Abstract
Melanin is a kind of versatile biopolymer with strong binding capacities for cationic pollutants. The effects of melanin extraction from biomass on cationic pollutant adsorption of biomass remain unknown. Methylene blue (MB) was herein used as a model cationic pollutant, and chestnut shells were used as a melanin-containing byproduct of biomass. The MB biosorption behaviors onto the raw and melanin-extracted chestnut shells were comparatively studied. The biosorbents were contrastively characterized by BET surface area, scanning electron microscopy, energy-dispersive spectrometry, infrared spectroscopy, pH point of zero charges (pHpzc), and cation exchange capacity (CEC). The MB adsorption capacity of each biomass was evaluated at different pH, contact time, initial MB concentration, and temperature. The melanin extraction resulted in higher values of adsorbent surface area, pHpzc, and CEC. It toned down the sensitivity of biomass to the solution pH, expanding the applicable pH range. It also introduced new types of binding sites or adsorption mechanisms. The biosorption process was well described by the chemisorption model of the pseudo-second-order equation and accelerated by the melanin extraction. The biosorption equilibrium data are well modeled by the Langmuir isotherm. The melanin extraction more than doubled the monolayer adsorption capacity. The improvement in the biosorption performance of chestnut shells by the melanin extraction is mainly owed to the chemical activation of binding sites and secondary to the physical modification. Melanin-containing biomass such as chestnut shells can be used in cascade for melanin production and subsequently for adsorptive removal of basic dyes such as MB.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Dyes play very important roles in today’s world, which make the world more colorful and make people more enjoyable. They are extensively used to color textiles, leather, hair, paper, and so on [1]. A large amount of colored wastewater is produced during dye production and application processes, which can cause esthetic degradation, disrupt the aquatic ecosystem, and threaten human health. Therefore, the effluent should be effectively treated before discharging into the water body. Adsorption offers a responsible option, although some treatment technologies have been developed, such as physical/chemical/biological degradation, precipitation, and coagulation. Adsorption technology strongly depends on adsorbents. Their performance, cheapness, and environmental benignity are taken into account in industrial practice. Agricultural wastes have been intensively investigated as biosorbents for dye removal due to their merits of low cost, high efficiency, degradability, and strong selectivity [2]. Additionally, exhausted biosorbents can be disposed of by burning to generate heat or energy [3, 4].
Chestnut is a popular nut species, and its shells are residual from the nut processing. Most of the residuals are usually discarded by incineration or landfills, wasting the resource and stressing the environment. Hence, many researchers dedicated to utilizing chestnut shells. Our laboratory has reported previously using chestnut shells to absorb heavy metals from water [5, 6]. Unfortunately, the adsorption capacity is too low to meet the requirements of industrial applications. Sarici-Özdemir [7] found that the chestnut shells provided the best biosorption of methylene blue (MB), with an adsorption capacity of 126.5 mg g−1, which was higher than the counterparts of the fruit shells from hazelnuts and walnuts. It shows the potential of chestnut shells for cationic dye removal. Chestnut shells contain about 15% melanin [8], a kind of versatile natural pigment. The melanin exhibits antioxidant and antibacterial activities [9, 10], promising a multifunctional food colorant. It can also be used as an adsorbent for heavy metals [11] and as a dyestuff for textiles [12]. Cascade utilization is an economically attractive way to use biomass resources sufficiently. However, there is little knowledge regarding utilizing the spent of the chestnut shells after melanin extraction.
Alkali treatment is a traditional modification method for biosorbents, which can improve their adsorption performance through activating binding sites on biomass and improving physical structures and chemical composition of biosorbents [13]. Many agricultural wastes such as chestnut shells [11], banana peels [14], and seed coats of Xanthoceras sorbifolia [15] can be used as biosorbents and for melanin production. Can these agricultural wastes be sequentially used for melanin extraction first and then for biosorption? Melanin is usually extracted with alkaline solutions. Can the extraction work as an activating process for biosorption? Melanin can bind various inorganic and organic species, and has a potential as adsorbents. For example, the MB adsorption capacity of melanin from sunflower husk was 190.9 mg g−1 [16]. What influence does the melanin removal from the agrowastes have on their biosorption behavior? It is hard to answer these questions as yet because there is no report stating the significance of melanin in biosorption by agricultural waste.
