Abstract
Soil amelioration is a challenging task in bulk civil engineering applications such as embankment slopes, landfill liner, pavement subgrade, retaining wall back fill. The conventional chemical stabilization techniques (i.e., cement, calcium hydroxide, sodium chloride, calcium chloride, etc.) inherently suffer from associated carbon emissions during their production stages. With the advent of biopolymers derived from natural sources having low embodied energy levels, they can replace conventional stabilizers. The current review article highlights the significant properties of two such biopolymers, i.e., xanthan gum (XG) and guar gum (GG), and their innate potential in stabilizing different soil types including mine tailings. The issues arising with wet and dry mixing of these biopolymers and suggested measures have been critically addressed. The degradation characteristics of biopolymers, which limit their use for bulk civil engineering applications, have been critically discussed, and the potential solutions to overcome durability issues are suggested. Future applications of these biopolymers in geoenvironmental engineering relying on the metal encapsulation properties are discussed in detail. It is believed that the selected biopolymers in this review are renewable, sustainable and remarkable materials with low embodied energy levels and low carbon footprint values compared to existing conventional stabilizers.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The rapid development of infrastructure promotes the socioeconomic growth of any country. The infrastructure development includes the construction of roads, buildings, thermal power plants, waste management facilities and others, which usually under civil engineering applications. Most of the time, the encountered soils (problematic) may not meet the requirements for these applications necessitating their modification relying on conventional stabilizers including cement, lime, fly ash, etc. [1,2,3,4,5,6,7,8]. However, these conventional stabilizers release a significant amount of CO2 emissions, contributing to increased greenhouse gas emissions [9, 10]. These conventional stabilizers, when mixed with soil, alter the natural groundwater pH hindering the growth of flora and fauna [11]. In order to circumvent the problems associated with these conventional stabilizers, Bio-geo-engineering solutions have emerged and are gaining attention. These bio-geo-engineering solutions include ‘microbial-induced calcium precipitation’ (MICP) and ‘enzyme-induced calcite precipitation (EICP).’ These two novel techniques rely on microbes present in the in situ soil to hydrolyze urea resulting in the precipitation of carbonate ions enhancing the geotechnical properties of soil [12,13,14,15,16]. The bio-engineering solutions together with biopolymers can be applied to improve cohesionless soil properties such as water retention, shear strength, contaminant mitigation and reduce erosion phenomena [17, 18]. However, the effectiveness of EICP/MICP depends on the amount of microbes present and is most suitable for cohesionless soils [19, 20]. To address these issues, researchers have exploited novel natural materials to stabilize the soil [21]. Recent investigations have proposed the use of biopolymers which facilitate the direct application in the field, for problematic soils to improve their engineering properties. The wealth of earlier published research provides insight on various aspects related to production, properties of different biopolymers and their feasibility for limited applications [22]. These applications include, as a stabilizer, thickener and emulsifier in food, textile, pharmaceutical and cosmetic industries [23,24,25]; as drilling fluid in hydraulic fracturing and as an additive in slurry explosives [26, 27]; as a lubricant in petroleum production and mineral extraction [28]; as moisture retainer in agriculture [29, 30]; and as heavy metal removal sorbent [31, 32].
Among these biopolymers, guar gum and xanthan gum have been found suitable to overcome the issues related to soils (cohesive and cohesionless) satisfactorily. Guar solutions are widely used in huge quantities, about 20,000 tons annually as drilling fluid to improve higher production rates in oil and gas recovery wells. However, 10,000 M tons of XG solution is used annually as grouts and plaster, oil well drilling fluids [33].
Background
The word ‘Gum’ represents an adhesive material secreted by plant(s) or other organisms, which readily dissolves in water forming a viscous colloidal solution [34]. Apart from using it as a gluing material, gums have found many applications for food and medicinal purposes over the past few decades due to drastic advancements in concerned fields. Furthermore, these gums undergo physical, chemical and biological changes during their production phase and are commercially termed as ‘biopolymers.’ The name originates due to their production from living species such as plants and other organisms and comprises long chains of polysaccharides [35]. The current review paper specifically deals with XG and GG biopolymer inclusions in enhancing the engineering properties of soils for various applications and highlights the various mechanisms involved. Also, the review summarizes the potential applications of XG and GG in bulk civil engineering applications and the challenges encountered by practicing engineers based on a thorough literature survey.
Biopolymers
The different kinds of biopolymers that are excessively used for various applications include agar gum, beta-glucan, carrageenan, calcium alginate, casein, cellulose, chitosan, dextran, gellan gum, guar gum, sodium alginate, starch, polylysine and xanthan gum [11, 13, 20, 36, 37]. In the present study, due emphasis is laid on only GG and XG due to their practical applicability to bulk civil engineering applications [38].
Guar gum
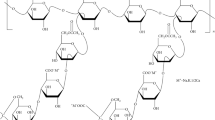
Guar gum or guaran is produced from guar beans with the botanical name Cyamopsis tetragonoloba, a leguminous plant. The NMR (nuclear magnetic resonance) studies of galactomannan revealed that the water-soluble polysaccharide consists of approximately 36.6% of D-galactose and 63.1% of D-mannose anhydrides [39]. The structure of GG (Fig. 1(a)) is a straight chain of mannose units linked via glycosidic bond (α-1 and 4 positions) and on every alternate mannose unit, a single D-galactose unit joined by β (1 and 6 positions) glycoside linkage [34]. The absence of the carboxyl group (COOH−) imparts a neutral charge to GG. It has a high molecular weight of up to 2 × 106 [38]. India produces about 90% of the total GG produced worldwide [27] and exported about 381,880 metric tons of GG to other countries in the last year (2019–2020) [40].
