also indicate an acceptable linearity for the whole range of concentration, but, comparison of the obtained plots from the two models, determined that the Langmuir’s model results showed a better correlation (R2 > 0.99 for both adsorbents) compared to the Freundlich’s model. The removal efficiency (%R) and adsorption capacity (qe) of crystal violet were calculated from equations given as follows (Table 1):
where V is the crystal violet solution volume, and M is the adsorbent mass contacted with analyte. The maximum capacity of adsorbents for MMWCNT and MGO was calculated as 384 mg/g and 222 mg/g, respectively.
The Adsorption kinetics
The adsorption kinetics can match with equations of order rate as pseudo-first and pseudo-second. The equation of pseudo-first-order rate is as follows:
where, qt and qe are adsorption amount, and equilibrium adsorption amount, respectively, at different times (t). k1 is pseudo-first-order rate constant that was calculated from slope of diagram in the plot log(qe − qt) versus t.
Also, the pseudo-second-order rate equation is,
where k2 is of the pseudo-second-order rate constant that was obtained from the diagram intercept and slope of plot \(\frac{t}{qt}\) versus t.
The experimental results for crystal violet adsorption kinetics were compatible with the equation of pseudo-second-order rate with values R2 as 0.9930 and 0.9803 for magnetite MWCNT and modified graphene oxide, respectively, shown in (Table 2).
Conclusion
In this study, the modified sulphonic acid multi-walled carbon nano-tube (MWCNT) and modified graphene oxide (GO) were prepared, characterized and applied as a novel carbonic adsorbent for the crystal violet dye removal from aqueous solutions. The effects of pH, adsorbent dosage, initial dye concentration and contact time on the efficiency of adsorption were studied by batch technique. The Freundlich, Langmuir and Temkin isotherm models were used in the experimental data analysis to show that, the data matched with the Langmuir model. The maximum adsorption capacity of magnetite MWCNT and modified GO for crystal violet were 384 mg/g) and 222 mg/g, respectively. The optimal pH for adsorption process of both adsorbents was 6 at 25 ℃. In addition, optimal adsorbent dosages for magnetite MWCNT and modified GO were 40 mg and 60 mg. Subsequently, it could be concluded that the studied magnetite MWCNT sorbent can be easily used as an eco-friendly, low-cost material, effective for crystal violet removal dye from aqueous solutions.
References
Critchell K, Bauer-Civiello A, Benham C, Berry K, Eagle L, Hamann M, Hussey K, Ridgway T (2019) Plastic pollution in the coastal environment: current challenges and future solutions, coasts and estuaries. Elsevier, Amsterdam, pp 595–609
Kumar S, Terashima C, Fujishima A, Krishnan V, Pitchaimuthu S (2019) A new generation material graphene Photocatalytic degradation of organic pollutants in water using graphene oxide composite: applications in water technology. Springer, Berlin, pp 413–438
Vojoudi H, Badiei A, Bahar S, Ziarani GM, Faridbod F, Ganjali MR (2017) A new nano-sorbent for fast and efficient removal of heavy metals from aqueous solutions based on modification of magnetic mesoporous silica nanospheres. J Magn Magn Mater 441:193–203
Vojoudi H, Badiei A, Amiri A, Banaei A, Ziarani G, Schenk-Joß K (2018) Efficient device for the benign removal of organic pollutants from aqueous solutions using modified mesoporous magnetite nanostructures. J Phys Chem Solids 113:210–219
