Abstract
Commercial MK is widely used in the synthesis of geopolymer binders because of its high kaolinite content and reactivity. However, the possibility of producing geopolymers with low reactive MK is not sufficiently experienced and deserves to be verified. The present study deals with the potential use of a low reactive traditionally elaborated metakaolin (MK) and a local ground-granulated blast-furnace slag (GGBFS) as precursors for geopolymer synthesis. The precursor composition (MK/GGBFS: 100/0, 80/20 and 50/50 wt%) and the activator properties (SiO2/Na2O molar ratio (MR): 2; 1.5 and density: 1.4; 1.3 g/cm3) are the variables used to formulate twelve geopolymer mixes. Furthermore, various curing conditions (temperatures of 60, 80 and 100 °C and durations of 6, 24 and 48 h) were chosen for the hardening of geopolymer pastes. An optimization approach has been used in the attempt to deduce the optimal formulation and curing conditions allowing the synthesis of geopolymer with good performances. Tests of setting times and compressive strength were performed on geopolymer pastes in order to assess the effects of different MK/GGBFS contents, activator properties and curing conditions on the performances of geopolymer pastes. The hardened samples were analyzed by scanning electron microscopy (SEM). It has been demonstrated that the used MK, despite its low reactivity, may lead to geopolymer of good performances. The best formulation was 80/20 of MK/GGBFS activated with a sodium silicate solution, the MR and the density of which were 1.5 was 1.4 g/cm3, respectively. On the other hand, the suitable curing temperature was often 60 °C, especially for curing duration of 48 h.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Geopolymer binders have received considerable attention over the last few decades; they are widely studied as potential alternatives to the conventional cements. Basically, geopolymer binders are synthesized by the reaction of active aluminosilicate powder with a highly concentrated alkaline solution. Metakaolin (MK), fly ash (FA), ground-granulated blast-furnace slag (GGBFS) and certain clay wastes are the most common aluminosilicates used as precursors in geopolymer binders [1, 2], while sodium silicate (Na2SiO3), potassium silicate (K2SiO3), sodium hydroxide (NaOH), and potassium hydroxide (KOH) are the main chemicals used as alkali activators [2,3,4]. The reaction between the aluminosilicate precursor and the alkaline activator triggers a complex chemical process, called geopolymerization. It begins with the dissolution of the reactive silicate and aluminate tetrahedra from the precursor in the alkaline solution, and the released SiO4 and AlO4 tetrahedrons combine to form monomers and then oligomers by sharing one oxygen atom. The condensation reaction of oligomers occurs to form a small network structure (gelation), and the matrix will subsequently reach its hardened state (polycondensation) to form 3D geopolymer system [3, 5].
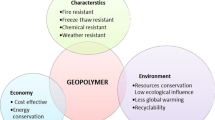
Due to lower environmental impact of the aluminosilicate precursors, whether natural or treated clays, wastes or by-products, geopolymers promise high ecological benefit comparatively to ordinary Portland cement. Moreover, several technical benefits have been commonly reported; these benefits are strongly dependent on the type of the synthesized geopolymer. It has been reported that geopolymer-based materials exhibited higher tensile, flexural and compressive strengths, higher modulus of elasticity, better fire, frost and freeze–thaw cycles resistance, better resistance to chemical attacks, carbonation and corrosion, lower drying shrinkage, sorptivity and thermal conductivity, lower porosity, water absorption and permeability compared to cement mortars and concretes [2, 5, 6].
According to various published researches, the performance of geopolymers is affected by many parameters that can be grouped into four types such as precursor, activator, mixture and curing conditions. The main criteria required for the aluminosilicate precursor are: appropriate chemical and mineralogical compositions, high content of amorphous phases, sufficient fineness and low water demand [4]. Ren et al. [3] added that particle size and the Si/Al molar ratio are crucial factors in determining the precursor reactivity. It is well known that the mechanical properties and microstructure of geopolymer are strongly influenced by the Si/Al ratio. In most of the cases, optimum performances have been reported for mixtures with Si/Al ratio ranged from 3 to 3.8 [4, 7]. In turn, type, concentration and density of the alkaline solution remain the principal parameters affecting the final characteristics of resulted geopolymer [4, 8]. However, geopolymerization reaction is mainly related to the activator’s alkalinity (M2O/H2O) and its silica modulus (SiO2/M2O), where M represents the alkali metal [9]. In the mixture design, a wide range of factors and ratios are involved in defining criteria and properties of the geopolymer product, namely mixture proportions, mixing regime, ratios of liquid to solid, aggregate to binder, SiO2/Na2O, use of additives and superplasticizer [1,2,3, 9]. Finally, it has been widely shown that geopolymerization process is strongly dependent on the parameters of curing regime, such as mode, temperature and duration [1, 8, 10] (Fig. 1).