Xanthan gum
Xanthan gum (chemical formula of monomer –C35H49O29) is a natural anionic polysaccharide produced by fermentation of sugars using the bacteria Xanthomonas campestris [41]. It was discovered in 1950s at the United States Department of Agriculture. The in situ production of XG is possible by bacterial fermentation of industrial waste sugars such as sucrose, fructose, etc. [42]. The primary structure of XG comprises repeated units of two glucose units, two mannose units and one glucoronic unit (pentasaccharide units) in the molar ratio of 2.8:2.0:2.0 (Fig. 1b); molecular weight is in the order of 2 million [38]. The microstructural studies revealed that the stiff rod-like helical structure of XG shows insensitivity to temperature, pH, shear, enzyme degradation, possesses high viscosity even at very low concentration and exhibits pseudo-plastic behavior [43]. The carboxyl group (COOH−) present in the chemical structure of XG easily dissociates to form carboxylate (COO−) anion and hydrogen (H+) cation.
Physicochemical properties
In order to use biopolymers for soil stabilization, the properties of gum need to be identified, especially in aqueous medium, which affects the behavior of soil. The final properties of biopolymers (XG or GG) depend on the prevailing conditions maintained during their manufacture. Brief description of these properties is covered in the following sections.
Viscosity
Biopolymers exhibit varying degrees of viscosity due to temperature (dissolution and measurement), pH, the concentration of gum, etc. The pseudo-plastic behavior or shear thinning of GG solution is due to a decrease in apparent viscosity with increase in shear rate. Also, the viscosity value increased with an increase in dissolution temperature till 60–85 °C; later, and it reduced due to breaking or weakening of intermolecular bond [44]. Experimental studies of Nugent et al. [38] and Chen et al. [45] revealed that the zero shear rate viscosity of 2% wt of guar solution is approximately 1200 Pa-second (Pa.s).
In similar lines, XG solutions show shear thinning or pseudo-plastic behavior means viscosity decreases with an increase in shear rate [46]. At a dissolution temperature between 40 and 60 °C, the xanthan solutions exhibit higher viscosity. Salinity influences the viscosity at low polymer concentration [43]. Some studies have reported that the effect of salt is negligible beyond 0.1% (w/v) in xanthan solution [47]. Experimental studies indicate that the zero shear rate viscosity of XG solution at 3% and 4.5% wt is 680 and 1037 Pa.s, respectively [38, 45]. Also, viscosity of the xanthan solution shows stability over a wide range of pH. However, loss of pyruvic acid and acetyl groups causes a slight reduction in viscosity at extreme pH values (< 3 and > 9) [48, 49].
Rate of hydration
‘Hydration’ is a chemical reaction in which the gum molecules react with water to form chemical bonds and hydrate. Since the biopolymers (XG/GG) are hydrophilic, they readily react with water and form numerous hydrogels. The optimal hydration rate of GG is observed at pH 6 ~ 9 and is lowest at 3.5. The presence of salts in the solution decreases or slightly increases the hydration rates of different grades of GG [34]. GG molecules require a minimum of 2 h to complete the hydration process to obtain maximum viscosity [28]. Similarly, XG reacts with water and becomes saturated. The hydration rate depends on the associated functional groups of biopolymer, prevailing temperature, pH of a solution, presence of salts and other compounds that have a higher affinity toward water.
Hydrogen bonding activity and cation bridging
The chemical reaction between functional groups of biopolymer and other molecules results in an ionic or covalent bond. In case of GG, the presence of numerous hydroxyl ions forms a hydrogen bond with hydrated minerals and organic surfaces lead to an increase in degree of linking. In addition, cross-linking of guar hydrogels with Ca2+ ions results in better aggregation [11]. XG being an anionic polysaccharide participates in hydrogen bonding and cation bridging with clay particles [50]. When XG dissolved in water, the carboxyl ion dissociates into carboxylate ion (COO−) and hydrogen ion (H+). The monovalent H+ ion acts as a bridge between COO− and OH− on clay surfaces known as cation bridging. Also, COO− may link with cations present in clay resulting in the clay–polymer network. Hydrogen bonding and electrostatic attraction forces can be observed in clay particles only. Since cohesionless soils carry no charge, these types of nature of bonding are not present [51].
pH
The nonionic behavior of GG solution shows stability over a wide range of pH, i.e., 4–10.5. However, it exhibits acidic nature between pH of 5.5 and 6.1. XG is acidic and exhibits pKa between 4.5 and 5.5 [52]. Reddy et al. [11] carried out experiments on the measurements of pH and turbidity of red mud–biopolymer (XG and GG)-immersed water. Results indicate that the pH reduced with an increase in gum concentration.
Degradation
Degradation of polymer is measured in terms of loss of viscosity and weakening of the intermolecular bond between monomer units when biopolymers are subjected to a wide range of temperature and adverse conditions in the field [13, 53]. According to Gopferich [54], types of degradation are photo, thermal, mechanical and chemical. Since biopolymers comprise hydrogen bonds, they subject to chemical degradation via enzyme-catalyst hydrolysis. Like other natural polymers, GG and XG experience degradation. Thermogravimetric analysis (TGA) results of Zohuriaan and Shokrolahi [55] reported that XG shows thermal stability below 250 °C. Evaluating the CO2 gas evolved by soil microbes during biopolymer breakdown gives details of biodegradation [56]. Since the biopolymers show stability over a wide range of pH, decrease in viscosity is low (Sect. 4.1). Biodegradation studies on cross-linking (with borax and CaCl2) biopolymers (XG and GG) reveals that 15% of CO2 gas is released for a period of 10 weeks, showing faster degradation under wet conditions than dry conditions. Biopolymers with sorbed metals release less CO2 than without sorption [56].
Cross-linking phenomenon
The repeated monomer units in a biopolymer make them reactive and form cross-linking networks with metals, soil particles and other biopolymer with different functional groups. According to Knox et al. [56], cross-linking is a process of joining two or more molecules by ionic or covalent bond by an external agent(s). These agents include borax and calcium chloride, which form an interpenetrating polymer network leading to an increase in the molecular weight of the polymer. GG contains OH− as its functional group which will link to Ca2+ ions of CaCl2 forming cross-linking network and aids in increased bonding. The borate ions link to four hydroxyl groups of two chain molecules of GG forming complex network [57]. Cross-linking results in increased strength, higher resistance to biodegradation, increased heavy metal encapsulation capacities followed by greater stability over a wide range of temperatures and pH values [31, 44].
