Abstract
There is an increasing need for portable sleep monitoring in clinical practice, but there is no comparative study that used the same device for home and in-laboratory sleep monitoring and device close to full polysomnography (PSG) to evaluate the feasibility and preference of home unattended sleep monitoring. Twenty male participants with high risk for moderate to severe OSA based on the STOP-BANG questionnaire were included. The participants were randomly assigned to group A (home unattended monitoring after in-laboratory monitoring) or group B (in-laboratory monitoring after home unattended monitoring). A 2-week washout period was implemented between the sleep tests. All hook-up procedures were performed in laboratory. Participants were asked to complete a questionnaire after finishing each sleep test. There was no difference in sleep efficiency, arousal index, or time spent in each sleep stage between the two monitoring modes using Nox-A1. Additionally, other respiratory parameters such as apnea–hypopnea index (AHI), supine AHI, and snoring time did not differ. A high and similar sensor quality for airflow, oxygen, and respiratory effort was observed in both monitoring groups. Patient’s feelings and satisfaction with the test were similar between in-laboratory and home monitoring, but preference rate for the in-laboratory test was higher than that for home monitoring (70% vs. 30%, respectively). These data suggest that home unattended monitoring with Nox-A1 type 2 ambulatory device is a feasible alternative diagnostic mode for high risk of moderate to severe OSA, yielding reliable quality recordings and high patient satisfaction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obstructive sleep apnea (OSA) is a very common nocturnal respiratory symptom affecting 2–4% of middle-aged adults in the general population [1]. Untreated OSA is independently associated with numerous health problems and increased morbidity and mortality including cardiovascular diseases, diabetes mellitus, stroke, and reduced quality of life [2,3,4]. As the obesity rate and the proportion of aged population increase, well-known risk factors for OSA, the prevalence of OSA is also elevated [3, 5]. Considering the high prevalence and clinical significance of OSA, easily accessible and accurate diagnostic methods and appropriate treatment based on the objective diagnosis are essential.
Nocturnal polysomnography (PSG) attended by a technologist in a sleep laboratory is the current standard of practice for the diagnosis of OSA. However, due to several limitations of PSG such as a technically complex, time-consuming, and relatively costly procedure that requires a hospital stay, portable monitoring (PM) has been given attention as an alternative diagnostic test for OSA with numerous advantages, which can offset the limitations of PSG [6,7,8].
PM is divided into four types (i.e., type 1–type 4) based on the presence or absence of an attended technologist and the number of channels available for recording [9]. The Portable Monitoring Task Force of the American Academy of Sleep Medicine recommends that PM use is considered in patients with a high pre-test probability of moderate to severe OSA without comorbid medical conditions and other sleep disorders [9].
Several studies have been performed to evaluate the diagnostic accuracy and feasibility of PM devices for the diagnosis of OSA. There are many consistent findings that show strong agreement between apnea and hypopnea index (AHI) values from PM and in-laboratory monitoring [10,11,12,13], demonstrating good diagnostic performance of PM for suspected OSA patients. In addition to diagnostic accuracy, feasibility should also be considered for PM, especially when the test is performed without a technologist. Compared to studies that examined the accuracy of PM, there are relatively few research findings showing the feasibility of PM. Portier et al. [10] reported a higher failure rate in home sleep monitoring than in-laboratory PSG in adults (20% vs. 5%), and they concluded that home sleep tests (HST) are not a feasible method for one-third of patients involved in the study due to disability or difficulties regarding transportation. However, home sleep monitoring showed similar levels of interpretability and technical acceptance compared to those of hospitalized patients in another study that examined the feasibility of unattended PSG in children [14]. Results on the feasibility of PM are likely to be influenced by the definition of feasibility, age of subjects, device used in the study, and hook-up location.
To date, several well-designed studies using PM devices of different levels have been performed to compare home unattended and in-laboratory PSG in a prospective randomized crossover design [10, 15,16,17]. However, direct comparison of research findings from these randomized crossover trials was difficult because there are some methodological differences or limitations in the studies as follows: (1) different devices were used for home PSG and in-laboratory PSG [10, 16], which might be a bias on the assessment of subjective preference or diagnostic accuracy and (2) only limited electroencephalography (EEG) leads were assessed (not more than 2) [10, 15, 17]. According to Chesson et al. [18], results obtained from a particular device cannot be generalized to other devices, even those of the same class.