Cationic dyes are an important category of synthetic dyes and contribute a large part of dye wastewater. MB is used as a model of cationic dyes in wastewater treatment research and is widely used by industries for various purposes. Here is a try to answer the questions raised above by using MB as the adsorbate and chestnut shells as the melanin-containing agricultural waste. The authors also try to answer the potential of melanin-extracted chestnut shells for biosorption. To fulfill these aims, we compared the MB adsorption behaviors onto the chestnut shells before and after the melanin extraction. Related mechanisms were also investigated.
Materials and Methods
Materials
Chestnut shells (Castanea mollissima) were provided by Luotian Liangli Food Co., Ltd (Luotian, China). MB was an analytical reagent from Sinopharm Group (Shanghai, China).
Biosorbent Preparation
The melanin extraction was conducted according to the method developed by Yao et al. [8]. Briefly, the chestnut shells were immersed in a 0.2 mol L−1 NaOH solution with a liquid–solid ratio of 15 mL g−1 and exposed to 50 °C in an electric incubator for 24 h. Then the chestnut shells were separated from the crude melanin solution by filtrating with a cotton bag, and the extraction was repeated. After that, the spent chestnut shells were flushed with distilled water until the effluent was neutral and dried in a forced-air oven at 50 °C for 24 h. Finally, it was smashed to 40–60 mesh and named as the melanin-extracted chestnut shells (MECS) for the MB biosorption. The raw chestnut shells (RCS) for the biosorption were treated as the same procedure except for the melanin extraction.
Biosorbent Characterization
The Brunauer–Emmett–Teller (BET) surface areas of chestnut shells before and after melanin extraction were determined employing nitrogen adsorption/desorption at 77 K on an automatic surface area analyzer (Mike ASAP2020, USA). The surface morphology and elemental composition of the samples were investigated on a Quanta FEG 250 scanning electron microscope (SEM, FEI Inc., USA) equipped with energy-dispersive spectroscopy (EDS, Thermo Fisher K-Alpha, USA). The surface functional groups were detected using the KBr method on a Nicolet iS50 Fourier transform infrared (FT-IR) spectrometer (Thermo Fisher, USA).
The pH point of zero charges (pHpzc) of the adsorbent was determined by the immersion technique [17]. Briefly, 50 mg RCS or MECS was suspended in 50 mL of 0.03 mol L−1 KCl solutions adjusted to different initial pH values (pHinitial). The suspensions were agitated at 25 °C in a shaker at 120 RPM for 48 h. Then, the final pH values of suspensions (pHfinal) were measured, and the values of ∆pH (= pHfinal – pHinitial) were calculated. The pHpzc was identified as the pHinitial with minimum ∆pH.
The cation exchange capacity (CEC) was determined by the ammonium acetate method [18]. One gram of the biosorbent was shaken with 20 mL potassium acetate of 1 mol L−1 for 30 min at 75 RPM. Then the solid was recovered by centrifugation for 10 min, and treated with the solution again. The process was repeated twice more but replacing the solution with distilled water. After that, the solid was further shaken with 25 mL of 1 mol L−1 ammonium acetate (pH 5.5) for 30 min at 75 RPM. The supernatant and the biomass were separately collected by the centrifugation for 10 min. This process was repeated twice, and the supernatants were put together, and the potassium concentration in the mixed supernatant was measured by a Perkin Elmer AAnalyst 100 flame atomic absorption spectrometer (Shelton, USA). CEC value was determined from the potassium concentration.