Surface morphological characteristics
Morphology of biopolymer is essential to study the binding of soil particles with gum molecules. The scanning electron micrographs of GG and XG are presented in Fig. 2, which reveals flaky and continuous morphological texture.
Effect of biopolymer inclusion on the geotechnical properties
Soil treated with either XG or GG has shown remarkable improvement in their geotechnical properties, i.e., consistency limits, compaction characteristics, shear strength, hydraulic conductivity, metal encapsulation capacity, erosion resistivity, durability, moisture retention capacity, etc. These aspects are critically discussed in the following sections, and a brief overview is provided in Table 1.
Consistency limits
The consistency of cohesive soil is usually expressed in terms of liquid limit (wL), plastic limit (wP) and shrinkage limit (wS) and is known as Atterberg’s limits. Earlier investigations have revealed that both wL and PI values increase with an increase in biopolymer content for soils exhibiting different mineralogy (Fig. 3). At higher gum concentrations, the rapid change in viscosity leads to a nonlinear increase in liquid limit for pure kaolinite and red mud waste [11, 38]. When the biopolymer-treated soils are mixed with water, the initiation of cross-linking network of monomers consumes unusually higher moisture contents resulting in increased wL and PI values. Singh and Das [58] reported that for highly plastic silt, the highest PI value is observed at 0.5% XG and later decreased due to the flocculation effect of soil particles as seen from Fig. 3. The presence of salts in pore fluid decreases liquid limit values of biopolymer (XG/GG)-treated kaolinite soils [38]. For clays of high and low plasticity, the linear shrinkage values reduced when treated with GG and XG [9, 58].
Compaction characteristics
The dry density of soil–biopolymer mixture depends on mixing moisture content, nature and type of biopolymer and its concentration, the amount of fines content, the binding mechanism involved and the relative packing of soil particles [51]. As seen from Fig. 4a, b, with the increase in biopolymer dosage (GG/XG), the maximum dry density values (MDD) decreased and the corresponding optimum moisture content (OMC) values were found to increase [59,60,61]. This is attributed to the filling of monomers in the void spaces between the soil particles, which absorb more water, causing a net reduction in the particle interaction resulting in decreased dry density values. These mechanisms are schematically presented in Fig. 5. On the contrary, a few select studies have reported higher MDD values with an increase in biopolymer dosage [51]. For highly plastic silty clay, the MDD values increased up to 1% GG dosage and reduced thereupon up to 2%; OMC values decreased with an increase in gum dosage [62]. For clayey soils, interaction exists between clay and gum molecules through hydrogen bond, which significantly dictates the variation in MDD with OMC [63]. In case of cohesionless soils, biopolymer interaction seems to be indirect as it forms a coating around the individual particles and fills the void spaces between them (Fig. 5).
Shear strength
The shear strength is the resistance offered to the applied shear stress and depends on the cohesion and angle of internal friction of the material (i.e., soil). Unconfined compressive strength (UCS), direct shear test (DST) and triaxial shear tests are used in the laboratory to determine these soil parameters. Unconsolidated undrained triaxial shear tests are preferred over others as the addition of water usually hinders the rate of strength gain for soil–biopolymer mixtures [20]. The addition of biopolymer (GG/XG) under saturated conditions aids in the binding of individual soil grains (cohesive and cohesionless alike) resulting in hard matrix as seen from Fig. 0.5. For cohesive soils, the formation of hydrogen and electrostatic bonds between individual clay particles and hydrogels of biopolymer results in increased shear strength. For cohesionless soils, the increase in the dosage of biopolymer augments cohesive property and enhances the elastic modulus [50].
The nature of bonding between biopolymer (GG/XG) and the soil depends on their respective functional groups, ionic nature [36]. At lower curing periods, gelatinous form of the soil–biopolymer mix does not contribute to any remarkable increase in strength. The shear strength value of red yellow soil at 1% XG content increased to 7240 kPa at 750 days from 6550 kPa at 28 days of curing period due to hardening of soil–biopolymer matrix [51]. The nature and type of curing conditions affect the rate of gain in strength. The independent studies carried on biopolymer (XG/GG)-treated red mud waste and low-compressible silts (ML) showed greater resistance compared to respective untreated case(s) when subjected to outside atmospheric conditions (exposed to daily variations of temperature, rainfall and relative humidity) resulting in twofold increase in UCS values (Fig. 6) [20, 64]. The rate of gain in strength (compressive and tensile) and the mechanism responsible are biopolymer specific. Peat amended with 2% XG showed six times increase in UCS value when compared to untreated case at 28 days of curing [65]. Increase in compressive strength was more significant during first 28 days of curing. At higher curing periods, the increase in strength was found to level off.
DST test results on clays of low plasticity, clays of high plasticity and collapsible soils showed that the inclusion of GG/XG causes an increase in the effective cohesion followed by a reduction in the angle of internal friction values [9, 61]. The phenomenon is more pronounced at higher gum dosage contents. For mine tailings, the surface shear strength and undrained shear strength improved by twofold upon biopolymer treatment (2% GG & 3% XG) [36]. The undrained shear strength of mine tailings increased by three and 13 times with XG and GG treatment compared to untreated cases [45]. Silty sand exhibited higher strength with XG (2%), compared to cement and fly ash (at 7%) treatment [66]. Ni et al. [63] studied the effect of mixing moisture content on biopolymer (XG)-treated shanghai clay and concluded that the ideal amount of water to get optimum strength (UCS) is 30% within the tested range (0–5%). It is preferred to keep the dosages of biopolymers around optimal levels [20]. The optimum dosage of biopolymer for getting ideal strength depends on type of soil, type of biopolymer, their properties and mixing water content employed.
Hydraulic conductivity
The ability of a material to allow water to flow through it is termed as hydraulic conductivity (HC). The HC values usually decrease with an increase in biopolymer dosage values due to filling up of the available pore voids with gum particles (Fig. 5). When biopolymer-treated soils are cured, the development of shrinkage cracks leads to an increase in void spaces, which in turn increases HC values [60, 61, 67]. On the contrary, the HC values of XG-/GG-treated sand–bentonite mixtures were slightly higher compared to untreated case [68]. This is attributed to the thin coating of biopolymer around the sand–bentonite particles leading to higher aggregation, increased particle size resulting in increased pore voids. This increase in interaggregate pores increases HC values. The phenomenon is more pronounced with cationic permeating fluid which reduces the diffused double-layer thickness leading to boarder flow paths [68] (Fig. 7). The reduced hydraulic conductivity property of soil–biopolymers has potential applications for contaminant and seepage barriers, grouting material, in the construction of slurry walls and landfill liners, etc. [62].