Kaolin clay is very abundant in many countries around the globe, and flash calcination is the most reliable method allowing converting kaolin into highly reactive MK. Several prior studies investigated the use of solely MK or mixture of MK and GGBFS as suitable precursors for geopolymers binders. The existing literature demonstrates that in addition to its kaolinite content and purity level, the efficiency of MK is primarily proportional to the content of reactive silica and alumina, which in turn depends on the effectiveness of kaolin to MK conversion process. However, the possibility of producing geopolymers with low reactive MK is not sufficiently experienced and deserves to be verified. The main objective of this investigation is to synthesize geopolymer materials based on traditionally elaborated MK with moderate reactivity and GGBFS. As there is no a standard mix design method, the synthesis was performed using an optimization approach in which, setting time and compressive strength were examined as functions of various factors including MK/GGBFS proportion; concentration and density of the alkaline solution and curing conditions.
Materials and experimental methods
Materials
GGBFS with a glass content of about 97% utilized in this study was recovered from the unit of El-Hadjar in the north-east of Algeria. The raw kaolin (KT2) was obtained from the deposit of Tamazert in the east of Algeria; its kaolinite content is only 58%. Both materials were dried to constant weight at 105 °C in an electrical oven and then were milled in steel ball mill to fine powders. KT2 was converted into reactive metakaolin named MKT2 after suitable thermal treatment, the details of which were described in a previous study [11]. Chemical compositions of GGBFS and MKT2, determined by X-ray fluorescence analysis (XRF), are given in Table 1. The materials were also characterized by laser granulometry and X-ray diffraction (XRD); the results are depicted in Figs. 2 and 3, respectively.
According to results of Table 1, the basicity coefficient ((CaO + MgO)/(SiO2 + Al2O3)) of GGBFS is 0.97 (< 1), while its hydraulicity index ((CaO + MgO)/(SiO2)) is 1.18 (< 1.4) indicating its acidic character and moderate reactivity [12, 13]. MKT2 is mostly composed of SiO2 and Al2O3, while it contains less than 1% by weight, of carbonates. In the previous work [11], it has been found that the strength activity index (SAI) of MKT2 was 1.06, whereas its lime consumption was only 843 mg/g which reflects its moderate pozzolanic reactivity compared to commercial MK. Particle size distributions of GGBFS and MKT2 are presented in Fig. 2. Both materials display similar distribution profiles, and the median size values D50 of GGBFS and MKT2 are 11.48 µm and 8.07 µm, respectively. However, their Blaine values are 4155 and 7000 cm2/g, indicating that MKT2 is finer than GGBFS. According to Fig. 3, the hump peak in the interval 2θ between 25° and 35° indicates that GGBFS is mainly composed of an amorphous phase; however, calcite is the unique detected crystallized mineral. The XRD diagram of MKT2 does not present any kaolinite peak, which proves that the thermal treatment has successfully converted the raw kaolin into metakaolin.
Two chemicals were used in preparing the alkali activators, such as sodium silicate solution and sodium hydroxide pellets. The sodium silicate solution (SS) was purchased from a local laboratory with chemical composition of 29.8% SiO2, 14.43% Na2O and 55.77% H2O, and it has a molar ratio (MR = SiO2/Na2O of 2.06) and a density at 20 °C of 1.53 g/cm3. The sodium hydroxide (NaOH), with 99% of purity, was added to SS until achieving the fixed molar ratios.