Biosorption Experiments
The batch technique was employed in the biosorption studies. Several 200-mL flasks containing 50 mL MB solution of desired concentration and pH in each vessel were agitated with 50 mg biosorbent at 120 RPM on a thermostatic rotary shaker for pre-determined contact time. The pH studies were conducted at 298 K for 48 h, in which the initial pH values of the MB solutions of 400 mg g−1 were ranged from 2 to 10 adjusted with HNO3 or NaOH solutions. Kinetic experiments were carried out at 298 K for 2.5–1080 min using 400 mg g−1 MB of pH 5.6. To determine the sorption isotherms, the MB solutions of pH 5.6 with concentrations from 100 to 600 mg g−1 were used, and the biosorption was done at 298 K for 48 h. To check the effect of temperature on the biosorption, the processes were implemented at temperatures ranging from 288 to 328 K for 48 h using 400 mg g−1 MB of pH 5.6. After the biosorption, the mixtures were quickly filtered through a 1.2-μm pore-sized microporous membrane, and the residual MB concentration of the filtrates was determined by measuring the absorbance at 664 nm using a Mapad UV − 1800 BPC spectrophotometer (Shanghai, China). The amount of MB adsorbed, q (mg g−1), was calculated as follows:
where C0 and C are the initial and final MB concentration (mg g−1), respectively, V is the volume of MB solution (L), and m is the mass of the adsorbent (g). All the experiments were performed in triplicate and the average values ± standard deviations (error bars) are presented. The data fitted to the adsorption isotherms or kinetic equations were solved by linear regression using Microsoft ® Excel.
Results and Discussion
Physicochemical Properties of Adsorbents
The physical structure and chemical properties of biosorbent are crucial to its adsorption performance. SEM–EDS technology was applied to characterize the surface morphology (Fig. 1) and elemental compositions (Fig. 2) of RCS and MECS before and after MB loading. As shown in Fig. 1, the chestnut shells exhibit rough layered surface structures with slit holes. By contrast with RCS (Fig. 1a), the melanin extraction narrowed the slits, blurred the layers, and made a few small irregular pores (Fig. 1b). After MB biosorption (Fig. 1c and d), the surface became smoother and less porous and lamellar. The melanin extraction made the BET specific surface area increased by 10.96%, from 1.798 m2 g−1 for RCS to 1.995 m2 g−1 for MECS, possibly due to opening up pore spaces and expanding the internal surface area of the chestnut shells.
Figure 2 shows the elemental compositions based on EDS analysis. The melanin extraction decreased C, N, and O contents because the melanin contains benzene rings, and amino, carboxyl, and hydroxyl groups, which are rich in these elements. On the contrary, it increased the Na content, indicating Na+ ions had been bound to the surface of MECS. After MB adsorption, the Na content of the two biosorbents was decreased, especially in the MECS, which indicates that the adsorption of the cationic MB took place by ion exchange with Na+ ions. The biosorption enhanced the O content could result from the formation of MB hydrate. The decrease in the C content of RCS after the biosorption might result from the increase in the O content. The increase in C and N content of MECS during the biosorption could be attributed to the N in the MB molecules (Chemical formula: C16H18ClN3S) and the loss of Na.
The surface charge of adsorbents was characterized by pHpzc measurement, which is helpful to understand the biosorption mechanisms. When the solution pH equals the pHpzc, the positive and negative charges on the surface of the adsorbent are balanced, resulting in an electrically neutral surface. Alternatively, the adsorbent surface is overall positively charged in a solution of pH below pHpzc, favoring the adsorption of anionic species. On the contrary, a solution pH over the pHpzc results in an overall negatively charged surface preferable to catch cations. The pHpzc of RCS was found to be 4.1 (Fig. 3), exhibiting the acidic property of this biomass may be due to the melanin, an ingredient containing abundant acidic groups including phenolic hydroxyls and carboxyls. After the melanin extraction, the pHpzc was increased to 7.0 (Fig. 3), owing to the removal of acidic melanin and neutralization of the acidic groups with NaOH. It is consistent with the EDS results above.