Compressibility characteristics
Soil compressibility is the relative ease with which a decrease in soil volume occurs when subjected to an external load, and the process is termed ‘compression’ [69]. At lower concentration of biopolymer (1% GG), the formation of hydrogen bond/cation bridging reduces the compressibility of soil–biopolymer mixture [70]. At higher biopolymer concentrations, the gum strands replace the soil grains (Fig. 5) of low-plastic clays absorbing more water and cause a reduction in the interaction between gum and soil particles resulting in higher compressibility values (Fig. 8) [59]. For kaolinite particles, the electrostatic repulsion reduces the effective stress leading to increased void ratio values [70]. An increase in curing period reduces the compression index values (Cc) [50]. One-dimensional consolidation test results on bentonite revealed 76% reduction in Cc values with 1% XG at 90-day curing period, whereas it was 60% with 1.5% XG for kaolinite at a similar curing period. This is attributed to the hardening of soil–biopolymer links with an increase in curing time.
Collapse potential
Collapsible soils exhibit large volume changes upon saturation without any increase in applied vertical stress [71], and the phenomenon is termed as ‘collapse potential’ (Cp). Ayeldeen et al. [72] reported that Cp values decreased by 13% with 2% addition of biopolymer (XG and GG alike). The biopolymer inclusion significantly reduced the collapse potential (Cp) from ‘severe trouble’ to ‘no problem stage.’ For low-plastic silty clays, Cp value is reduced by 6% and 4% with 2% addition of XG and GG, respectively [61]. This is attributed to the fact that the saturated biopolymer decreases the permeability of soil–biopolymer mixture at outer layers hindering the passage of water from inside of specimen to the outside, causing a net reduction in Cp.
Temperature effect
Differential temperatures encountered in the filed affect the performance of biopolymer-treated soil. The laboratory and field studies conducted on XG-/GG-treated soil showed greater resistance to the prevailing temperatures. Chen et al. [73] conducted laboratory studies to evaluate the drying effect on strength property of XG-treated sand. Increase in curing temperature up to 40 °C increased the UCS value by 150 kPa. In similar lines, Muguda et al. [64] evaluated the performance of XG- and GG-treated soil under prevailing environmental conditions. Continuous evaporation of water increases viscosity and leads to transformation of gelatinous structure to glassy structure resulting in increased adhesive strength of biopolymer-treated soil. However, at elevated temperatures (i.e., > 250 °C), an increase in matric suction results in the formation of hair cracks in soil–biopolymer mixtures causing slight reduction in resultant strength.
Young’s modulus
Young’s modulus quantifies the relationship between applied stress and strain produced within the soil in elastic region. The relative stiffness of compacted soil–biopolymer mixtures is quantified using this parameter. Chang et al. [51] reported that the elastic moduli values of red yellow soil increased by 110 MPa at 750 days with 1% XG compared to 28 days of curing. Soldo et al. [20] reported that the Young’s modulus value of well-graded sand with silt reached an optimum value of 236 MPa at 1% GG and 264 MPa at 2% XG compared to untreated soil of 88 MPa.
California bearing ratio (CBR) and resilient modulus
CBR and resilient modulus are the key parameters to assess the effectiveness of soil stabilizer for pavement applications [37]. Lee et al. [66] have proposed indirect correlations between UCS and CBR for inorganic silty sand treated with 2% XG. The mixture fulfilled the IRC (Indian Road Congress) design criteria requirements for both shoulder and a pavement application as the obtained UCS value was 4.5 MPa. Another study carried out by Elkafoury and Azzam [74] revealed that CBR characteristics of poorly graded sand increased by three times when treated with 0.9% XG.
Metal encapsulation capacity
Encapsulation of toxic metals by impervious/nonreactive materials is one of the most sought-after waste disposal techniques. Soils treated with biopolymer (XG/GG) have shown better metal encapsulation abilities with relatively low leachability. Experimental studies revealed that the copper ions get adsorbed on sand treated with biopolymer (XG/GG) and show better retention abilities even in the presence of aggressive chelants like 5% HCl solution [31]. Cross-linked biopolymers (XG linked with GG) possess higher sorption capacity and are quite effective in immobilizing metals (up to 90%) such as arsenic, lead, nickel, chromium, copper, cobalt, zinc, etc. [56]. Since the biopolymer(s) are naturally degradable materials, combining two or more biopolymers has shown better resistance to aggressive conditions encountered in the field. This characteristic feature of biopolymer-treated soils aids their applicability for landfill liner applications.
Durability studies
Biopolymers absorb more amount of water than natural soil(s) due to their hydrophilic nature. Both XG and GG are found effective in reducing moisture loss from soils. The long-chain polymer network of biopolymer absorbs adequate water due to hydrogen bonding forming a thin coating around the soil particles improving their binding ability [75]. When mine tailings were amended with GG and XG (at 1.6%), the erosion rates reduced by 15 and 10 times, respectively, at the end of five cycles of wetting and drying [36]. The moisture retention capacity is directly proportional to the dosage of biopolymers. Experimental studies on soft clayey soils treated with GG in the presence of lime and polyester revealed that UCS values remained unchanged when the samples were subjected to 10 freeze–thaw cycles at the end of 150-day curing period [21]. Highly plastic silts when amended with 1% XG exhibited better moisture retention levels with 12 freeze–thaw cycles [58]. The ability of XG/GG biopolymer-treated soils in resisting weathering action ascertains their use for erosion control applications along slopes.