Geopolymer mixtures and sample testing
Four alkali activators having two molar ratios (2 and 1.5) and two densities (1.4 and 1.3 g/cm3) were prepared by mixing SS solution, solid NaOH pellets and distilled water (Table 2). The mixtures were manually stirred during 5 min for homogenization and then cooled to room temperature for 24 h. Precursors of three different MKT2/GGBFS proportions (50/50, 80/20 and 100/0 by wt%), dry mixing beforehand for 5 min, were mixed with the previous alkali activators in a laboratory blender during 5 min. Twelve geopolymer pastes were synthesized by adding gradually to 500 g of precursor, the quantity of alkali activator necessary to achieve a standard consistency as described in NF EN 196-3. The detailed mix designs can be seen in Table 3. The labeling system of geopolymer pastes (GP) includes indications of the three variable parameters. Each variant is named according to the contents of MKT2 and GGBFS in the precursor, the molar ratio and the density of the activator. For example, GP80/20:2(1.4) means geopolymer paste of which the precursor contains 80 wt% of MKT2 and 20 wt% of GGBFS; however, MR and density of the activator are 2 and 1.4, respectively. At the fresh state, tests of setting times using the Vicat apparatus test were performed as described in the NF EN 196-3 standard. The mixtures so prepared were introduced into 25 × 25 × 25 mm3 steel molds, vibrated for 2 min to eliminate the entrapped air bubbles, and then placed in the oven to be heated at 60, 80 and 100 °C. The heating periods were 6, 24 and 48 h. The reference geopolymer pastes were prepared in the same manner. They were then covered with plastic film and stored at 20 °C. After 24 h, samples were demolded and cured at ambient room temperature until testing. At the hardened state, the compressive strength was evaluated using a 250 kN Matest compression testing machine, with a loading speed of 0.1 MPa/s. The reported results are the average of six tests. Scanning electron microscopy with energy-dispersive X-ray analysis (SEM–EDX) was implemented to examine the morphology and the microstructure of the hardened geopolymer pastes. The observations were performed at 20 kV using a Microscope Tescan Vega 3. Small samples were previously dried up and metalized with nickel to provide a conductive surface.
Results and discussion
Setting time of geopolymer pastes
The results of the setting time tests, conducted on geopolymer pastes having a standard consistency, are presented in Table 4. It can be seen that whatever the activator molar ratio and density, the setting times of mixtures made with 100% MKT2 are the highest. However, when MKT2 was partially substituted by GGBFS, both initial and final setting times significantly decrease. Compared to the plain MKT2 pastes, the initial setting times of mixes containing 20% of GGBFS decrease between 40.63 and 56.25%, while at 50% replacement, the decrease is at around 83%. A similar trend is observed in final setting time results, where the decrease ranges from 32 to 49% when the GGBFS content is 20% and from 73 to 79% when the GGBFS content is 50%. It should be noted that the difference between initial and final setting times decreases with the increase of the GGBFS level in pastes. Two factors may have to be the prominent causes for the accelerated setting such as GGBFS content and MKT2 content. It is well known that the inclusion of GGBFS in geopolymer systems accelerates the geopolymerization reaction and thereby the setting process [10, 14]. First of all, from results shown in Table 1 it can be seen that GGBFS contains more CaO than MKT2, thus the higher is the GGBFS proportion the higher is the CaO content. As reported by several authors [14,15,16,17], in alkaline medium, cations of Ca2+ combine easily with reactive silica and alumina which advantageously promotes the rapid formation of C-S-H and C-A-S-H gels and subsequently accelerates the hardening process and shortens the setting time. Secondly, and according to Table 3, the increase in GGBFS content increases the Si/Al ratio, and consequently, the setting times decrease. Kuri et al. [18] reported that the increase of Si/Al ratio accelerated the polycondensation process by producing more alkaline aluminosilicate gel which may reduce the setting times. The effect of GGBFS content on setting time is presented in Fig. 4.