Rabodonirina S, Rasolomampianina R, Krier F, Drider D, Merhaby D, Net S, Ouddane B (2019) Degradation of fluorene and phenanthrene in PAHs-contaminated soil using Pseudomonas and Bacillus strains isolated from oil spill sites. J Environ Manag 232:1–7
Tian J, Wei J, Zhang H, Kong Z, Zhu Y, Qin Z (2019) Graphene oxide-functionalized dual-scale channels architecture for high-throughput removal of organic pollutants from water. Chem Eng J 359:852–862
Liu P, Zhu C, Mathew AP (2019) Mechanically robust high flux graphene oxide-nanocellulose membranes for dye removal from water. J Hazard Mater 371:484–493
Tony MA, Mansour SA (2019) Removal of the commercial reactive dye Procion Blue MX-7RX from real textile wastewater using the synthesized Fe2O3 nanoparticles at different particle sizes as a source of Fenton’s reagent. Nanoscale Adv. 1(4):1362–1371
Lü J, Li Z, Hu Z, Ge M (2019) Highly efficient removal of organic dyes by novel as-synthesized agbr/montmorillonite composite. J. Wuhan Univ. Technol.-Mater. Sci. Ed. 34:35–40
Wang Q, Ju J, Tan Y, Hao L, Ma Y, Wu Y, Zhang H, Xia Y, Sui K (2019) Controlled synthesis of sodium alginate electrospun nanofiber membranes for multi-occasion adsorption and separation of methylene blue. Carbohydr Polym 205:125–134
Venkatesh S, Venkatesh K (2019) Ozonation for degradation of acid red 14 effect of buffer solution. Proc. Natl. Acad. Sci. India Sec A: Phys. Sci. 90:1–4
Ghasemi F, Kimiagar S, Shahbazi M, Vojoudi H (2018) Removal enhancement of basic blue 41 by Rgo–Tio2 nanocomposite synthesized using pulsed laser. Surf Rev Lett 25:1850041
Yagub MT, Sen TK, Afroze S, Ang HM (2014) Dye and its removal from aqueous solution by adsorption: a review. Adv Colloid Interface Sci 209:172–184
Yuan R, Ramjaun SN, Wang Z, Liu J (2012) Photocatalytic degradation and chlorination of azo dye in saline wastewater: kinetics and AOX formation. Chem Eng J 192:171–178
Forgacs E, Cserhati T, Oros G (2004) Removal of synthetic dyes from wastewaters: a review. Environ Inter 30:953–971
Crini G, Badot P-M (2008) Application of chitosan, a natural aminopolysaccharide, for dye removal from aqueous solutions by adsorption processes using batch studies: A review of recent literature. Prog Polym Sci 33:399–447
Li Y, Du Q, Liu T, Peng X, Wang J, Sun J, Wang Y, Wu S, Wang Z, Xia Y (2013) Comparative study of methylene blue dye adsorption onto activated carbon, graphene oxide, and carbon nanotubes. Chem. Eng. Res. Des. 91:361–368
Ai L, Jiang J (2012) Removal of methylene blue from aqueous solution with self-assembled cylindrical graphene–carbon nanotube hybrid. Chem Eng J 192:156–163
Huertas-Hernando D, Guinea F, Brataas A (2006) Spin-orbit coupling in curved graphene, fullerenes, nanotubes, and nanotube caps. Phys Rev B. 74:155426
Zhang D, Ye K, Yao Y, Liang F, Qu T, Ma W, Yang B, Dai Y, Watanabe T (2019) Controllable synthesis of carbon nanomaterials by direct current arc discharge from the inner wall of the chamber. Carbon 142:278–284
Mehrabi M, Reyhani A, Parvin P, Mortazavi S (2019) Surface structural alteration of multi-walled carbon nanotubes decorated by nickel nanoparticles based on laser ablation/chemical reduction methods to enhance hydrogen storage properties. Int. J. Hydrog Energy 44:3812–3823
Fleming E, Du F, Ou E, Dai L, Shi L (2019) Thermal conductivity of carbon nanotubes grown by catalyst-free chemical vapor deposition in nanopores. Carbon 145:195–200