Mineral composition and surface morphological characteristics
X-ray diffraction studies indicate that the addition of biopolymer to soil enhances the binding and aggregation of soil particles without changing its mineralogical composition [11]. Scanning electron microscopy studies have revealed that the biopolymer forms a thin coating around the coarse-grained soils enabling their physical aggregation. For fine-grained soils, direct electrostatic bonding (hydrogen bonding followed by cation bridging) between individual clay particles and biopolymers is confirmed by forming soil lumps with pellet-like structures [20, 61, 63]. The electrostatic bonding results in reduced specific surface area values due to dense soil–biopolymer fabrics formed at higher curing periods [59].
Practical and economic feasibility of biopolymer
Cement and lime have been used as effective soil stabilizers, but their production contributes to huge quantities of CO2 gas emissions. One ton of cement production is responsible for 0.5t of CO2 gas [76]. Global clinker-to-cement ratio increased on an average value of 1.6% per year from 2014 to 18 and led to an increase in CO2 intensity at 0.5% per annum. Therefore, to reduce the detrimental effects of CO2 on the environment, clinker production should be reduced or alternate binding materials have to be introduced with innovative technologies [76]. Several researchers highlighted that cement in soil stabilization creates an impervious barrier at the subsurface [77, 78]. This affects the effective infiltration rate of water and increases surface water runoff which in turn reduces groundwater recharge near the subsurface. The heat island effect due to concrete- or cement-based structures (e.g.: pavements) causes an adverse impact on the environment and climate change. Furthermore, the elevated pH levels due to the hydration of cement/lime affect the growth of vegetation. Also, at the service end life, the recycling/permanent disposal of cement-/lime-treated soil(s) is difficult and creates a great ecological imbalance. These issues associated with cement and lime can be circumvented using natural biopolymers as the inherent CO2 emissions are relatively less compared to cement/lime [13]. Since biopolymers are naturally produced materials, they are nontoxic [70], environmentally friendly and do not contribute to greenhouse gases. The hydration process of biopolymer involves absorption of more water; hence, these are widely used in agricultural applications as a moisture retainer. At the end of their service period, the natural biopolymers degrade and do not cause any changes in the subsurface layers of the earth [11].
The cost analysis reveals that XG costs 3.6% more than cement for soil stabilization applications as per carbon trade exchange [13]. Biopolymer of superior grade/quality is used for food and medicinal applications, and hence the associated prices are a bit higher [60]. In addition to this, the lack of additional production facilities and varying standards across the world are the major factors affecting the price of a biopolymer. However, the market trend of biopolymers is expected to increase from 484.7 kilotons in 2017 to 984.8 kilotons by 2022, showing a compound annual growth rate of 15.2%, and is expected to reduce the global biopolymer prices [79].
Challenges/limitations in field applications
The limitations and challenges associated with biopolymers stem from their biodegradation and selection of mixing methods for various field applications. The majority of natural polymers are subjected to biodegradation due to the weakening of intermolecular bond strength between the monomer units. The biodegradation of biopolymer(s) depends on intermolecular bonding of monomer units, type of native bacteria present, prevailing temperature, relative humidity and pH conditions [53, 80]. The rate of degradation in the field varies from hours to years, and the usual final products produced after degradation are carbon dioxide, water and a small amount of ammonia [81]. The long-term performance should be investigated simulating aggressive environmental conditions before proceeding to field application. Combining two or more biopolymers has shown better degradation resistance due to enhanced interlocking of monomers [31]. Also, the selection of the right mixing method for specific field application is a decisive factor. Dry mixing is preferred if the prevailing field conditions (due to extreme heat, relative humidity) do not favor the biopolymer solution preparation [66] and the dosages of biopolymers required are relatively high. Wet mixing involves adding the biopolymer to water (at dissolution temperature) to obtain a uniform solution of the right consistency and is usually preferred to achieve optimum performance of biopolymer. This method is usually employed as long as the biopolymer dosage is within the solubility range. Moreover, due care should be taken to keep the hydrogels in suspension, which are a prime source in triggering cross-linking monomer chains with soil particles.
Conclusions
The current review describes the effective application of guar gum and xanthan gum biopolymers to improve various soil properties. It is observed that the selection of a biopolymer for a given specific application depends on soil type, gradation, fines content, type and properties of biopolymer, prevailing field conditions.
-
The physicochemical properties of biopolymer(s) such as viscosity, hydration rate, hydrogen bonding and cation bridging, cross-linking ability, surface characteristics have significant effect on the final properties of soil–biopolymer mixtures.
-
Well-graded soils respond better to biopolymer treatment compared to poorly graded soils. For medium- to high-plastic clays, GG treatment showed moderate improvement in shear strength and effectively mitigated desiccation cracking. For sand and silty sand, XG improves the shear strength, permeability properties significantly.
-
Experimental studies have shown that fine soils exhibit a better strengthening effect compared to coarse soils.
-
The dosage of biopolymer depends on the nature and type of soil. Majority of soils have shown remarkable improvement in their geotechnical properties when dosage of XG is kept at 1–3% and 1–2% for GG. Within these specified ranges:
-
2–4 times increase in shear strength for cohesive soils and 6–8 times for cohesionless soils are observed.
-
10–100 times reduction in permeability for cohesive soils and 1000 times for cohesionless soils are noticed.
-
For cohesive soils, dry density values reduced by 1–2 kN/m3 and the proportionate increase in OMC values is within 2–6%.
-
-
The ability of XG-/GG-treated soils in resisting weathering action facilitates their use for erosion control applications along slopes.
-
The degradation characteristics of biopolymer(s) depending on chemical structure and prevailing field conditions limit their applicability for a majority of field applications. In order to circumvent this problem, it is suggested to combine two or more biopolymers in the presence of cross-linking agents like borax and calcium chloride which will increase their resistance under aggressive field conditions.
Biopolymers are renewable, sustainable and remarkable materials with low embodied energy levels with very low carbon footprint values compared to existing conventional stabilizers. At the end of service period, the CO2 released due to biopolymer degradation is usually reabsorbed by surrounding flora and fauna and hence remains carbon neutral.