With this in mind, we designed a randomized crossover study to simultaneously fulfill the following two conditions to evaluate the feasibility based on interpretability and patients’ satisfaction/preference of Nox-A1 type 2 ambulatory device for home unattended sleep monitoring in patients with high risk for moderate to severe OSA, which overcome the limitations of previous studies using the same device for home and in-laboratory monitoring and evaluating with the ambulatory device which consists of six EEG leads.
Methods
Subjects
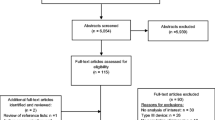
One hundred fifty-one individuals who visited the Seoul Sleep Clinic and Department of Otorhinolaryngology at the Seoul National University Hospital between July 2017 and November 2017 for evaluation of snoring and/or OSA were initially screened in this study. All patients underwent a clinical examination regarding their medical conditions and anthropometric measurements prior to recruitment to the study. Inclusion criteria were male gender, no history of previous sleep testing or treatment for OSA, and high risk of moderate to severe OSA. Individuals who were at high risk of moderate to severe OSA were selected based on a Korean-translated STOP-BANG questionnaire. Exclusion criteria included age under 20 years, heavy drinkers (more than 3 times per week), and individuals who had or were diagnosed with a skin allergy, congestive heart failure, chronic obstructive pulmonary disease, neuromuscular disease, or psychiatric disease. Twenty-four male patients were initially selected based on these criteria. Prior to sleep monitoring, 4 participants were withdrawn because of acute illness (n = 1) or time constraints (n = 3). Twenty subjects were finally included in this study. The flow of participant selection is shown in Fig. 1. All participants provided written informed consent, and the study was approved by the Institutional Review Board at the Seoul National University Hospital (No. 1706-041-858).
Study design
The enrolled participants were randomly assigned to group A or group B using a block design. This is used to allocate equal number of subjects to the two groups. Individuals in group A first underwent in-laboratory sleep monitoring with the Nox-A1 device followed by home unattended monitoring using the same device (home unattended monitoring after in-laboratory monitoring). Individuals in group B underwent home unattended monitoring first, then subsequent in-laboratory sleep monitoring (in-laboratory monitoring after home unattended monitoring). Home unattended sleep PSG was performed in participants’ homes in principle, but for 3 participants, recording in a motel room was allowed because their homes were > 2 h away. All subjects completed the 1st questionnaire (version for 1st examination) the morning after sleep testing. A 2-week washout period was implemented between the first and the second sleep tests. Following the washout period, the sleep monitoring methods in groups A and B were switched, and sleep examination and the 2nd questionnaire (version for 2nd examination) were repeated. This randomized cross-over trial was registered at Clinical Research Information Service (KCT0002914). Complete date range for participant recruitment and follow-up was 4 months.
In-laboratory and home unattended sleep monitoring
All hook-up procedures for in-laboratory attended and home unattended sleep monitoring were performed by one certified polysomnographic technologist to minimize a bias, which could affect primary outcomes of this study due to the possibility of differences in the hook-up techniques, duration, and attitude toward the participants. Participants were asked to visit the sleep laboratory located within the clinical trial center of the Seoul National University Hospital between 8 and 9 p.m. The Nox-A1 ambulatory monitoring system (Nox Medical, Inc., Reykjavik, Iceland) was used for this sleep study, which consists of six EEG channels (F3-A2, F4-A1, C3-A2, C4-A1, O1-A2, and O2-A1), 2 electrooculogram (EOG) channels, 3 submental electromyogram (EMG) channels, 2 anterior tibialis EMG channels, an electrocardiogram, a nasal pressure sensor, thorax and abdomen movement sensors, a built-in microphone, and wireless pulse oximetry. For attended in-laboratory PSG, recordings started between 10 and 11 p.m., after completion of sensor application and bio-calibration, and ended when the participant woke up. They were awakened before 8 a.m. the next morning and were asked to complete a questionnaire regarding subjective feelings, satisfaction, and/or preference. A technologist monitored all recording processes in the next room. For home unattended monitoring, all hook-up procedures were also conducted at the same location as in-laboratory monitoring. The technologist applied the sensors to the subjects with the same protocol and confirmed whether they were functioning. After being educated by a technologist on how to detach the applied sensors, the participants were returned home fitted with the device. They were asked to go to bed at their usual bedtime. It took fewer than 1.5 h to get from the laboratory to their homes. After finishing the home unattended overnight sleep test, they detached the sensors themselves and completed a questionnaire. Analysis start and stop times were manually set as 11 p.m. and 8 a.m., respectively, for home monitoring. Types of PM recommended by American Sleep Disorders Association [9] are briefly described in Table 1.
