Abstract
Thiophene-based π-conjugated organic small molecules and polymers are the research subject of significant current interest owing to their potential use as organic semiconductors in material chemistry. Despite simple and similar molecular structures, the hitherto reported properties of thiophene-based organic semiconductors are rather diverse. Design of high performance organic semiconducting materials requires a thorough understanding of inter- and intra-molecular interactions, solid-state packing, and the influence of both factors on the charge carrier transport. In this chapter, thiophene-based organic semiconductors, which are classified in terms of their chemical structures and their structure–property relationships, are addressed for the potential applications as organic photovoltaics (OPVs), organic field-effect transistors (OFETs) and organic light emitting diodes (OLEDs).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Organic semiconductors (OSCs) are organic materials with the ability to conduct electrons, and their electronic conductivities lay between that of metals and insulators, spanning a broad range from 10−9 to 103 Ω−1 cm−1. Discovery of the photoconduction of solid anthracene in 1906 is the first study involving the conductivity of organic compounds [1]. Development of new high performance OSCs, with perfect potential in plastic electronics applications, including organic light-emitting diodes (OLEDs), organic field-effect transistors (OFETs), sensors or organic photovoltaic cells (OPVs), has been one of the most active research fields in last decade [2,3,4]. Synthetic flexibility, low production cost, large area application, easy processibility through attached solubilizing side chains, and the possibility of an exact tailoring of the electronic and mechanical properties to achieve the desired functions make semiconducting organic oligomers and polymers attractive candidates for future applications in electronic devices [5,6,7]. Moreover, the physical properties such as optical behavior, charge carrier mobilities, highest occupied molecular orbital (HOMO)/lowest unoccupied molecular orbital (LUMO) energy levels, and structural arrangements of these organic semiconductors can be tuned by various chemical functionalizations.
Thiophenes are one of the most essential classes of heterocyclic compounds in the field of material chemistry. Thiophene-based materials are beneficial due to their semiconducting natures, nonlinear optical responses and effective electron transport properties [8]. Oligomers and polymers based on thiophenes have attracted considerable attention both experimentally and theoretically. Their well-defined physicochemical relationships provide outstanding synthetic control of their molecular electronic structures through control of the thiophene chain length, and substituent variety can be used to tune the molecular orbital energies, charge carrier mobilities, and spectral properties of the individual molecular species [9,10,11]. In addition, a number of thiophene-based fused systems can be constructed and utilized in the field of semiconducting materials. In this chapter, basic research studies on thiophene-based small molecules and polymers and their applications as organic semiconductors are focused.
2 Thiophene
Thiophene chemistry has been attracting considerable interest for a long time now, which is mainly due to its applicability toward tuning the optical and electronic properties of the desired organic materials through easy variations in the molecular structure. Thiophene, a flat sulfur-embedded, aromatic, five-membered ring, represents one of the most investigated heterocyclic compounds, displaying interesting electronic properties. Thiophene sulfur has a formal oxidation state and two lone-pair electrons, one of which participates in ring aromatization. The sulfur atom has a high polarizability due to the outer-shell electrons orbiting in the large 3s and 3p orbitals, which facilitate its electron donating properties and make it available for nonbonding interactions. Moreover, sulfur can have extended octet upon contribution of its 3d orbitals [12, 13]. Hypervalency and extended octet property enables the functionalization of thiophene sulfur with oxygen atoms, as in oligo- and polythiophene-S,S-dioxides, which display peculiar electrical and optical properties [14, 15].
Thiophene-based oligomers and polymers are described by their conformations produced by the rotations around the single bond linking two adjacent thienyl rings (Fig. 1). Moreover, multiple short intermolecular S···S nonbonding interactions bring about highly aligned molecular arrays in the solid state, possessing a high charge carrier mobility along π-stacking direction. When thiophenes are linked together via C2 and C5 positions, an extended delocalization is achieved, in which the electron-rich thiophene rings are conjugated together exhibiting a coplanar conformation (Fig. 1). Quinoid resonance structure of thiophene-based conjugated oligomers and polymers leads to an increase in rigidification and planarization of the main chain owing to the significant restriction of the rotation between the rings, thus, quinoid form exhibit a higher energy and a lower band gap (E g ) compared to its corresponding aromatic form [16].