References
Eades JL, Grim RE (1960) Reaction of hydrated lime with pure clay minerals in soil stabilization. Highw Res Board Bull 262:51–63
Ramana Murty V, Hari Krishna P (2007) Amelioration of expansive clay slopes using calcium chloride. J Mater Civ Eng 19:19–25. https://doi.org/10.1061/(ASCE)0899-1561(2007)19:1(19)
Moghal A, Sivapullaiah P (2012) Role of lime leachability on the geotechnical behavior of fly ashes. Int J Geotech Eng 6:43–51. https://doi.org/10.3328/IJGE.2012.06.01.43-51
Elkady TY (2016) The effect of curing conditions on the unconfined compression strength of lime-treated expansive soils. Road Mater Pavement Des 17:52–69. https://doi.org/10.1080/14680629.2015.1062409
Moghal AAB (2017) State-of-the-art review on the role of fly ashes in geotechnical and geoenvironmental applications. J Mater Civ Eng 29:04017072. https://doi.org/10.1061/(ASCE)MT.1943-5533.0001897
Mahedi M, Cetin B, White DJ (2018) Performance evaluation of cement and slag stabilized expansive soils. Transp Res Rec 2672:164–173. https://doi.org/10.1177/0361198118757439
Moghal AAB, Rehman AU, Vydehi KV, Umer U (2020) Sustainable perspective of low-lime stabilized fly ashes for geotechnical applications: promethee-based optimization approach. Sustainability 12:6649. https://doi.org/10.3390/su12166649
Ghorbani A, Hasanzadehshooiili H, Mohammadi M et al (2019) Effect of selected nanospheres on the mechanical strength of lime-stabilized high-plasticity clay soils. Adv Civ Eng 2019:1–11. https://doi.org/10.1155/2019/4257530
Acharya R, Pedarla A, Bheemasetti TV, Puppala AJ (2017) Assessment of guar gum biopolymer treatment toward mitigation of desiccation cracking on slopes built with expansive soils. Transp Res Rec 2657:78–88. https://doi.org/10.3141/2657-09
Andrew R (2019) Global CO2 emissions from cement production, 1928–2019. Earth Syst Sci Data 11:1675–1710
Reddy NG, Rao BH, Reddy KR (2018) Biopolymer amendment for mitigating dispersive characteristics of red mud waste. Géotech Lett 8:201–207. https://doi.org/10.1680/jgele.18.00033
Whiffin VS, van Paassen LA, Harkes MP (2007) Microbial carbonate precipitation as a soil improvement technique. Geomicrobiol J 24:417–423. https://doi.org/10.1080/01490450701436505
Chang I, Im J, Cho G-C (2016) Introduction of microbial biopolymers in soil treatment for future environmentally-friendly and sustainable geotechnical engineering. Sustainability 8:251. https://doi.org/10.3390/su8030251
Chittoori BCS, Rahman T, Burbank M, Moghal AAB (2019) Evaluating Shallow Mixing Protocols as Application Methods for Microbial Induced Calcite Precipitation Targeting Expansive Soil Treatment. Geo-Congress 2019. American Society of Civil Engineers, Philadelphia, Pennsylvania, pp 250–259
Moghal AAB, Lateef MA, Mohammed SAS et al (2020) Efficacy of enzymatically induced calcium carbonate precipitation in the retention of heavy metal ions. Sustainability 12:7019. https://doi.org/10.3390/su12177019
Moghal AAB, Lateef MA, Abu Sayeed Mohammed S et al (2020) Heavy metal immobilization studies and enhancement in geotechnical properties of cohesive soils by EICP technique. Appl Sci 10:7568. https://doi.org/10.3390/app10217568
Zhao Z, Hamdan N, Shen L et al (2016) Biomimetic Hydrogel Composites for Soil Stabilization and Contaminant Mitigation. Environ Sci Technol 50:12401–12410. https://doi.org/10.1021/acs.est.6b01285
Almajed A, Lemboye K, Arab MG, Alnuaim A (2020) Mitigating wind erosion of sand using biopolymer-assisted EICP technique. Soils Found 60:356–371. https://doi.org/10.1016/j.sandf.2020.02.011
Ivanov V, Chu J (2008) Applications of microorganisms to geotechnical engineering for bioclogging and biocementation of soil in situ. Rev Environ Sci Biotechnol 7:139–153. https://doi.org/10.1007/s11157-007-9126-3
Soldo A, Miletić M, Auad ML (2020) Biopolymers as a sustainable solution for the enhancement of soil mechanical properties. Sci Rep 10:267. https://doi.org/10.1038/s41598-019-57135-x
Arasan S, Bagherinia M, Akbulut RK, Zaimoglu AS (2017) Utilization of polymers to improve soft clayey soils using the deep mixing method. Environ Eng Geosci 23:1–12. https://doi.org/10.2113/gseegeosci.23.1.1
Chang I, Lee M, Tran ATP et al (2020) Review on biopolymer-based soil treatment (BPST) technology in geotechnical engineering practices. Transportation Geotechnics 24:100385. https://doi.org/10.1016/j.trgeo.2020.100385
Sutton RL, Wilcox J (1998) Recrystallization in ice cream as affected by stabilizers. J Food Sci 63:104–107. https://doi.org/10.1111/j.1365-2621.1998.tb15686.x
Raina CS, Singh S, Bawa AS, Saxena DC (2005) Textural characteristics of pasta made from rice flour supplemented with proteins and hydrocOLLOIDS. J Texture Stud 36:402–420. https://doi.org/10.1111/j.1745-4603.2005.00024.x
Prabaharan M (2011) Prospective of guar gum and its derivatives as controlled drug delivery systems. Int J Biol Macromol 49:117–124. https://doi.org/10.1016/j.ijbiomac.2011.04.022
Rosalam S, England R (2006) Review of xanthan gum production from unmodified starches by Xanthomonas comprestris sp. Enzym Microb Technol 39:197–207. https://doi.org/10.1016/j.enzmictec.2005.10.019