Sleep analysis
Sleep scoring was performed by a different registered polysomnographic technologist from the technologist who performed the hook-up procedures. The scorer was blinded to whether these data were derived from in-laboratory or home monitoring. Noxturnal software was used for manual scoring of sleep data. Obstructive apnea was defined as the absence of airflow ≥ 90% of the previous baseline for at least 10 s. Hypopnea was defined as a substantial decrease of ≥ 30% in air flow for at least 10 s associated with a ≥ 4% reduction in oxygen saturation. The AHI was defined as the total number of apnea and hypopnea events per hour of total sleep time. Arousal index was defined as the mean number of occurrences per hour of total sleep time.
Evaluation of feasibility
Feasibility of unattended home sleep test using Nox-A1 type 2 ambulatory device was assessed in terms of need for an additional recording because of non-interpretability. As previously reported [10], if at least one of the following criteria was fulfilled, the data were judged to be non-interpretable, requiring a new recording: (1) > 70% of the data were lost in the recording, (2) > 80% of total recording time had a poor airflow signal, (3) impossible evaluation of sleep stage, and (4) insufficient total sleep time (≤ 3 h).
Questionnaires on patient’s feeling, satisfaction, and preference
Patient’s feeling, satisfaction, and preference were assessed using two different questionnaires: 1st (S2) and 2nd questionnaires (S3). 1st and 2nd questionnaires included 6 different questions regarding the participant’s subjective feelings after awakening, frequency of arousals during sleep, restriction of activity while wearing the device, and satisfaction with the test. Detailed questions were as follows: (1) ‘I felt refreshed when I got up’, (2) ‘I slept well’, (3) ‘I felt tired and sleepy when I got up’, (4) ‘I awoke frequently during sleep’, (5) ‘I was restricted in my activities until I fell asleep while wearing the device’, and (6) ‘I was satisfied with the test’. Subjects could answer each question as follows: strongly disagree, disagree, neutral, agree, or strongly agree. 2nd questionnaire had 2 additional questions regarding the participant’s preferred monitoring type and the reason why they chose this type: (1) ‘I will choose the following monitoring mode if I have to take a sleep test next time’ and (2) ‘Please choose a reason why you prefer in-laboratory monitoring or home sleep monitoring’.
Statistical analysis
To confirm the appropriateness of the sample size, we performed a power analysis using the G*Power program (version 3.1.9.2; HHU, Düsseldorf Universität, Germany). Using the sleep efficiency data from the study of Bruyneel et al. [19], we confirmed that a sample of 14 subjects would provide at least an 80% power at a two-sided significance level of 5% for both the paired t test and the non-parametric Wilcoxon signed-rank test. Considering the average dropout rate known as 30% in clinical trials, at least 19 subjects were needed. With a sample of 20 subjects, a 90% power at a two-sided significance level of 5% was calculated. All data were expressed as the mean ± standard deviation (SD) because their distribution was close to normal based on the results of a normality test (Shapiro–Wilk test) and a distribution plot. Nonetheless, we conducted parametric and non-parametric analyses and presented p values from both analyses. To reduce the effects of outliers, some data were log-transformed for parametric tests. To compare a mean difference in variables between groups A and B, the independent t test and the non-parametric Mann–Whitney U test were used. To compare paired data of polysomnographic parameters between the two monitoring methods, the paired t test and the non-parametric Wilcoxon signed-rank test were used. The Fisher’s exact test was used to compare qualitative variables. A Bland–Altman plot was constructed to examine agreement of AHI between in-laboratory and home monitoring. All statistical analyses were performed using IBM SPSS, version 20.0 (SPSS; Chicago, IL, USA), and a two-sided significance level of 5% was used.
Results
General characteristics of study participants
Twenty male participants were classified into group A or group B, and sleep analysis was performed. Participants in group A were older than those in group B (Table 2). Body mass index (BMI) and STOP-BANG scores were not significantly different between the two groups. We selected subjects who have a high risk for moderate to severe OSA based on STOP-BANG questionnaire, but among participants who were enrolled in the present study, 3 participants were diagnosed as mild OSA and 1 participant was classified as normal following in-laboratory or HST. In addition, we observed that 100% and 84.2% of participants who had STOP-BANG score ≥ 3 had AHI ≥ 5 and AHI ≥ 15, respectively.