Conversely, both initial and final setting times increase with the increase of MKT2 content in geopolymer pastes. From Table 3, it can be seen that the activator quantity increases with the increase of MKT2 content whatever the activator molar ratio and density. As the activator contains the dry extract of SS solution, NaOH pellets and distilled water, it is believed that the most important in increasing its quantity is the increase in the liquid part rather than the dry extracts. Metakaolin is known to have a high water demand [10, 19], which is why mixtures elaborated with 100% MKT2 required more liquid to achieve the same consistency as other mixtures. Many researchers have reported that the increase in liquid content should lead to delayed setting times [14, 15, 20]. Nath and Sarker [20] reported that increasing the alkaline liquid content caused abundance of liquid in the mixture, which eventually slowed the condensation process for geopolymer formation and affected the setting time. While decreasing the alkaline liquid content caused according to Yaseri et al. [21], an accelerated dehydration of water accelerated and geopolymerization, which led to a faster setting. On the other hand, MKT2 is significantly finer than GGBFS according to Table 1 and Fig. 2. Increasing the MKT2 content improves the fineness of the precursor, which prolongs the setting time of geopolymer pastes. This observation is supported by the findings of Huseien et al. [22], where the setting time increased with the partial replacement of GGBFS (60% of particles finer than 10 µm) by MK (75% of particles finer 10 µm). The authors established that MK is greatly effective to decelerate the setting time of GGBFS/MK-based geopolymer.
It should be highlighted that for mixtures having the same MKT2/GGBFS proportions, the variation of molar ratio and density of the activator seems to be without significant effect on setting time. For example, when RM decreases from 2 to 1.5, both initial and final setting times of GP80/20:1.5(1.4) increase compared to those of GP80/20:2(1.4); however, the setting times of GP50/50:1.5(1.3) decrease compared to those of GP50/50:2(1.3). Similarly, when the activator's density decreases from 1.4 to 1.3, both initial and final setting times of GP80/20:2(1.3) increase compared to those of GP80/20:2(1.4); however, the setting times of GP50/50:1.5(1.3) decrease compared to those of GP50/50:1.5(1.4).
Compressive strength of geopolymer pastes
Compressive strengths of hardened geopolymer pastes were determined after 6, 24 and 48 h of heat curing regimes at 60, 80 and 100 °C and then compared to those of the reference pastes obtained after 180 days of ambient curing. The results are presented in Table 4.
Effect of GGBFS content
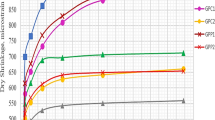
The testing results of compressive strength are significantly influenced by the contents of MKT2 and GGBFS in the precursor (Fig. 5). It is easily seen that the geopolymer pastes made with 100% MKT2 exhibit overall the lowest strengths, whatever the activator properties and the curing conditions (temperature and duration). Strengths of only 9 MPa are recorded after 6 h of curing at 60 and 100 °C. Despite its greater fineness compared to GGBFS, MKT2 could not achieve better strengths. These findings should be linked to the moderate reactivity of MKT2 [11], its low Ca2+ content [15] and the high liquid/binder ratios of plain MKT2 pastes (Table 3), as the excess of liquid hinders the diffusion of dissolved species, decreases the polycondensation rate [23], and increases the number of pores in the hardened pastes [24] which leads to lower strengths. The compressive strength is remarkably enhanced with the inclusion of GGBFS independently of the activator properties and the curing conditions. The highest enhancement (436.26%) is recorded when GGBFS was incorporated at 50% in the paste GP2(1.4) cured for 6 h at 60 °C; however, the lowest one (1.15%) is obtained when GGBFS was used at 50% in the paste GP1.5(1.4) cured for 48 h at 60 °C. The greatest strength (82.16 MPa) is recorded for the GP80/20:1.5(1.4) paste after 48 h of curing at 100 °C; moreover, the best results are often obtained when the GGBFS content was 20%. It should be remembered that these mixes have a molar ratio Si/Al of 2.75, which reflects the availability of a sufficient amount of Si and Al and, thus, can be considered as the optimum ratio; however, it is difficult to identify the ideal liquid to binder ratio due to its variation with the activator properties. The adequate GGBFS content and therefore the optimum Si/Al ratio, enhances the formation of silicate oligomers and effectively involves Al leading to improved strengths [18]. It is well known that the strength development is mainly related to the formation of geopolymerization products, namely C-S-H, C-A-S-H and N-A-S-H. Since GGBFS promotes the formation of further C-S-H and/or C-S-H gels [10, 25], thus, its inclusion may have been the prominent reason for increased compressive strength. On the other hand, increasing the Si/Al ratio by incorporating 50% of GGBFS leads to an excess in Si content and a lack in Al content, which probably promoted the formation of Si-rich gels leading to highly amorphous geopolymer pastes with lower strengths [26]. Moreover, pastes with 50% of GGBFS recorded the fastest setting (Fig. 4), which seems unfavorable for the bonding maturity of geopolymer pastes.