Mudgil D, Barak S, Khatkar BS (2014) Guar gum: processing, properties and food applications—A Review. J Food Sci Technol 51:409–418. https://doi.org/10.1007/s13197-011-0522-x
Singh AV, Singh R (2010) Synthesis, characterization and rheological properties of guaran grafted polystyrene (g-g-ps) copolymer. J Eng 3:47–51
Thombare N, Jha U, Mishra S, Siddiqui MZ (2016) Guar gum as a promising starting material for diverse applications: a review. Int J Biol Macromol 88:361–372. https://doi.org/10.1016/j.ijbiomac.2016.04.001
Thombare N, Mishra S, Siddiqui MZ et al (2018) Design and development of guar gum based novel, superabsorbent and moisture retaining hydrogels for agricultural applications. Carbohyd Polym 185:169–178. https://doi.org/10.1016/j.carbpol.2018.01.018
Etemadi O, Petrisor IG, Kim D et al (2003) Stabilization of metals in subsurface by biopolymers: laboratory drainage flow studies. Soil Sedim Contam: Int J 12:647–661. https://doi.org/10.1080/714037712
Singh V, Kumari P, Pandey S, Narayan T (2009) Removal of chromium (VI) using poly(methylacrylate) functionalized guar gum. Biores Technol 100:1977–1982. https://doi.org/10.1016/j.biortech.2008.10.034
Plank J (2005) Applications of Biopolymers in Construction Engineering. In: Biopolymers Online. American Cancer Society, Online; Wiley-VCH Verlag GmbH & Co. KGaA:Weinheim, Germany, /https://doi.org/10.1002/3527600035.bpola002
Chudzikowski RJ Guar gum and its applications. Journal of the Society of Cosmetic Chemists 22:43–60
Yen TF, Yang ICY, Karimi S, Martin GR (1996) Biopolymers for Geotechnical Applications. ASCE, pp 1602–1607
Chen R, Lee I, Zhang L (2015) Biopolymer stabilization of mine tailings for dust control. J Geotech Geoenviron Eng 141:04014100. https://doi.org/10.1061/(ASCE)GT.1943-5606.0001240
Arab MG, Mousa RA, Gabr AR et al (2019) Resilient behavior of sodium alginate-treated cohesive soils for pavement applications. J Mater Civ Eng 31:04018361. https://doi.org/10.1061/(ASCE)MT.1943-5533.0002565
Nugent RA, Zhang G, Gambrell RP (2009) Effect of exopolymers on the liquid limit of clays and its engineering implications. Transp Res Rec 2101:34–43. https://doi.org/10.3141/2101-05
Grasdalen H, Painter T (1980) N.M.R. studies of composition and sequence in legume-seed galactomannans. Carbohyd Res 81:59–66. https://doi.org/10.1016/S0008-6215(00)85677-3
ReportList. http://agriexchange.apeda.gov.in/indexp/reportlist.aspx. Accessed 30 Dec 2020
Katzbauer B (1998) Properties and applications of xanthan gum. Polym Degrad Stab 59:81–84. https://doi.org/10.1016/S0141-3910(97)00180-8
Karimi S (1998) A Study of Geotechnical Applications of Biopolymer Treated Soils with an Emphasis on Silt. PhD thesis, University of Southern California
Garcı́a-OchoaSantosCasasGómez FVEJAE (2000) Xanthan gum: production, recovery, and properties. Biotechnol Adv 18:549–579. https://doi.org/10.1016/S0734-9750(00)00050-1
Casas J, Mohedano A, Garcıa-Ochoa F (2000) Viscosity of guar gum and xanthan/guar gum mixture solutions. J Sci Food Agric 8(12):1722–1727
Chen R, Zhang L, Budhu M (2013) Biopolymer stabilization of mine tailings. J Geotech Geoenviron Eng 139:1802–1807. https://doi.org/10.1061/(ASCE)GT.1943-5606.0000902
Milas M, Rinaudo M, Tinland B (1985) The viscosity dependence on concentration, molecular weight and shear rate of xanthan solutions. Polym Bull 14:157–164. https://doi.org/10.1007/BF00708475
Kang KS, Pettitt D (1993) XANTHAN, GELLAN, WELAN, AND RHAMSAN In: Whistler RL, BeMiller JN, editors. Industrial gums. New York: Academic Press, 341–98. https://doi.org/https://doi.org/10.1016/B978-0-08-092654-4.50017-6
Bradshaw IJ, Nisbet BA, Kerr MH, Sutherland IW (1983) Modified xanthan—its preparation and viscosity. Carbohyd Polym 3:23–38. https://doi.org/10.1016/0144-8617(83)90010-3
Tako M, Nakamura S (1984) Rheological properties of deacetylated xanthan in aqueous media. Agric Biol Chem 48:2987–2993. https://doi.org/10.1271/bbb1961.48.2987
Latifi N, Horpibulsuk S, Meehan CL et al (2017) Improvement of problematic soils with biopolymer—an environmentally friendly soil stabilizer. J Mater Civ Eng 29:04016204. https://doi.org/10.1061/(ASCE)MT.1943-5533.0001706
Chang I, Im J, Prasidhi AK, Cho G-C (2015) Effects of Xanthan gum biopolymer on soil strengthening. Constr Build Mater 74:65–72. https://doi.org/10.1016/j.conbuildmat.2014.10.026
Welch SA, Barker WW, Banfield JF (1999) Microbial extracellular polysaccharides and plagioclase dissolution. Geochim Cosmochim Acta 63:1405–1419. https://doi.org/10.1016/S0016-7037(99)00031-9
Hou CT, Barnabe N, Greaney K (1986) Biodegradation of xanthan by salt-tolerant aerobic microorganisms. J Ind Microbiol 1:31–37. https://doi.org/10.1007/BF01569414
Gijpferich A (1996) Mechanisms of polymer degradation and erosion. 17:12 Biomaterials, 17(2): 103–114. https://doi.org/10.1016/0142-9612(96)85755-3
Zohuriaan MJ, Shokrolahi F (2004) Thermal studies on natural and modified gums. Polym Testing 23:575–579. https://doi.org/10.1016/j.polymertesting.2003.11.001
Knox AS, Petrisor IG, Turick CE, et al (2010) Life Span of Biopolymer Sequestering Agents for Contaminant Removal and Erosion Resistance. In: Elnashar M (ed) Biopolymers. Sciyo 81–108