The fascinating versatility of thiophene-based π-conjugated molecules allows numerous possibilities, such as ring functionalization and main chain elongation (Fig. 2). While a few rings form monodisperse oligomers, a large number of rings are used to construct polydisperse polymers. In addition, thiophenes can be selectively modified at α- and β-positions and even at sulfur atoms to establish useful structures. For example, incorporation of α-linked thiophene units into oligomers renders an efficient conjugation along the main chain, while the attachment of long alkyl side chains at the α- or β-position enhances the solubility; as a result, it increases solution processability and self-assembly capability. Moreover, deformation of bond and angle of thiophene ring takes place easily, owing to the bonded and lone pair electrons of high polarizable sulfur and the long C–S bonds [17, 18].
The electronic properties of thiophene-based oligomers and polymers depend on the degree of π-conjugation, which affects frontier molecular orbital (FMO) energies, energy gaps, optical, electrochemical, and electrical properties. Among many promising organic semiconductors used for electronic applications, thiophene-based materials exhibit an irreplaceable position due to their conductivities and optical properties [19,20,21]. Tuning HOMO/LUMO energy levels and band gaps of thiophene-based functional π-conjugated systems has been placed in the center of the synthetic chemistry. Also, band gaps play a dominant role in determining the physical properties of the conducting molecules; more research has been devoted to the synthesis by considering the structure and function relations of these materials.
In general, one of the strategies for fine-tuning the HOMO–LUMO gaps of organic electronic materials involves the utilization of thiophene subunits [22] and particularly those rigid structures possessing an extended π-conjugation in the ground state [23], such as thienothiophenes (TTs) and dithienothiophenes (DTTs). Thienoacenes (TAcs) formed by fusing thiophenes together are known to be effective units for increasing the intermolecular interactions and adjusting the band gap of organic materials in solid state [23].
3 Thiophene-Based Small Molecules/Oligomers As Organic Semiconductors
Small conjugated molecules and well-defined oligomers with distinct molecular structures and controllable purity alleviate the lack of chemical and structural characterization of conjugated polymers [24, 25]. Therefore, the use of well-characterized oligomers and reproducible processing methods improved understanding of structure–property relationships [26]. The introduction of electron-donating or -withdrawing building blocks onto thiophenes or oligothiophenes is the most straightforward way to construct new oligomers with well-defined structures and tunable FMOs. Thus, thiophene-based oligomers have been considered to be encouraging organic semiconducting materials due to their proper syntheses, excellent transport properties, high polarizabilities, as well as their tunable optical and electrochemical properties [22].
α-Linked oligothiophenes (Th n , n is the number of thiophene units), which are among the most well-known p-type π-conjugated materials, have been used as the core components in various optoelectronic and electronic devices such as OPVs, OLEDs, and OFETs, owing to their light-emitting and absorbing, hole transporting, and charge carrier properties (Fig. 3) [27,28,29]. Furthermore, they have multiple intermolecular interactions through electropositivite sulfur atoms. Single crystal X-ray diffraction and structure analysis have indicated that the self-assembly approaches of oligothiophenes are directed by van der Waals interactions, π–π stacking, and a variety of anisotropic oriented donor (D)–acceptor (A) intra- and intermolecular C–H···S, C–H···O, C–H···π, and S···S interactions [30].
Oligothiophenes have been widely implemented for the production of organic semiconductor building blocks in OPVs. Among these building blocks, fused-thiophenes, such as TT, DTT, and tetrathienoacenes (TTA) have been indicated to be attractive π-bridge cores due to their strong intermolecular S···S interactions, pronounced intermolecular π–π stackings and extensive intramolecular π-conjugation [23, 31]. In addition, thiophene-based π-conjugated oligomers, star-shaped [32], X-shaped [33] and dendritic [34] oligothiophenes have been the motivation of recent research [22, 23] as promising future organic semiconductors. Unsubstituted oligothiophenes have been investigated comprehensively as they mostly act as p-type (hole-transporting) materials [35, 36], whereas n-type (electron-transporting) thiophene-based semiconductors are less studied, but they are certainly required for the production of bipolar transistors, as they may display a relevance in the development of photovoltaic cells [37].