Effect of curing conditions
From results of Fig. 5 and irrespective to the curing temperature, it can be seen that in most cases, compressive strength increases with the increase in curing duration. For example, compressive strength of GP50/50:2(1.3) cured at 100 °C increases by 76.2% and 301.4% after 24 h and 48 h, respectively, compared to its value after 6 h of curing. Although the heat curing enhances the dissolution of Si and Al species and facilitates the polycondensation process [1], it can be observed that longer curing duration has also a significant effect in enhancing and accelerating the compressive strength, as reported by many authors [7, 25, 27].
When examining the trend between compressive strength development relative to curing temperature for the same curing time, GGBFS content and activator properties, a strong correlation is not obvious. In 52.78% of cases, the best strength is obtained when geopolymer pastes were cured at the lowest temperature (60 °C), followed by the curing at 80 °C in 25% of cases, although the highest compressive strength (82.16 MPa) is recorded after curing GP80/20:1.5(1.4) at 100 °C. Meanwhile, the heat curing results are, in 58.33% of cases, significantly higher than those obtained after 180 days of curing at ambient room temperature. The beneficial effect of heat curing consists in improving the dissolution of aluminosilicate particles which enhances and accelerates the geopolymerization process [28]. Moreover, the additional geopolymerization products fill in the pores resulting from the evaporated water, leading to a denser matrix and improved strengths [18, 28]. The slight decrease in compressive strength observed after curing at 100 °C is attributed to an insufficient dissolution of the aluminosilicate particles and a faster geopolymerization as stated Muraleedharan and Nadir [9].
Effect of the activator properties
As it can be seen from Table 4 and Fig. 5, the compressive strength strongly depends on the molar ratio MR of the activator. For the same MKT2/GGBFS proportions and curing conditions, the MR of 1.5 allows obtaining, in most cases, the highest compressive strengths, whatever the activator density. It is believed that lowering MR from 2 to 1.5 means that more NaOH is available in the alkaline solution; therefore, a supplementary sodium aluminosilicate gel (N-A-S-H) is formed. According to Kuri et al. [18], a higher Na2O content improves the binding mechanism of geopolymer which may lead to higher compressive strength. It should be noted that MR of 1.5 agrees well with the values of 1.4 [24] and 1.7 [29] stated as optimum MR in the case of MK/GGBFS-based geopolymers, and values of 1.27 and 2 in the case of geopolymers based on plain MK [30] and plain GGBFS [31], respectively.
On the other hand, the high activator density appears to have positive impact on compressive strength of geopolymer pastes, independently of its MR. The lower density of activator leads to a decrease in compressive strengths in 66.67% and 88.89% of cases, when MR was 2 and 1.5, respectively. For example, the compressive strength of GP50/50:2(1.4) obtained after 6 h of curing at 100 °C decreases by 62.31%, when lowering the activator density to 1.3. Since the low density is obtained by adding water to the alkaline solution rather than silicate and sodium, the dissolution of precursor constituents is consequently affected, and less geopolymerization products are formed, which is the main reason for the recorded low strengths. On the basis of the above analysis, it can be concluded that the best activator properties are: MR of 1.5 and density of 1.4.