CEC characterizes the number of negative charged groups or Lewis base functional groups at the biosorbent surface. It is an important parameter dealing with the ion uptake onto the biosorbent. The carboxyl, amino, and hydroxyl groups on the melanin from the chestnut shells are capable of cation exchange. The value of CEC measured for RCS was 47.18 meq 100 g−1, whereas a value of 123.9 meq 100 g−1 was obtained for MECS. These results suggest that more sites were activated for cation exchange, although the potential sites on the melanin were removed by the melanin extraction.
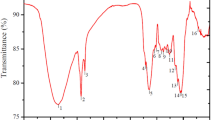
Figure 4 shows the FT–IR spectra of RCS and MECS before and after MB biosorption. In the spectrum of RCS before the biosorption, the broad and intense absorption peak at around 3500–3000 cm−1 corresponds to the O–H and N–H stretching vibrations. The absorption peak at 2916 cm−1 is attributed to the C–H stretching vibration of aliphatics. The absorption peak at 1734 cm−1 can be assigned to the C = O stretching of COOH. It disappeared after the melanin extraction because most COOH groups lie on the melanin and the left carboxyls turned into the carboxylic sodium, further confirming the EDS and pHpzc analyses. The peak at 1615 cm−1 is due to the asymmetric stretching vibration of C = O. After the MB biosorption, some distinct changes are noted. The absorption peaks at 3500–3000, 1734, and 1615 cm−1 were remarkably shifted in positions or were changed in intensity, indicating the MB binding was mostly at the carboxyl, hydroxyl, and amino groups. The peaks at 1485 and 1328, and 879 cm−1 are ascribed to C = S+ stretching, C–N (in N–CH3) stretching, and H-bonds of the type N···H–O···S+, respectively. The occurrence of these peaks was observed in the spectra of MB-loaded RCS and MB-loaded MECS, implying the formation of dimers and H-aggregates due to the interaction of sulfur and nitrogen atoms with OH groups of water molecules [19]. The new weak absorption bands formed at 2782 cm−1 in these two spectra for the vibrations of the N(CH3)2 groups engaged in H-bonds [19], indicating the formation of MB hydrate as depicted by the EDS analysis above.
Effect of pH on Biosorption
The pH of adsorbate solution exerts profound effects on the biosorption, including the surface charge of biosorbent and the degree of ionization/aggregation and speciation of the adsorbate molecules. Figure 5 reports the MB biosorption onto RCS and MECS as functions of the initial solution pH. At pH 2, there were many hydrogen ions in the solution, which competed with MB, a cationic dye, for the binding sites on the biomass, lowering the adsorption capacities. By increasing the solution pH, the competition is progressively weaker, and more dye molecules can be captured by the biosorbents. The MB adsorption capacity of RCS increased gradually from 96.9 mg g−1 at pH 2 to a maximum of 229.7 mg g−1 at pH 10. While that of MECS sharply increased from 139.7 mg g−1 at pH 2 to 355.7 mg g−1 at pH 4, and then slightly further increased to 385.3 mg g−1 at pH 10. As depicted in Fig. 5, MECS performed better than RCS at all the pH levels tested. Furthermore, MECS worked very well at a wide range of solution pH from 4 to 10. These results demonstrate that the melanin extraction is not only able to enhance the MB adsorption capacity of the chestnut shells but also to tone down the sensitivity of the biomass to the pH of adsorbate solution, expanding its applicable pH range. Generally speaking, the more positive charges adsorbent surface has, the fewer cations can be bound by electrostatic attraction. As noted above, the pHpzc of MECS (7.0) is higher than that of RCS (4.1). Consequently, there are more positive charges on the former’s surface than the latter’s at the same pH, while the former did the biosorption better. MECS shows a good adsorption performance at 4–6, where the surface is positively charged. These suggest that the biosorption via coulombic interaction might not be the main adsorption mechanism. The competition between MB and H+ for adsorption sites may be more important. To further clarify the mechanisms, the authors measured the equilibrium pH of the system after the adsorption. The final pH as the function of the initial pH in the 0.03 mol L−1 KCl electrolyte solution was used as the control, whose data are from the pHpzc measurement experiment. The results are shown in Fig. 6 where it can be seen that the pH values of the solution after adsorption were lower than those of the control at most initial pHs, indicating that protons were released from the biomass during adsorption via ion-exchange. It further confirms the result of the EDS analysis. The adsorption capacities are similar throughout pH 4 to 10 for MECS, maybe due to the domination of the ion-exchange rather than the coulombic interaction.