3.1 Organic Photovoltaics
Organic photovoltaics (OPVs) have earned remarkable research interest as a new category of organic semiconductor materials, having the potential for realizing flexible, light weight, easily-processible, and low-cost solar energy sources. Organic photovoltaic device structures can be created with two type fundamental architectures, namely, bilayer structure and bulk heterojunction (BHJ). The device performance of a photovoltaic cell is defined by a short circuit current (J SC) related to the photoinduced charge transport density, the charge carrier mobility within the OSCs, the open-circuit voltage (V OC) depending on the energy difference between the HOMO of the donor and the LUMO of the acceptor units, the fill factor (FF) correlates with the charge carriers, attaining the electrodes and the device quality. Typical organic photovoltaic cells consist of a donor (p-type semiconductor) and an acceptor (n-type semiconductor) as an active layer. In view of these points, thiophene-based π-conjugated oligomers and their related compounds as donor materials have been widely examined as active semiconducting materials in organic photovoltaics [33, 38].
Concerning the improvement of device performance of OPVs, thiophene-based conjugated oligomers with low band gap, broad absorption and suitable energy levels are necessary. The introduction of electron-efficient (as an acceptor, A) or -rich (as a donor, D) building blocks to thiophenes or oligothiophenes leads to the oligomers with well-defined structures and tunable FOMOs. Moreover, D–A conjugated oligomeric systems can behave as perfect donor components in OPVs due to their unique features such as synthetically reproducibilities, strong intermolecular interactions, light-absorbing regions, and both vacuum-deposition and solution-processibility, which can be implemented to fabricate devices [39]. In last few years, several new families of D–A oligomers consisting of electron rich thiophenes/oligothiophenes have been developed as donor materials for high-performance OPVs [40].
In addition to D–A systems, A–D–A small molecules have also attracted great interest in recent years due to their excellent photovoltaic performances [41,42,43]. Bäuerle and co-workers reported A–D–A type DCV5T (1) possessing strong electron withdrawing dicyanovinyl (DCV) units at both peripherals of the molecule, and the OPV devices fabricated using vacuum processing indeed showed a PCE of 6.9% (Fig. 4) [44]. The UV–vis absorption spectra displayed that the introduction of DCV units resulted in not only a large red shift of the absorption band but also higher absorption coefficients with respect to the unsubstituted oligothiophenes. Consequently, it reduced the band gap leading to a high photovoltaic performance. They also synthesized a series of DCV-substituted oligothiophenes lacking of alkyl side chains, and used them as electron rich groups in vacuum-processed bilayer heterojunction and BHJ OPVs [44]. OPV devices of 2 with C60 demonstrated a PCE of 5.2% for BHJs under simulated AM1.5 100 mW cm−2 illumination.
In order to improve the solubility and film quality of oligothiophene derivatives, Liu et al. designed and synthesized a series of septithiophene main chain end-capped with electron withdrawing alkyl cyanoacetate groups instead of DCV and examined the relationship between these different end groups and their BHJ device performances (Fig. 5) [45]. OPVs with 3:PC61BM (2:1) blended film exhibited a high PCE of 5.08%, with a J SC of 10.74 mA cm−2, V OC of 0.86 V and FF of 0.55. For better solar absorption, a new family of oligothiophenes 4 were prepared, using a strong acceptor, 3-ethylrhodanine, as the end group [46]. As expected, the absorption band of septithiophene-based 4 with a red-shift of about 100 nm demonstrated a stronger solar absorption than that of 3, which improved the value of J SC. The OPV device fabricated using 4:PC61BM blend film rendered a higher PCE of 6.10% with a high V OC of 0.92 V and a J SC of 13.98 mA cm−2 , but a relatively low FF of 0.474. Terminal acceptor groups of A–D–A molecules also have a great influence on molecular packing in solid state, which affects the morphologies of the active layers and, in turn, device performances [47]. Zhang et al. designed a new molecule 5 using 2-(1,1-dicyanomethylene)-rhodanine as the terminal units providing a noticeably high PCE of 9.30% with V oc of 0.91 V, J sc of 14.87 mA cm−2 and FF of 0.687 [48].