SEM analysis
SEM analysis was carried out on the samples which resulted in the highest compressive strengths at the same activator properties (MR of 1.5 and density of 1.4). The effects of GGBFS content, curing time and curing temperature were examined. Figure 6 shows the SEM images of the plain MKT2 sample and those containing 20% and 50% of GGBFS. As it can be seen, the micrograph of GP100/0 reveals a densified microstructure, although the morphology of its surface appears irregular and less homogenous. The presence of micropores, micro-cracks, air bubbles and many unreacted MKT2 particles may be associated with the low strength achieved by this mixture compared to those containing GGBFS. However, images of GP80/20 and GP50/50 samples show that the inclusion of GGBFS leads to a denser and more homogenous microstructure, almost without micro-cracks. Although many agglomerations of MKT2 particles are still visible, particularly in GP80/20, some angular-shaped particles of GGBFS are observed in the GP50/50 sample. In both MKT2/GGBFS-based geopolymer samples, the geopolymer gel seems to be denser owing to the higher polymerization of these mixtures, which agrees with their higher compressive strengths. According to the EDS analysis, the elemental composition of the geopolymer gel of GP100/00 is dominated by Si, Al and Na, which indicates that the main hydration product is N-A-S-H gel with ratios Si/Al and Na/Al of 1.71 and 0.55, respectively. Concerning the MKT2/GGBFS-based geopolymers samples, the EDS results indicate that the geopolymer gels are composed mainly by Si, Al and Na; however, the Ca content increases with increasing GGBFS content in the mix. Burciaga-Diaz et al. [32] evidenced the localized formation of products of different natures, such as N-A-S-H gel around the MK particles and C-A-S-H gel, possibly intermixed with other products, around GGBFS particles. On the other hand, Xiang et al. [16] reported that by increasing the Si/Al ratio, more Si–O–Si bands should be formed, which improved the compressive strength. The Si/Al ratios of GP80/20 and GP50/50 are 1.98 and 1.28, respectively, which explains the strength results shown in Table 4. The effect of curing time on the microstructure of GP80/20:1.5(1.4) cured at 100 °C is shown in Fig. 7. The three images reveal a densified and packed structure; the geopolymer gel has been clearly precipitated on the surface due to the geopolymerization reactions. However, significant morphological differences can be observed after 48 h of curing, where the micropores have completely disappeared and an integrally homogeneous binder occupies the upper surface of the sample. A heavy presence of unreacted GGBFS and MKT2 particles is obvious at 6 h of curing; however, the agglomerations of unreacted particles decrease significantly after 48 h of curing. This means that increasing the curing period enhances the dissolution of precursors and increases the polycondensation rate, which leads to higher compressive strengths as shown in Fig. 5. The effect of curing temperature on the microstructure of GP80/20:1.5(1.4) cured during 48 h at 60 °C, 80 °C and 100 °C in comparison with the reference geopolymer paste cured at 20 °C for 180 days is shown in Fig. 8. Although few agglomerations of unreacted particles are obvious, the SEM images reveal that a large area of geopolymer gel is visible in all samples, indicating that the selected temperatures are sufficient to enhance the geopolymerization reactions. Moreover, by increasing the curing temperature, the microstructure becomes denser, less porous and contains less micro-cracks, especially in the sample cured at 100 °C, which explains its higher compressive strength compared to those cured at 60 °C and 80 °C. However, and despite its high compressive strength (79.75 MPa), the GP80/20:1.5(1.4) cured at 20 °C during 180 days seems to be undensified, uncompacted and contains more micropores, compared to that one cured for 48 h at 100 °C.
Conclusions and recommendations
The possibility of using low reactive MK in the synthesis of MK/GGBFS-based geopolymer was investigated. The effects of MK/GGBFS contents, activator properties and curing conditions on setting times and compressive strength were examined. On the basis of obtained results, the following findings are drawn:
-
The geopolymer based entirely on MKT2 recorded the longest setting times and the lowest compressive strengths. This result is due to the low MKT2’s reactivity, CaO content and Si/Al ratio and the high water content in these mixes.
-
Inclusion of GGBFS as a partial substitution of MKT2 decreased the setting times of geopolymer paste whatever the activator molar ratio and density; however, the properties of the activator did not present a significant effect on setting times.
-
The compressive strengths of geopolymer pastes were remarkably enhanced with the inclusion of GGBFS, independently of the activator properties and curing conditions, but the higher enhancements were often recorded for the GGBFS content of 20%.
-
The compressive strength of geopolymer pastes increased with the increase in curing duration, while the trend between compressive strength and curing temperature was not obvious. In most cases, highest strengths were obtained for curing at 60 °C, although the best strength (82.16 MPa) was obtained for the paste cured at 100 °C.