Effect of Contact Time on Biosorption and Kinetics
When adsorbent is suspended in a solution, adsorbate is continuously captured with a prolonged contact time until getting an equilibrium state, and hence contact time is important to adsorption process design. Figure 7 demonstrates the amount of adsorbed MB as functions of the contact time onto RCS and MECS. The equilibrium was attained within 360 min for RCS and 600 min for MECS. Profoundly more MB was adsorbed by MECS than by RCS for any contact time evaluated.
Kinetic properties are usually employed to unravel adsorption mechanisms and to provide theoretical bases for applications in practice. Therefore, the authors fitted the experimental data before the equilibrium to three well-known adsorption kinetic models, namely, the pseudo-first-order and pseudo-second-order kinetic models, and the intra-particle diffusion model.
The pseudo-first-order kinetic model is based on solid-phase capacity and has been widely used to describe the adsorption of liquid–solid systems. Its linear form is generally expressed as [20]:
where qe (mg g−1) and qt (mg g−1) are the adsorption capacity at equilibrium and time t (min), respectively; k1 (min−1) is the rate constant during the pseudo-first-order kinetic adsorption process.
The pseudo-second-order kinetic model is based on chemical adsorption, and its linear form is written as [21]:
or
where k2 (g mg−1 min−1) is the adsorption rate constant, h0 (mg g−1 ·min−1) is the initial adsorption rate constant, and qe, qt, and t are the same as in Eq. 2.
The intra-particle diffusion model can be used to study the diffusion and migration characteristics during an adsorption process, which can be expressed as [22]:
where kd (mg g−1 min−1/2) is the intra-particle diffusion rate constant, I (mg g−1) is the boundary layer thickness constant, and qt and t are the same as in Eq. 2.
The linear fits of the data to the kinetic models are plotted in Fig. 8, and the model parameters are listed in Table 1. As shown in Fig. 8, the pseudo-second-order kinetic model provided better fits compared with the pseudo-first-order kinetic model, which had R2 value closer to unity than the latter did (Table 1). The theoretical values (qe, cal) calculated from the former less deviated from the experimental values (qe, exp) than those from the latter, further confirming the serviceability of the pseudo-second-order equation to describe the MB adsorption process onto RCS and MECS. Accordingly, MB molecules were captured by RCS and MECS via chemisorption, probably via the ion exchange with Na+ and H+ ions as indicated above by the EDS results and the pH change during the biosorption. The MB biosorption onto MECS has a faster initial adsorption rate (h0) than that onto RCS, corroborating that the melanin-extraction accelerated the biosorption process.
a The pseudo-first-order, b the pseudo-second-order, and c the intra-particle diffusion kinetic plots for methylene blue (MB) biosorption onto raw chestnut shells (RCS) and melanin-extracted chestnut shells (MECS) (pH: 5.6, MB concentration: 400 mg L−1, adsorbent dosage: 1 mg mL−1, temperature: 298 K)
To further investigate the diffusion mechanism, the authors analyzed the kinetic data by fitting to the intra-particle diffusion model, and the plots are presented in Fig. 8c. The MB biosorption onto RCS gives a linear plot that does not pass through the origin, suggesting the biosorption process was partially controlled by the intra-particle diffusion. Unlike this, the biosorption onto MECS is multi-linear, where the initial portion is the MB diffusion through the solution to the external surface of the biosorbent, and the final portion corresponds to the intra-particle diffusion [23]. In summary, the adsorption processes of MB onto RCS and MECS are affected by intra-particle diffusion, but it is not the sole rate-controlling step.