Comparison of polysomnographic parameters between Nox-A1Lab and Nox-A1Home monitoring
We compared the polysomnographic variables between the two monitoring methods with the paired t test and the non-parametric Wilcoxon signed-rank test for paired samples (Table 3). Total sleep time was significantly higher in Nox-A1Home monitoring. In the paired t test, there were no significant differences in sleep latency, sleep efficiency, wake after sleep onset time (WASO), arousal index, periodic leg movement index, percent time spent in each sleep stage and supine position, AHI, supine AHI, and snoring time (%). However, in the Wilcoxon signed-rank test, WASO was significantly higher in the Nox-A1Home monitoring. The mean difference in AHI between Nox-A1 in-laboratory and Nox-A1 home monitoring was 1.2 h−1, 95% CI (− 3.7 to 6.1; P = 0.627). A Bland–Altman plot showed limits of agreement at + 21.8 and − 19.4 for 1.96 × SD; the correlation coefficient was 0.836 (p < 0.001) (Fig. 2), indicating that there was strong agreement between Nox-A1 in-laboratory-measured AHI and Nox-A1 home monitoring-measured AHI and differences were tightly distributed.
Comparison of data quality and feasibility between Nox-A1Lab and Nox-A1Home monitoring
Data quality regarding the oxygen sensor, nasal sensor, abdomen and thorax respiratory inductance plethysmography (RIP) was automatically determined by the Noxturnal software (Nox Medical, Inc., Reykjavik, Iceland) and confirmed by visual assessment (Table 4). The quality of the nasal sensor, abdomen RIP, and thorax RIP data was significantly higher in Nox-A1Home monitoring than in Nox-A1Lab, but these differences in less than l% of the nasal, abdomen RIP, and thorax RIP sensor quality were not clinically significant. There was no non-interpretable test in either monitoring type.
Difference in patient satisfaction according to Nox-A1Lab and Nox-A1Home monitoring
All participants were asked to complete the questionnaire in the morning after finishing in-laboratory and home sleep monitoring. More than 60% of participants who performed both in-laboratory and home sleep monitoring answered ‘neutral’ to the question ‘I felt refreshed when I got up’ (S1 Table). The rate of positive answers (‘agree’ plus ‘strongly agree’) on the same question was 15% and 10% in Nox-A1Lab and Nox-A1Home monitoring, respectively. The percentage of participants who indicated that they slept well last night following Nox-A1Home monitoring was similar to that following Nox-A1Lab monitoring. The percentage of participants who felt tired and sleepy after waking up was higher in Nox-A1Lab monitoring compared to Nox-A1Home monitoring (35% vs. 20%). The frequency of positive answers (‘agree’ plus ‘strongly agree’) on frequent nocturnal awakenings was also higher in Nox-A1Lab monitoring than in Nox-A1Home monitoring. Approximately one-third of participants responded that they felt restriction of activities while wearing the device following both sleep tests. There was no significant difference in the proportion of preference type between group A and group B (Table 5), but the preference rate for in-laboratory monitoring was 2- and 2.7-fold higher than that for home sleep monitoring in the group A and group B, respectively (Table 5). The reasons for preferring in-laboratory monitoring with Nox-A1 were (1) difficulties related to transportation (35%), (2) difficulties in emergency measures (30%), and (3) apprehension regarding acquisition of data during home monitoring (5%), while familiar bedding environment (25%) was the main reason for preferring home sleep monitoring.
Discussion
The main purpose of this study was to investigate the feasibility and patients’ satisfaction/preference of the type 2 ambulatory full polysomnography device for HST without supervision. Home unattended sleep monitoring with Nox-A1 was feasible in terms of data interpretability. Moreover, there was a similar trend in overall assessments regarding patient’s satisfaction and subjective feelings after completing both PSG tests. In addition, even though it was not the primary aim of this study, there was no significant difference in PSG parameters including time spent in each sleep stage, snoring time, and AHI between in-laboratory and home unattended monitoring with Nox-A1.
There was no data loss or poor data quality during home unattended monitoring with this type 2 PM, though this study was mainly conducted during the summer season in which participants would be expected to get sweaty during their commutes home, thereby potentially reducing the quality of the data. These results suggest that Nox-A1 home sleep monitoring without continuous supervision was feasible for all our patients. According to previous randomized crossover studies, the failure rate of home unattended PSG ranges from 5 to 7% when hook-up was conducted at home [15, 19]. However, when the hook-up location was the laboratory and patients spent the night at home, as in our study, the failure rate increased to 20% [10]. The discrepancy between our findings and the result of Portier et al. is possibly due to the difference in sample size (n = 20 vs. n = 103), the method of thermistor connection, and use of a different device. In addition, it is possible that features of the Nox-A1 device itself (e.g., visually identifiable initial sensor impedance, short-length EEG cables, and specialized cable leads) influence the data integrity.