-
Despite its low reactivity, MKT2 may be used in the synthesis of MK/GGBFS-based geopolymer. Overall, setting time and compressive strength of the synthesized geopolymer paste were positively affected by the decrease of the activator MR and density and the increase in curing duration; however, the effect of increasing curing temperature was not obvious.
-
The microstructure of the GP80/20:1.5(1.4) cured at 100 °C for 48 h is dense and compact with large area of the geopolymer gel on the upper surface of the sample. The variation in GGBFS content, curing time and curing temperature affects negatively the microstructure of geopolymer pastes.
-
The best performances of this MK/GGBFS-based geopolymer were obtained when the density of the activator was 1.4 and its MR was 1.5 with a GGBFS content of 20%.
-
The present study was carried out on a laboratory scale; the best performances of the synthesized geopolymer were obtained using heat curing. It should be highlighted that in real-scale applications, several sources of energy, non-renewable (fossil fuels, electrical energy, etc.) and renewable (solar energy) can be exploited in producing the curing temperatures.
Due to the encouraging obtained results and the abundance of KT and GGBFS in Algeria, the developed MK/GGBFS-based geopolymer deserves to be valued as an alternative binder for mortar and concrete. Compared to ordinary Portland cement, this binder with low environmental impact and energy consumption allows to get rid of GGBFS wastes, while ensuring high early age compressive strength. However, the optimal performances of this geopolymer binder must be investigated for other fineness and proportions of MK and GGBFS, types and properties of activator and design methods. Moreover, the use of resulting binder in producing geopolymer concrete is strongly recommended, the fresh and hardened properties of which need to be investigated thoroughly.
References
Farhan KZ, Johari MAM, Demirboga R (2020) Assessment of important parameters involved in the synthesis of geopolymer composites: a review. Constr Build Mater 264:120276
John SK, Nadir Y, Girija K (2021) Effect of source materials, additives on the mechanical properties and durability of fly ash and fly ash-slag geopolymer mortar: a review. Constr Build Mater 280:122443
Ren B, Zhao Y, Bai H, Kang S, Zhang T, Song S (2021) Eco-friendly geopolymer prepared from solid wastes: a critical review. Chemosphere 267:128900
Singh B, Ishwarya G, Gupta M, Bhattacharyya SK (2015) Geopolymer concrete: a review of some recent developments. Constr Build Mater 85:78–90
Zhang P, Wang K, Li Q, Wang J, Ling Y (2020) Fabrication and engineering properties of concretes based on geopolymers/alkali-activated binders—a review. J Clean Prod 258:120896
Amran M, Debbarma S, Ozbakkaloglu T (2021) Fly ash-based eco-friendly geopolymer concrete: a critical review of the long-term durability properties. Constr Build Mater 270:121857
Rovnaník P (2010) Effect of curing temperature on the development of hard structure of metakaolin-based geopolymer. Constr Build Mater 24:1176–1183
Ranjbar N, Kuenzel C, Spangenberg J, Mehrali M (2020) Hardening evolution of geopolymers from setting to equilibrium: a review. Cement Concr Compos 114:103729
Muraleedharan M, Nadir Y (2021) Factors affecting the mechanical properties and microstructure of geopolymers from red mud and granite waste powder: a review. Ceram Int 47:13257–13279
Jindal BB (2019) Investigations on the properties of geopolymer mortar and concrete with mineral admixtures: a review. Constr Build Mater 227:116644
Mehsas B, Siline M, Zeghichi L (2021) Development of supplementary cementitious materials from Algerian kaolin: elaboration of metakaolin and assessment of pozzolanicity. Innov Infrastruct Solut 6:50
Behim M, Cyr M, Clastres P (2011) Physical and chemical effects of El Hadjar slag used as an additive in cement-based materials. Eur J Environ Civ Eng 15:1413–1432
Winnefeld F, Ben Haha M, Le Saout G, Costoya M, Ko SC, Lothenbach B (2015) Influence of slag composition on the hydration of alkali-activated slags. J Sustain Cem Based Mater 4:85–100