Effect of Initial Concentration and Adsorption Isotherm
Initial adsorbate concentration is an indispensable source of driving force to overcome mass transfer resistances of the adsorbate from the solution to the adsorbent. The effect of initial MB concentration on the biosorption was investigated, and the results are shown in Fig. 9. No obvious differences between RCS and MECS in the adsorption capacity at equilibrium, qe, were observed at the initial concentration of 100 mg L−1. As the concentration increased up to 200 mg L−1, the superiority of MECS to RCS could be fully demonstrated. RCS was almost saturated by MB with a qe-value of 147.9 mg g−1 at 300 mg L−1 while MECS was done with a qe-value of 398.2 mg g−1 at 600 mg L−1.
Adsorption isotherms present the equilibrium distribution of adsorbates between the liquid and solid phases at a constant temperature. The authors used the Langmuir and the Freundlich isotherms, two famous models intensively applied in adsorptive removal of pollutants, to describe the equilibrium data. The Langmuir model assumes that the adsorbent has a uniform surface and that the adsorbate forms a monolayer on the surface of the adsorbent. The linear equation is [24]:
where qm is the maximum adsorption capacity of MB by the biomass (mg g−1) when the monolayer is saturated; KL is the Langmuir adsorption coefficient (L mg−1) used to express the binding energy; Ce is the MB concentration in the solution at equilibrium (mg L−1); and qe is the same as in Eq. 2.
The Freundlich model is empirical and assumes that the surface of the adsorbent non-uniform and the linear expression is [25]:
where KF is the Freundlich adsorption constant (L mg−1); n is a constant shaping the isotherm; and qe and Ce are the same as in Eq. 2 and in Eq. 6, respectively.
Figure 10 is the linear regression plots of Ce/qe vs. Ce for the Langmuir and ln qe vs. ln Ce for the Freundlich. The parameters of the isotherms as well as the coefficient of determination (R2) are given in Table 2. In comparison to the Freundlich model, the Langmuir isotherm equation provides a better fit to the equilibrium data for both RCS and MECS according to Fig. 10 and R2-values in Table 2. This means that the biosorption is single-layered on the surface of both biosorbents. The melanin extraction enhanced the saturated monolayer adsorption capacity, qm, by 163.5%, from 154.6 mg g−1 on RCS to 407.3 mg g−1 on MECS (Table 2). It also increased the Langmuir adsorption coefficient, KL, by 168.1%, from 0.1488 to 0.3990 L mg−1, which contributes stronger binding energy and favors the biosorption. Adsorption capacity is strongly dependent on both the physical and chemical properties of adsorbent. After the melanin extraction, some small pores appeared on the surface of the chestnut shells, which increased the specific surface area by 10.96% as depicted above, physically providing more room to accommodate MB molecules. Meanwhile, the CEC increased by 162.6%, chemically supplying denser binding sites to capture the adsorbate. These facts hint the improvements in qm and KL, two significant parameters to evaluate adsorbent performance, are mainly owed to the chemical activation of binding sites and secondary to the physical modification.
Effect of Temperature on MB Biosorption
Temperature impacts on the kinetic energy of molecules, thereby affecting the solute diffusion rate to the surface and interior of the adsorbent. In chemisorption, it also influences the availability of adsorption sites and the activation of adsorbate molecules. Figure 11 presents the effect of the temperature on MB adsorption onto RCS and MECS. As the temperature increased, more MB was adsorbed by RCS, indicating the endothermic nature of the process. While the MB adsorption capacity of MECS went up 312.2 mg g−1 at 288 K to a maximum of 387.7 mg g−1 at 308 K and then decreased to 359.9 mg g−1 at 328 K, probably implying that the presence of at least two types of adsorption sites or adsorption mechanisms, in which some are endothermic and the others exothermic. This thermodynamic change illustrates that new types of binding sites or adsorption mechanisms were introduced by the melanin extraction process.