Zhang C, Zhang M, Li Y, Shuai D (2019) Visible-light-driven photocatalytic disinfection of human adenovirus by a novel heterostructure of oxygen-doped graphitic carbon nitride and hydrothermal carbonation carbon. Appl. Catal. B Environ. 248:11–21
Huo S, Liu M, Wu L, Liu M, Xu M, Ni W, Yan Y-M (2019) Synthesis of ultrathin and hierarchically porous carbon nanosheets based on interlayer-confined inorganic/organic coordination for high performance supercapacitors. J Power Sour 414:383–392
Devrim Y, Arıca ED (2019) Multi-walled carbon nanotubes decorated by platinum catalyst for high temperature PEM fuel cell. Int J Hydrog Energy 44(34):18951–18966
Ginestra P, Madou M, Ceretti E (2019) Production of carbonized micro-patterns by photolithography and pyrolysis. Precis Eng 55:137–143
Han W, Chu H, Ya Y, Dong S, Zhang C, (2019) Effect of fuel structure on synthesis of carbon nanotubes in diffusion flames. Fuller Nanotub Carbon Nanostructures 3:265–272
Jia S, Tang D, Peng J, Sun Z, Yang X (2019) β-Cyclodextrin modified electrospinning fibers with good regeneration for efficient temperature-enhanced adsorption of crystal violet. Carbohydr. Polym. 208:486–494
Zhang L, Zhang H, Guo W, Tian Y (2014) Removal of malachite green and crystal violet cationic dyes from aqueous solution using activated sintering process red mud. Appl. Clay Sci. 93:85–93
Saeed A, Sharif M, Iqbal M (2010) Application potential of grapefruit peel as dye sorbent: kinetics, equilibrium and mechanism of crystal violet adsorption. J. Hazard Mater. 179:564–572
Adak A, Bandyopadhyay M, Pal A (2005) Removal of crystal violet dye from wastewater by surfactant-modified alumina. Sep. Purif. Technol. 44:139–144
Streit AF, Côrtes LN, Druzian SP, Godinho M, Collazzo GC, Perondi D, Dotto GL (2019) Development of high quality activated carbon from biological sludge and its application for dyes removal from aqueous solutions. Sci. Total Environ. 660:277–287
Massoudinejad M, Rasoulzadeh H, Ghaderpoori M (2019) Magnetic chitosan nanocomposite: fabrication, properties, and optimization for adsorptive removal of crystal violet from aqueous solutions. Carbohydr. Polym. 206:844–853
Bai L, Liu Y, Ding A, Ren N, Li G, Liang H (2019) Fabrication and characterization of thin-film composite (TFC) nanofiltration membranes incorporated with cellulose nanocrystals (CNCs) for enhanced desalination performance and dye removal. Chem. Eng. J. 358:1519–1528
Paramasivan T, Sivarajasekar N, Muthusaravanan S, Subashini R, Prakashmaran J, Sivamani S, Koya PA (2019) Graphene family materials for the removal of pesticides from water, a new generation material graphene: applications in water technology. Springer, Berlin, pp 309–327
Sharma N, Purkait MK (2019) Improving the hydrophilicity of polysulfone membrane by the addition of imidazol with polyvinyl pyrrolidone for crystal violet dye removal, advances in waste management. Springer, Berlin, pp 395–407
Vojoudi H, Badiei A, Bahar S, Ziarani GM, Faridbod F, Ganjali MR (2017) Post-modification of nanoporous silica type SBA-15 by bis (3-triethoxysilylpropyl) tetrasulfide as an efficient adsorbent for arsenic removal. Powder Technol. 319:271–278
Chen C, Hu J, Shao D, Li J, Wang X (2009) Adsorption behavior of multiwall carbon nanotube/iron oxide magnetic composites for Ni (II) and Sr (II). J. Hazardous Mater. 164:923–928
Huang Y, Zhu J, Liu H, Wang Z, Zhang X (2019) Preparation of porous graphene/carbon nanotube composite and adsorption mechanism of methylene blue. SN Appl Sci. 1:37