Chen and co-workers synthesized a series of A–D–A molecules 6–10 (Fig. 6) with 2-(1,1-dicyanomethylene)rhodanine, having the same end groups and similar backbones, containing different conjugation lengths and spatial symmetric units for solution-processed solar cells [49]. BHJ devices of 6–10 were constructed through a solution-process method and their photovoltaic performances were investigated thoroughly. Their current density–voltage (J–V) curves are depicted in Fig. 7a. Among these five molecules, the optimal device fabricated using 6 provided an excellent PCE of 10.08% (certified at 10.10%, with an average PCE of 9.80%) with a V oc of 0.92 V, a FF of 0.69, and a high J sc of 15.88 mA cm−2; this PCE was reported to be the highest to date for single junction small molecule OPVs. The devices containing 7 and 9 with centrosymmetric chemical structures demonstrated moderate PCEs of 6.33 and 6.50%, respectively, while axisymmetric 10 showed a relatively low V OC of 0.81 V due to its higher HOMO level, but a high FF of 0.68, J SC of 13.91 mA cm−2 and comparatively higher PCE of 7.86%. The other axisymmetric molecule 8 provided a V OC of 0.90 V emerging from comperatively high HOMO level, but a high FF of 0.68, relatively high J sc of 14.77 mA cm−2 and the second highest PCE of 9.30%. The EQE curves obtained from the optimized devices of 6–10 are demonstrated in Fig. 7b, indicating the broad spectra covering the range between 300 and 800 nm. The EQE performances were obtained to be relatively higher for 6, 8 and 10 possessing devices compared to those of 7 and 9. Particularly, the device fabricated using 6 gave a broader band with a peak at around 700 nm. J sc values were recorded to be much higher for the axisymmetric 6, 8 and 10 based devices (14–16 mA cm−2) and consequently higher PCEs with respect to the devices prepared using sentrosymmetric 7 and 9 (J sc ~ 11 mA cm−2) (Fig. 7c). Since a large dipole moment change (Δμ ge) lowers the Coulombic binding energy of exciton, which enables intramolecular charge dissociation and subsequently results in high J sc, it was observed in graph illustrated in Fig. 7c that J sc values are directly proportional to those of Δμ ge.

(Reprinted (adapted) with permission from Kan et al. Copyright 2015 American Chemical Society [49])
a Current density vs voltage (J–V) curves for the optimized devices using 6–10 under simulated AM 1.5 G irradiation (100 mW cm−2). b EQE curves for the optimized devices. c Correlation between Jsc and dipole moment change (Δμ ge: difference between the ground (μ g) and excited (μ e) states)
Peng and co-workers revealed the influence of the modification of 2,2′-bithiophene core units on the photovoltaic properties of oligothiophene materials [50]. Without extra post-treatments, the conventional devices, fabricated using 11-H and 11-F, showed high PCEs of 5.00 and 5.80%, respectively (Fig. 8). The PCE of 7.14% recorded using 11-F was remarkably higher than that of 11-H (5.89%) when an inverted device structure was used. The improved efficiency of 11-F could be attributed to the presence of high electronegative atom fluorine.
Other electron withdrawing units such as benzothiadiazole (BT), thiadiazolopyridine (TP), diketopyrrolopyrrole (DPP) and thienopyrroledione (TPD) were also introduced into oligothiophene systems as electron acceptor for OPV applications (Fig. 9) [51,52,53,54,55,56]. However, they were reported to provide low PCEs ranging from 1.7 to 3.4%.
Optical, electrochemical and OPV properties of oligothiophene-based donors are listed in Table 1.