Conclusions
The melanin in the chestnut shells is a multifunctional biopolymer with potential in a wide range of applications. It has been proved that melanin is an efficient adsorbent for cationic pollutants. Herein, the authors checked out the effects of the melanin-extraction on the MB (a cationic dye) biosorption of chestnut shells. The extraction resulted in a higher adsorbent surface area, pHpzc, and CEC. It toned down the sensitivity of the biomass to the solution pH and expanded the pH range of applications. It also changed the thermodynamic behavior of the biosorption, which is probably attributed to introducing new types of binding sites. The biosorption process was well described by the chemisorption model of the pseudo-second-order kinetic equation and accelerated by the melanin extraction. The biosorption equilibrium data can be well modeled by the Langmuir isotherm. The melanin extraction over doubled the monolayer adsorption capacity. The improvement in the biosorption performance of chestnut shells by the melanin extraction is mainly owed to the chemical activation of binding sites and secondary to the physical modification. Chestnut shells are a low-cost residual of food processing. It represents a kind of byproducts of biomass abundantly containing melanin. Although melanin has strong binding capacities to cationic pollutants, the melanin extraction did not reduce the MB adsorption capacity of chestnut shells. On the contrary, it improved the adsorption performance of chestnut shells. The melanin-extracted chestnut shells have a potential for MB biosorption. Melanin-containing biomass such as chestnut shells can be used in cascades for melanin production and subsequently for adsorptive removal of basic dyes such as MB.
References
Singh SN (2015) Microbial degradation of synthetic dyes in waste waters. Springer. 187–204. https://doi.org/10.1007/978-3-319-10942-8
Sharmeen A, Tushar KS (2018) A review on heavy metal ions and dye adsorption from water by agricultural solid waste adsorbents. Water Air Soil Pollut 229(7):1–50. https://doi.org/10.1007/s11270-018-3869-z
Santiago-De la Rosa N, Mugica-Álvarez V, Cereceda-Balic F et al (2017) Emission factors from different burning stages of agriculture wastes in Mexico. Environ Sci Pollut Res Int 24:24297–24310. https://doi.org/10.1007/s11356-017-0049-4
Sana D, Jalila S (2017) Adsorptive removal of methylene blue from aqueous solution using different agricultural wastes as adsorbents. Korean J Chem Eng 34(4):1037–1043. https://doi.org/10.1007/s11814-017-0008-2
Qie LM, Yao ZY, Li GW et al (2018) Equilibrium, kinetics and thermodynamics of Cu(II) biosorption on Chinese chestnut shell pretreated with steam explosion. Water Sci Technol 78(4):868–877. https://doi.org/10.2166/wst.2018.359
Yao ZY, Qi JH, Wang LH (2010) Equilibrium, kinetic and thermodynamic studies on the biosorption of Cu(II) onto chestnut shell. J Hazard Mater 174(1–3):137–143. https://doi.org/10.1016/j.jhazmat.2009.09.027
Özdemİr ÇS (2018) Adsorptive removal of methylene blue by fruit shell: Isotherm studies. Fuller Nanotub Car N 26(9):570–577. https://doi.org/10.1080/1536383X.2018.1472083
Yao ZY, Qi JH, Wang LH (2012) Isolation, fractionation and characterization of melanin–like pigments from chestnut (Castanea mollissima) shells. J Food Sci 77(6):671–676. https://doi.org/10.1111/j.1750-3841.2012.02714.x
Yao ZY, Qi JH (2016) Comparison of antioxidant activities of melanin fractions from chestnut shell. Molecules 21(4):article487. https://doi.org/10.3390/molecules21040487