Wang S, Tang H, Guo J, Wang K (2016) Effect of pH on the rheological properties of borate crosslinked hydroxypropyl guar gum hydrogel and hydroxypropyl guar gum. Carbohydr Polym 147:455–463. https://doi.org/10.1016/j.carbpol.2016.04.029
Singh SP, Das R (2020) Geo-engineering properties of expansive soil treated with xanthan gum biopolymer. Geomechan Geoeng 15:107–122. https://doi.org/10.1080/17486025.2019.1632495
Cabalar AF, Awraheem MH, Khalaf MM (2018) Geotechnical properties of a low-plasticity clay with biopolymer. J Mater Civ Eng 30:04018170. https://doi.org/10.1061/(ASCE)MT.1943-5533.0002380
Ayeldeen MK, Negm AM, El Sawwaf MA (2016) Evaluating the physical characteristics of biopolymer/soil mixtures. Arab J Geosci 9:371. https://doi.org/10.1007/s12517-016-2366-1
Dehghan H, Tabarsa A, Latifi N, Bagheri Y (2019) Use of xanthan and guar gums in soil strengthening. Clean Techn Environ Policy 21:155–165. https://doi.org/10.1007/s10098-018-1625-0
Sujatha ER, Saisree S (2019) Geotechnical behaviour of guar gum-treated soil. Soils Found 59:2155–2166. https://doi.org/10.1016/j.sandf.2019.11.012
Ni J, Li S-S, Ma L, Geng X-Y (2020) Performance of soils enhanced with eco-friendly biopolymers in unconfined compression strength tests and fatigue loading tests. Constr Build Mater 263:120039. https://doi.org/10.1016/j.conbuildmat.2020.120039
Muguda S, Booth SJ, Hughes PN et al (2017) Mechanical properties of biopolymer-stabilised soil-based construction materials. Géotech Lett 7:309–314. https://doi.org/10.1680/jgele.17.00081
Latifi N, Horpibulsuk S, Meehan CL et al (2016) Xanthan gum biopolymer: an eco-friendly additive for stabilization of tropical organic peat. Environ Earth Sci 75:825. https://doi.org/10.1007/s12665-016-5643-0
Lee S, Chung M, Park HM et al (2019) Xanthan gum biopolymer as soil-stabilization binder for road construction using local soil in Sri Lanka. J Mater Civ Eng 31:06019012. https://doi.org/10.1061/(ASCE)MT.1943-5533.0002909
Cabalar AF, Wiszniewski M, Skutnik Z (2017) Effects of xanthan gum biopolymer on the permeability, odometer, unconfined compressive and triaxial shear behavior of a sand. Soil Mech Found Eng 54:356–361. https://doi.org/10.1007/s11204-017-9481-1
Biju MS, Arnepalli DN (2020) Effect of biopolymers on permeability of sand-bentonite mixtures. J Rock Mechan Geotech Eng 12:1093–1102. https://doi.org/10.1016/j.jrmge.2020.02.004
Chittoori BCS, Moghal AAB, Pedarla A, Al-Mahbashi AM (2017) Effect of unit weight on porosity and consolidation characteristics of expansive clays. J Test Eval 45:20160451. https://doi.org/10.1520/JTE20160451
Nugent RA, Zhang G, Gambrell RP (2011) The Effect of Exopolymers on the Compressibility of Clays. Geo-Frontiers 2011. American Society of Civil Engineers, Dallas, Texas, United States, pp 3935–3944
Mansour ZM, Chik Z, Taha MR (2008) On the procedures of soil collapse potential evaluation. J Appl Sci 8:4434–4439. https://doi.org/10.3923/jas.2008.4434.4439
Ayeldeen M, Negm A, El-Sawwaf M, Kitazume M (2017) Enhancing mechanical behaviors of collapsible soil using two biopolymers. J Rock Mechan Geotech Eng 9:329–339. https://doi.org/10.1016/j.jrmge.2016.11.007
Chen C, Wu L, Perdjon M et al (2019) The drying effect on xanthan gum biopolymer treated sandy soil shear strength. Constr Build Mater 197:271–279. https://doi.org/10.1016/j.conbuildmat.2018.11.120
Elkafoury A, Azzam W (2021) Utilize Xanthan gum for enhancing CBR value of used cooking oil-contaminated fine sand subgrade soil for pavement structures. Innov Infrastruct Solut 6:25. https://doi.org/10.1007/s41062-020-00389-6
Khayat KH (1998) Viscosity-enhancing admixtures for cement-based materials—An overview. Cement Concr Compos 20:171–188. https://doi.org/10.1016/S0958-9465(98)80006-1
Cement – Analysis. In: IEA. https://www.iea.org/reports/cement. Accessed 30 Dec 2020
Bellezza I, Fratalocchi E (2006) Effectiveness of cement on hydraulic conductivity of compacted soil–cement mixtures. Proc Inst Civ Eng Ground Improvem 10:77–90. https://doi.org/10.1680/grim.2006.10.2.77
Deng Y, Yue X, Liu S et al (2015) Hydraulic conductivity of cement-stabilized marine clay with metakaolin and its correlation with pore size distribution. Eng Geol 193:146–152. https://doi.org/10.1016/j.enggeo.2015.04.018
Global Market for Biodegradable Polymers to Obtain 15.2% CAGR by 2022. https://www.bccresearch.com/pressroom/pls/global-market-for-biodegradable-polymers-to-obtain-152-cagr-by-2022. Accessed 30 Dec 2020
Chandra R, Rustgi R (1998) BIODEGRADABLE POLYMERS. Elsevier Science Ltd., pp 1273–1335
Karlsson S, Albertsson A (1998) Biodegradable polymers and environmental interaction. Polym Eng Sci 38:1251–1253. https://doi.org/10.1002/pen.10294
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Rights and permissions
About this article
Cite this article
Moghal, A.A.B., Vydehi, K.V. State-of-the-art review on efficacy of xanthan gum and guar gum inclusion on the engineering behavior of soils. Innov. Infrastruct. Solut. 6, 108 (2021). https://doi.org/10.1007/s41062-021-00462-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41062-021-00462-8