3.2 Organic Field-Effect Transistors
Organic field-effect transistors (OFETs) are a type of device consisting of an organic semiconducting layer acting as a gate insulator and three terminals (drain, source, and gate electrodes). Moreover, the OFET device performance is assessed from the transfer current–voltage plots, which are crucial parameters such as the field-effect mobility (μ), current on/off ratio (I on/I off), threshold voltage (V T ), and subthreshold swing (S). In OFET applications, holes and electrons are given to the semiconducting polymer at HOMO and LUMO, respectively. Oligothiophenes (Th n ) form an important class of organic semiconductors widely used for the realization of OFETs. Thiophene based materials have advantageous such as planar structures, easy modification of thiophene ring, high stability and intermolecular interactions, which are required for OFET applications [57]. Concerning FET applications, while most of the thiophene containing semiconductors has been used as p-type materials having holes as charge carriers, several n-type thiophene possessing materials have electrons as charge transporters [57]. OFET applications of unfunctionalized oligothiophenes demonstrated that the mobility of α-Th 6 [58] has been improved from 10−4 to 0.1 cm2 V−1 s−1 within the past few decades, and that of octithiophene (α-Th 8 ) [59] has reached up to 0.28 cm2 V−1 s−1 (Fig. 3). Generally, extension of the conjugated system decreases the solubility of the oligomers, which is a drawback where solution-processed techniques are required for the fabrication of their devices and circuits. Thus, introduction of suitable alkyl chains attached to thiophene rings is important for the design of organic semiconductors with high solubilities. Halik et al. synthesized a series of quarterthiophene, quinquethiophene and sexithiophene derivatives (12–16) end-capped with alkyl groups together with parent sexitiophene (ST) (Fig. 10) [60]. The best OFET performances were recorded for sexithiophenes 15 and 16 substituted with relatively short alkyl chains (hexyl and ethyl, respectively) and charge carrier mobilities of them were obtained to be 1.0 and 1.1 cm2 V−1 s−1, respectively. Furthermore, based on many investigations, substitution of alkyl groups enhances the field effect performances of organic compounds [61]. In addition, structure–property relationship studies propose that the molecules presumably adopt arrangements with their long axis perpendicular to the surface of Si substrate. This molecular arrangement was found to result in higher OFET performances, and the mobilities were increased more than tenfold. Although the unsubstituted oligothiophenes [62] generally displayed mobility lower than 0.1 cm2 V−1 s−1, most of the derivatives end-capped with alkyl groups [63] showed higher mobilities of above 0.1 cm2 V−1 s−1.
In addition, several ambipolar oligothiophenes with balanced electron and hole transport were defined [64]. A series of α,α′-functionalized quaterthiophenes was prepared with perfluoroalkyl (-n–C6F13), perfluoroarene (–C6F5), acyl and perfluoroacyl substituents to tune the properties of oligothiophenes (Fig. 11) [65, 66]. While perfluoroalkyl-substituted oligothiophene 17 had an electron mobility of 5 × 10−4-10−6 cm2 V−1 s−1, compound 18 with perfluoroarene showed n-channel behavior with μ e = 0.43 cm2 V−1 s−1. However, high FET electron mobility of ∼ 0.6 cm2 V−1 s−1 was recorded for 20 films possessing perfluoroacyl substituents. Compound 21-based OFETs displayed high electron mobilities of 0.51 and 0.25 cm2 V−1 s−1 for vapor-deposited and solution-cast films, respectively. A value of 0.25 cm2 V−1 s−1 was recorded as the highest for a solution-processed organic semiconductor in vacuo. Also, 19 employs as an ambipolar semiconductor with high electron and hole mobilities of 0.1 and 0.01 cm2 V−1 s−1, respectively, resulting from balanced electron and hole injection barriers [64].
A thorough definition of OFET devices with charge transport properties of organic semiconductors is given in a comprehensive review including thiophene-based oligomers and polymers [57].