Another interesting point is that, unlike previous findings, there was a similar level of breathing indices to define OSA. Indeed, night-to-night variability of these respiratory parameters has been well described [20,21,22], but conflicting investigations showing high agreement in a respiratory disturbance index between an unattended home PSG and a subsequent PSG with several months between tests also exist [23].
Hook-up location is likely a critical factor for determining patients’ preferences as well as the failure rate of PSG. When the hook-up location was the laboratory and patients were asked to return home, the proportion of participants who preferred home monitoring was approximately twofold lower compared to the in-laboratory test [10, 16]. On the other hand, if the hook-up location was the participant’s home, the preference for home sleep testing was two- to threefold greater than laboratory monitoring [15, 19]. This preference for the laboratory test was mostly due to difficulties related to transportation and the inconveniences of home testing. From our findings and previous reports, it can be suggested that if participants are hooked-up at home, preference for home sleep monitoring will be similar or possibly superior to laboratory monitoring.
The major strength of this study is its design, consisting of a randomized crossover with a device of type 2 PM level. The present study also has several limitations. First, the sample size was relatively small compared with previous randomized crossover trials that compared home unattended and in-laboratory PSG. Thus, more data are clearly needed to prove superiority of home unattended sleep monitoring with Nox A1 type 2 device, including more participants and the use of another type 2 device subjected to an identical protocol. Second, we only included male subjects; thus, we cannot guarantee whether the findings on the data quality of home monitoring and preference type of the Nox-A1 device will be the same in female subjects.
In conclusion, this study utilized a randomized crossover design and demonstrates that unattended sleep monitoring with the type 2 ambulatory device can be performed in the patient’s home with reliable quality recordings and high patient satisfaction, which is comparable to in-laboratory PSG with overnight supervision. Thus, this system will be useful for patients with high risk of moderate to severe OSA who do not want to be examined at the hospital or those with disabilities who do not have significant comorbid medical conditions and there is no need for in-laboratory video motoring of sleep behaviors.
References
Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328(17):1230–5.
Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365(9464):1046–53.
Young T, Palta M, Dempsey J, Peppard PE, Nieto FJ, Hla KM. Burden of sleep apnea: rationale, design, and major findings of the Wisconsin Sleep Cohort study. WMJ. 2009;108(5):246–9.
Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165(9):1217–39.
Jennum P, Riha RL. Epidemiology of sleep apnoea/hypopnoea syndrome and sleep-disordered breathing. Eur Respir J. 2009;33(4):907–14.
Collop NA. Portable monitoring for the diagnosis of obstructive sleep apnea. Curr Opin Pulm Med. 2008;14(6):525–9.
Ghegan MD, Angelos PC, Stonebraker AC, Gillespie MB. Laboratory versus portable sleep studies: a meta-analysis. Laryngoscope. 2006;116(6):859–64.
Masa JF, Corral J, Pereira R, Duran-Cantolla J, Cabello M, Hernandez-Blasco L, Monasterio C, Alonso A, Chiner E, Rubio M, Garcia-Ledesma E, Cacelo L, Carpizo R, Sacristan L, Salord N, Carrera M, Sancho-Chust JN, Embid C, Vazquez-Polo FJ, Negrin MA, Montserrat JM. Effectiveness of home respiratory polygraphy for the diagnosis of sleep apnoea and hypopnoea syndrome. Thorax. 2011;66(7):567–73.
Practice parameters for the use of portable recording in the assessment of obstructive sleep apnea. Standards of Practice Committee of the American Sleep Disorders Association. Sleep. 1994;17(4):372–7.
Portier F, Portmann A, Czernichow P, Vascaut L, Devin E, Benhamou D, Cuvelier A, Muir JF. Evaluation of home versus laboratory polysomnography in the diagnosis of sleep apnea syndrome. Am J Respir Crit Care Med. 2000;162:814–8.
Santos-Silva R, Sartori DE, Truksinas V, Truksinas E, Alonso FF, Tufik S, Bittencourt LR. Validation of a portable monitoring system for the diagnosis of obstructive sleep apnea syndrome. Sleep. 2009;32(5):629–36.