Hadi MNS, Zhang H, Parkinson S (2019) Optimum mix design of geopolymer pastes and concretes cured in ambient condition based on compressive strength, setting time and workability. J Build Eng 23:301–313
Xie J, Wang J, Rao R, Wanga C, Fang C (2019) Effects of combined usage of GGBS and fly ash on workability and mechanical properties of alkali activated geopolymer concrete with recycled aggregate. Compos Part B 164:179–190
Xiang J, Liu L, He Y, Zhang N, Cui X (2019) Early mechanical properties and microstructural evolution of slag/metakaolin-based geopolymers exposed to karst water. Cem Concr Compos 99:140–150
Hanafi M, Ekinci A, Aydin E (2020) Triple-binder-stabilized marine deposit clay for better sustainability. Sustainability 12:4633
Kuri JC, Khan MNN, Sarker PK (2021) Fresh and hardened properties of geopolymer binder using ground high magnesium ferronickel slag with fly ash. Constr Build Mater 272:121877
Hasnaoui A, Ghorbel E, Wardeh G (2019) Optimization approach of granulated blast furnace slag and metakaolin based geopolymer mortars. Constr Build Mater 198:10–26
Nath P, Sarker PK (2015) Use of OPC to improve setting and early strength properties of low calcium fly ash geopolymer concrete cured at room temperature. Cem Concr Compos 55:205–214
Yaseri S, Hajiaghaei G, Mohammadi F, Mahdikhani M, Farokhzad R (2017) The role of synthesis parameters on the workability, setting and strength properties of binary binder based geopolymer paste. Constr Build Mater 157:534–545
Huseien GF, Mirza J, Ismail M, Ghoshal SK, Mohd Ariffin MA (2018) Effect of metakaolin replaced granulated blast furnace slag on fresh and early strength properties of geopolymer mortar. Ain Shams Eng J 9(4):1557–1566
Bature AS, Khorami M, Ganjian E, Tyrer M (2021) Influence of alkali activator type and proportion on strength performance of calcined clay geopolymer mortar. Constr Build Mater 267:120446
Peng H, Cui C, Cai CS, Liu Y, Liu Z (2019) Microstructure and microhardness property of the interface between a metakaolin/GGBFS-based geopolymer paste and granite aggregate. Constr Build Mater 221:263–273
Kubba Z, Huseien GF, Sam ARM, Shah KW, Asaad MA, Ismail M, Tahir MMd, Mirza J (2018) Impact of curing temperatures and alkaline activators on compressive strength and porosity of ternary blended geopolymer mortars. Case Stud Constr Mater 9:e00205
Wan Q, Rao F, Song S, García RE, Estrella RM, Patiño CL, Zhang Y (2017) Geopolymerization reaction, microstructure and simulation of metakaolin-based geopolymers at extended Si/Al ratios. Cem Concr Compos 79:45–52
Merabtene M, Kacimi L, Clastres P (2019) Elaboration of geopolymer binders from poor kaolin and dam sludge waste. Heliyon 5:e01938
Mahmoodi O, Siad H, Lachemi M, Sahmaran M (2021) Synthesis and optimization of binary systems of brick and concrete wastes geopolymers at ambient environment. Constr Build Mater 276:122217
Khalil MG, Elgabbas F, El-Feky MS, El-Shafie H (2020) Performance of geopolymer mortar cured under ambient temperature. Constr Build Mater 242:118090
Lyu SJ, Hsiao YH, Wang TT, Cheng TW, Ueng TH (2013) Microstructure of geopolymer accounting for associated mechanical characteristics under various stress states. Cem Concr Res 54:199–207
Bernal SA, Provis JL, Rose V, Mejía de Gutierrez R (2011) Evolution of binder structure in sodium silicate-activated slag-metakaolin blends. Cem Concr Compos 33:46–54
Burciaga-Díaz O, Magallanes-Rivera RX, Escalante-García JI (2013) Alkali-activated slag-metakaolin pastes: strength, structural, and microstructural characterization. J Sustain Cem Based Mater 2(2):111–127
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception, mix design, material preparation, data collection, and analysis.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of in performing present investigation.
Rights and permissions
About this article
Cite this article
Mehsas, B., Siline, M. & Zeghichi, L. The effect of using low reactive metakaolin on performances of geopolymer binder. Innov. Infrastruct. Solut. 7, 233 (2022). https://doi.org/10.1007/s41062-022-00833-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41062-022-00833-9