You TT, Zhou SK, Wen JL et al (2014) Chemical composition, properties, and antimicrobial activity of the water-soluble pigments from Castanea mollissima shells. J Agric Food Chem 62(8):1936–1944. https://doi.org/10.1021/jf4045414
Yao ZY, Qi JH, Hu Y et al (2016) Insolubilization of chestnut shell pigment for Cu (II) adsorption from water. Molecules 21(4):article405. https://doi.org/10.3390/molecules21040405
Jia Y, Jiang H, Liu Z et al (2017) An innovative approach to the preparation of coloured and multifunctional silk material with the natural extracts from chestnut shell and black rice bran. Color Technol 133(3):262–270. https://doi.org/10.1111/cote.12276
Nasuha N, Hameed BH (2011) Adsorption of methylene blue from aqueous solution onto NaOH–modified rejected tea. Chem Eng J 166(2):783–786. https://doi.org/10.1016/j.cej.2010.11.012
Amela K, Hassen MA, Kerroum D (2012) Isotherm and kinetics study of biosorption of cationic dye onto banana peel. Energy Procedia 19:286–295. https://doi.org/10.1016/j.egypro.2012.05.208
Yao ZY, Wang LH, Qi JH (2009) Biosorption of methylene blue from aqueous solution using a bioenergy forest waste: Xanthoceras sorbifolia seed coat. Clean–Soil Air Water 37(8):642–648. https://doi.org/10.1002/clen.200900093
Gracheva NV, Zheltobryukhov VF (2019) Sorption properties of sunflower husk melanins. Pharm Chem J 53(4):337–341. https://doi.org/10.1007/s11094-019-02002-2
Fiol N, Villaescusa I (2009) Determination of sorbent point zero charge: usefulness in sorption studies. Environ Chem Lett 7(1):79–84. https://doi.org/10.1007/s10311-008-0139-0
Chapman HD (1965) Cation exchange capacity. In Methods of soil analysis. American Society of Agronomy, Madison. 891–901. https://doi.org/10.2134/agronmonogr9.2.c6
Ovchinnikov OV, Evtukhova AV, Kondratenko TS et al (2016) Manifestation of intermolecular interactions in FTIR spectra of methylene blue molecules. Vib Spectrosc 86:181–189. https://doi.org/10.1016/j.vibspec.2016.06.016
Lagergren S (1898) About the theory of so-called adsorption of soluble substances. Chem Eng 24:1–39
Ho YS, McKay G (1998) The kinetics of sorption of basic dyes from aqueous solution by sphagnum moss peat. Can J Chem Eng 76:822–827. https://doi.org/10.1002/cjce.5450760419
Weber WJ, Morris JC (1963) Kinetics of adsorption on carbon from solution. J Sanit Eng Div 89(2):31–60. https://doi.org/10.1061/JSEDAI.0000420
Elmoubarki R, Mahjoubi FZ, Tounsadi H et al (2015) Adsorption of textile dyes on raw and decanted Moroccan clays: kinetics, equilibrium and thermodynamics. Water Resour Ind 9:16–29. https://doi.org/10.1016/j.wri.2014.11.001
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40(9):1361–1403. https://doi.org/10.1021/ja02242a004
Freundlich H (1906) Concerning adsorption in solutions. Z Phys Chem Stoechiom Verwandtschafts 57(4):385–470
Acknowledgements
Appreciation is sincerely expressed to an undergraduate, Xiao-Qi Fan, for doing some experimental work.
Funding
This work was supported by the Yunnan Agricultural Joint Foundation Projects (2018FG001-008), the National Science Foundation of China (31770673), and the Faculty Start-up Grant to Dr. Zeng-Yu Yao from Southwest Forestry University (111822).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, ZF., Liu, ZJ., Qie, LM. et al. Effects of Melanin Extraction on Biosorption Behavior of Chestnut Shells Towards Methylene Blue. Water Conserv Sci Eng 6, 163–173 (2021). https://doi.org/10.1007/s41101-021-00111-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s41101-021-00111-2