Xu L, Han F, Keenan BT, Kneeland-Szanto E, Yan H, Dong X, Chang Y, Zhao L, Zhang X, Li J, Pack AI, Kuna ST. Validation of the Nox-T3 portable monitor for diagnosis of obstructive sleep apnea in chinese adults. J Clin Sleep Med. 2017;13(5):675–83.
Zou J, Meng L, Liu Y, Xu X, Liu S, Guan J, Yin S, Yi H. Evaluation of a 2-channel portable device and a predictive model to screen for obstructive sleep apnea in a laboratory environment. Respir Care. 2015;60(3):356–62.
Brockmann PE, Perez JL, Moya A. Feasibility of unattended home polysomnography in children with sleep-disordered breathing. Int J Pediatr Otorhinolaryngol. 2013;77(12):1960–4.
Campbell AJ, Neill AM. Home set-up polysomnography in the assessment of suspected obstructive sleep apnea. J Sleep Res. 2011;20:207–13.
Fry JM, DiPhillipo MA, Curran K, Goldberg R, Baran AS. Full polysomnography in the home. Sleep. 1998;21(6):635–42.
Iber C, Redline S, Kaplan Gilpin AM, Quan SF, Zhang L, Gottlieb DJ, Rapoport D, Resnick HE, Sanders M, Smith P. Polysomnography performed in the unattended home versus the attended laboratory setting-Sleep Heart Health Study methodology. Sleep. 2004;27(3):536–40.
Chesson AL Jr, Berry RB, Pack A, American Academy of Sleep M, American Thoracic S. American College of Chest P. Practice parameters for the use of portable monitoring devices in the investigation of suspected obstructive sleep apnea in adults. Sleep. 2003;26(7):907–13.
Bruyneel M, Sanida C, Art G, Libert W, Cuvelier L, Paesmans M, Sergysels R, Ninane V. Sleep efficiency during sleep studies: results of a prospective study comparing home-based and in-hospital polysomnography. J Sleep Res. 2011;20(1 Pt 2):201–6.
Meyer TJ, Eveloff SE, Kline LR, Millman RP. One negative polysomnogram does not exclude obstructive sleep apnea. Chest. 1993;103(3):756–60.
Redline S, Tosteson T, Boucher MA, Millman RP. Measurement of sleep-related breathing disturbances in epidemiologic studies. Assessment of the validity and reproducibility of a portable monitoring device. Chest. 1991;100(5):1281–6.
Wittig RM, Romaker A, Zorick FJ, Roehrs TA, Conway WA, Roth T. Night-to-night consistency of apneas during sleep. Am Rev Respir Dis. 1984;129(2):244–6.
Quan SF, Griswold ME, Iber C, Nieto FJ, Rapoport DM, Redline S, Sanders M, Young T, Sleep Heart Health Study Research G. Short-term variability of respiration and sleep during unattended nonlaboratory polysomnography—the Sleep Heart Health Study (corrected). Sleep. 2002;25(8):843–9.
Acknowledgements
We thank Kwangwoo MeDix, Inc. for supplying the Nox-A1 portable devices, Cho-Hee Hong for conducting sleep analysis, and Min-Joo Jeon for performing all hook-up procedures for the in-laboratory and home sleep test. This randomized crossover trial was registered at Clinical Research Information Service (KCT0002914).
Funding
This work was supported by Grant no. 06-2017-2300 from the SNUH Research Fund and Kwangwoo Medix, Inc. D.W.Y received a scholarship from the BK21-plus education program provided by the National Research Foundation of Korea.
Author information
Authors and Affiliations
Contributions
Conception and design: DW and HW; data collection: DW and IH; analysis and interpretation of data: DW, IH, IK, and HW; drafting the manuscript: DW and IH; revision for important intellectual content: DW, IH, IK, and HW.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest with the contents of this article.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration. The Institutional Review Board at Seoul National University Hospital reviewed and approved the study protocol. The protocol was approved by the Institutional Review Board at the Seoul National University Hospital (No. 1706-041-858).
Informed consent
Written informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yoon, D.W., Hong, IH., Baik, I. et al. Evaluation of the feasibility and preference of Nox-A1 type 2 ambulatory device for unattended home sleep test: a randomized crossover study. Sleep Biol. Rhythms 17, 297–304 (2019). https://doi.org/10.1007/s41105-019-00213-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41105-019-00213-4