Xiong W, Zeng G, Yang Z, Zhou Y, Zhang C, Cheng M, Liu Y, Hu L, Wan J, Zhou C (2018) Adsorption of tetracycline antibiotics from aqueous solutions on nanocomposite multi-walled carbon nanotube functionalized MIL-53 (Fe) as new adsorbent. Sci. Total Environ. 627:235–244
Yu G, Lu Y, Guo J, Patel M, Bafana A, Wang X, Qiu B, Jeffryes C, Wei S, Guo Z (2018) Carbon nanotubes, graphene, and their derivatives for heavy metal removal. Adv. Compos. Hybrid Mater. 1:56–78
Deng Y, Ok YS, Mohan D, Pittman CU Jr, Dou X (2019) Carbamazepine removal from water by carbon dot-modified magnetic carbon nanotubes. Environ. Res. 169:434–444
Buffa A, Mandler D (2019) Adsorption and detection of organic pollutants by fixed bed carbon nanotube electrochemical membrane. Chem. Eng. J. 359:130–137
Lee H, Kang D, Kim J, Choi K, Chung W (2019) Void detection of cementitious grout composite using single-walled and multi-walled carbon nanotubes. Cement Concrete Compos. 95:237–246
Martínez-Paz P, Negri V, Esteban-Arranz A, Martínez-Guitarte JL, Ballesteros P, Morales M (2019) Effects at molecular level of multi-walled carbon nanotubes (Mwcnt) in Chironomus riparius (Diptera) aquatic larvae. Aquat. Toxicol. 209:42–48
Zang Z, Zeng X, Wang M, Hu W, Liu C, Tang X (2017) Tunable photoluminescence of water-soluble AgInZnS–graphene oxide (GO) nanocomposites and their application in-vivo bioimaging. Sens. Actuators B Chem. 252:1179–1186
Wang G, Wang B, Park J, Yang J, Shen X, Yao J (2009) Synthesis of enhanced hydrophilic and hydrophobic graphene oxide nanosheets by a solvothermal method. Carbon 47:68–72
Awasthi K, Singh D, Singh SK, Dash D, Srivastava O (2009) Attachment of biomolecules (protein and DNA) to amino-functionalized carbon nanotubes. New Carbon Mater. 24:301–306
Rattana S, Chaiyakun N, Witit-anun N, Nuntawong P, Chindaudom S, Oaew C, Kedkeaw P (2012) Limsuwan, preparation and characterization of graphene oxide nanosheets. Procedia Eng. 32:759–764
Hernández-Pérez A, Avilés F, May-Pat A, Valadez-González A, Herrera-Franco PJ, Bartolo-Pérez P (2008) Effective properties of multiwalled carbon nanotube/epoxy composites using two different tubes. Compos. Sci. Technol. 68:1422–1431
Banaei A, Farokhi Yaychi M, Karimi S, Vojoudi H, Namazi H, Badiei A, Pourbasheer E (2018) 2,2’-(butane-1,4-diylbis(oxy))dibenzaldehyde cross-linked magnetic chitosan nanoparticles as a new adsorbent for the removal of reactive red 239 from aqueous solutions. Mater. Chem. Phys. 212:1–11
Muthukumaran C, Sivakumar VM, Thirumarimurugan M (2016) Adsorption isotherms and kinetic studies of crystal violet dye removal from aqueous solution using surfactant modified magnetic nanoadsorbent. J. Taiwan Inst. Chem. Eng. 63:354–362
Mohanty K, Naidu JT, Meikap B, Biswas M (2006) Removal of crystal violet from wastewater by activated carbons prepared from rice husk. Ind. Eng. Chem. Res. 45:5165–5171
Acknowledgement
The authors would like to thank in particular Dr. Behrooz Majidi (P. N. University – Saqqez -- Kurdistan) for the valuable consultations and advisement during the experiments of this research, and for being an invited committee member in the PhD thesis defense of the first author as well.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest with any organization.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mustafanejad, F., Sajjadi, N., Marandi, R. et al. Efficient removal of crystal violet by sulphonic-modified multi-walled carbon nano-tube and graphene oxide. Nanotechnol. Environ. Eng. 6, 30 (2021). https://doi.org/10.1007/s41204-021-00121-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41204-021-00121-4