3.3 Organic Light-Emitting Diodes
The essential conditions for organic luminous materials in OLED applications are high luminescence quantum yield in solid state, high carrier mobility (both of p- and n-type), good quality film-forming properties, high thermal and oxidative stabilities and perfect color purity [67]. Oligothiophenes used in emissive applications fall behind various phenylene materials as a result of restricted fluorescent quantum yields in both solution and solid states [68,69,70]. The low emission properties of these materials are typically ascribed to large sulfur atoms, as well as efficient solid-state packing characteristic of thiophene-based materials [71]. In spite of this limitation, thiophene-based materials still provide a number of characteristics, which made them popular systems for technological applications. In addition to the stability and synthetic diversity, controllable colour tuning of the absorption and emission [58,59,60,61], good charge mobility [61], and the ability to produce materials with lower band gaps [72] make thiophene-based semiconductors promising candidates for light emitting applications.
The solution fluorescence of the simple unfunctionalized α,α-oligothiophenes Th 2 –Th 6 was studied (Fig. 3), which indicated that both absorption and emission bands shift to red and fluorescence quantum yield (Φ F ) increases with increasing conjugation length (Table 2) [73, 74]. The thin film emissions of Th 4 –Th 8 are also red-shifted compared to their solution states, but they showed a dramatically low Φ F as an outcome of the strong intermolecular interactions in solid state [75].
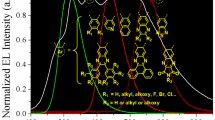
Oligothiophene S,S-dioxides have been widely examined as emitting materials for OLEDs by Barbarella [78], who reported that the insertion of one thienyl-S,S-dioxide unit into the aromatic backbone leads to high PL efficiencies of 45% for 22 and 37% for 23 in solid state (Fig. 12) [79]. Thus, thiophene-S,S-dioxide moiety in the main chain of oligothiophenes was reported to bring about a large increase in solid-state PL efficiency accompanied by an enhanced electron affinity, which deduced from electrochemical reduction potentials measured by cyclic voltammetry [70]. Recently, Barbarella and co-workers reported a variety of V-shaped oligothiophene S-oxide (24) and S,S-dioxides (25) containing thioalkyl side chains as an active material in electroluminescent devices [80]. Since the optoelectronic properties of the devices depend on the intrinsic charge transport properties of the active materials, it was reported that the S-oxide 24 has ambipolar charge carrier properties but 25 has dominant electron transporting behavior.
In order to improve the efficiency of OLEDs, the use of intermolecular interactions between D–A systems, which provide an exciplex formation, intermolecular triplet to singlet energy transfer from the host to guest, and a high singlet-to-triplet cross-section ratio in conjugated small molecules and polymers, is promising approach for realization of high efficiency and low operation voltage OLEDs [81]. Shirota and co-workers presented A–D–A type oligothiophenes 26 and 27, containing bithiophene and terthiophene as donors with dimesitylboron moieties as strong acceptors (Fig. 12) [82]. Compound 26 acted as an effective electron transporting material and an emissive layer with EQE of 0.17% at a luminance of 300 cd m−2 in OLED [83].
Recently, Lu and co-workers have synthesized four constitutional isomers of disubstituted thiophene containing tetraphenylethylene (TPE) groups at 2,5- (28), 3,4- (29), 2,3- (30) and 2,4- (31) positions, as active layers for OLED applications (Fig. 13) [84]. The main idea behind the use of TPE groups to enhance the OLED perfomances is aggregation induced emission (AIE), introduced by Tang [85]. Bulky TPE units hinder strong π–π stackings between planar aromatic moieties and, consequently, suppress the radiationless decay and thereby quenching. The resulting molecules provided high solid-state efficiencies, which are the precondition to obtain good OLED device performances. Compound 28, possessing more planar space conformation and displaying a relatively narrower optical band gap, led to the highest device performance with a maximum luminance (L max) of 65,210 cd m−2, a maximum current efficiency (LEmax) of 11.21 cd A−1 and a maximum EQE of 4.49% [84]. The other compounds 29–31 provided EQEs of 3.00, 2.53 and 3.48%, respectively, with L max of 9630–17,400 cd m−2 and LEmax of 5.79–8.62 cd A−1 (Fig. 14).

(Reprinted (adapted) with permission from Li et al. Copyright 2017 Royal Society of Chemistry [84])
a EQEs of 28–31 and their normalized EL spectra, and b their luminance–voltage–current density curves
4 Fused Thiophene-Based Small Molecules As Organic Semiconductors
Unlike thiophenes, fused thiophenes have more rigid structures with an elongated π-conjugation. Structural ring planarity, extended π-conjugation, chemical stability and intermolecular S···S interactions provide the efficient dimensionality of the electronic structures with enhanced electrical properties, consequently they are accepted as a promising new class of π-conjugated systems [23, 86]. The electron-rich properties of fused-thiophenes make them good electron donating and significant building blocks for a broad diversity of materials in optoelectronic applications [22]. Moreover, they have a strong tendency to form π–π stackings with a large overlapping area that is preferable not only for charge carrier through intermolecular hopping, but also for a high order molecular organization leading to large crystalline domains. The performance of fused-thiophenes is related to the role of d-orbitals of sulfur atom, which mix well with aromatic π-orbitals, so that electron-transfer over the π-center to the acceptor unit is made easier, which results in extended injection efficiency [23].
A number of fused thiophenes with diverse molecular structures have been synthesized and evaluated as organic semiconductors in recent years [87]. Fused thiophenes are categorized into the three classes; (i) thienothiophenes (TTs), consisting of two annulated thiophene units, (ii) dithienothiophenes (DTTs) fused three thiophene units to each other, (iii) thioacenes (TAcs) containing more than three annulated thiophene units [23]. Among the π-conjugated organic building blocks, fused-thiophenes are one of the most promising moieties for organic thin film transistors (OTFTs), OPVs and dye-sensitized solar cells (DSSCs) [31, 87].
4.1 Organic Photovoltaics
Recently, conjugated small molecules have drawn much interest due to well-characterized structures, high purity and thus low batch-to-batch variation, multidirectional chemical structures, and easier energy level control [22, 24]. Current studies are focused on the synthesis of high-performance small donor molecules that feature a broad absorption, appropriate energy levels, and efficient charge transport properties [88,89,90].
A prevailing design element of these efficient molecules is that they depend on supplementary electron rich (commonly, a thiophene-based donor, D) and electron deficient (acceptor, A) units in D–A–D and A–D–A types. Introduction of planar fused aromatic rings as donors into the small molecules benefited molecular crystallinity, lowered the band gaps and enhanced the hole mobilities, thus, provided higher J sc and improved photovoltaic performances [47]. For the successful development of D–A, narrow band-gap conjugated small molecules with different types of electron push–pull structures have been widely investigated [24, 37, 91, 92]. Furthermore, D–A systems have a great impact on the overall optical, electrochemical and charge carrier properties of the resulting small molecules for OPVs [47, 93, 94].
TTs, the simplest fused thiophene rings, are strong electron donating groups, which find wide applications in organic semiconductors. Among known fused ring structures, linearly symmetrical thieno[3,2-b]thiophene has been proven as a good organic building block for organic semiconductors owing to its coplanar rigidity and longer π-conjugation, facilitating intermolecular π–π stacking in the solid state and hence providing high charge carrier mobility [23]. Asymmetric D–π–A structures 32 and 33 possessed an electron rich triphenylamine (TPA) as a donor unit, TT or thiazole as the π-spacer, and a dicyanovinyl group as an acceptor unit (Fig. 15) [95]. They had compact packings in their thin films and good intramolecular charge-transfer characteristics for efficient OSCs, which rendered the PCEs up to 5.41 and 6.20% with J sc values of 11.04 and 12.01 mA cm−2, respectively (Fig. 16a). The high J SC recorded for the device based on 33 related to the higher absorption coefficient as demonstrated in Fig. 16b, which led to higher EQE over the whole spectrum range. Lee et al. synthesized an electron push–pull organic semiconductor 34 comprising TPA and dicyanovinyl, linked by a cross conjugated thieno[2,3-b]thiophene–thiophene unit [96]. Since the π-conjugation length was diminished by thieno[2,3-b]thiophene, molecule 34 exhibited a lower PCE of 2.09% in OPV devices. In addition, fused thiophene derivatives displayed excellent photo and thermal stabilities, providing enhanced performance as photosensitizers. Among the metal-free organic dyes, TT-based sensitizers, which had a validated efficiency of 1.23–9.8%, without any co-adsorbent and displaying long-term stabilities, are among promising candidates for highly efficient DSSCs [87]. Raposo and co-workers synthesized a series of compounds based on TT, possessing dicyanovinyl group as an acceptor and heterocyclic units (thiophene, bithiophene, furan and pyrrole) as π-spacers/donors [97].

(Reprinted (adapted) with permission from Kim et al. Copyright 2014 American Chemical Society [95])
a Current density–voltage characteristics of solar cells based on 32 and 33 and b incident photon-to-current efficiency (IPCE) data from each device
Bridged bithiophenes are a class of promising fused aromatic building blocks for OPV applications. These include dithienosilole (DTS), cyclopentadithiophene (CPDT), dithieno[3,2-b:2′3′-d]pyrrole (DTP), benzodithiophene (BDT), dithienogermole (DTG), dithieno[3,2-b:2′3′-d]phosphole (DTPh) and their derivatives (Fig. 17) [22, 98]. They exhibited a completely planar structures and good π-conjugation across the fused rings.
Successful OPV molecules can be designed by using electron-rich core units in the center substituted by relatively electron-deficient groups, having terminal π-conjugated end-caps. This system presents suitable synthetic way to a broad class of materials with tailored optical, electronic and physical properties. In 2011, DTS was used as a pivotal donor for different high performing molecular donor materials. Chen and co-workers reported the molecule 35 (Fig. 18) with a DTS unit as the central building block and alkyl cyanoacetate group as an electron-withdrawing, optimized device, which gave a relatively high PCE of 5.84% [99]. Heeger, Bazan and co-workers reported an efficient solution-processed small molecule BHJ solar cell, fabricated with a new molecular system, D1–A–D2–A–D1 36 (Fig. 18), containing DTS as a donor and pyridyl[2,1,3]thiadiazole (PT) as an electron acceptor [26]. DTS unit led to broad low-energy optical transitions with strong intramolecular charge transfer characteristics, and the four-coordinate Si atom allowed the association of aliphatic side chains to make the small molecule soluble in various organic solvents. High PCE of 6.7% was achieved with its BHJ device of 36:PC70BM. Later, this molecule was modified by electron-deficient mono-fluorinated benzothiadiazole (FBT) in place of PT to obtain slightly better performance [100]. Device based on PEDOT:PSS/37:PC71BM eventually led to an increases in device performance above 8% of PCE [101]. New A–π–D–π–A type small molecule 38 with DTS as a central donor unit and 3-ethyl-rhodanine as end-capping groups also provided the PCE of > 8% (Fig. 18) [102].
A molecule with alkylthio substituted BDT and rhodanin end-caps, 39, gave to the highest PCE of 9.95%, recorded the highest among BDT-based donors [90]. Recently, Zhou et al. modified 39 by replacing thioalkyl and terthiophenyl units with alkoxy and thieno[3,4-b]thiophenyl fragments, respectively, to achieve 40, of which device, however, demonstrated a PCE of 8.68% by using a solvent vapor annealing (SVA) method [103]. Nevertheless, 40 provided the best efficiency compared to its alkyl (PCE = 7.84%) and thioalkyl (PCE = 4.05%) substituted BDT derivatives. Comparable PCE of 7.4% with a V oc of 0.75 V, J sc of 14.95 mA cm−2 and FF of 0.67 was reported for the compound possessing TPD acceptor instead of central BDT donor, and bithiophene spacer between rhodamine and fused thiophene units in 40 [104]. Moreover, molecule 41 (Fig. 19) with alkylthio-thienyl side chains resulted in 9.2% of an efficient cell without solvent additive or post-processing [89]. Other BDT-T-based compounds such as 42 [105] and 43 [106] exhibited the best efficiencies of 9.6 and 9.3%, respectively, after careful thermal tunning and solvent vapour annealing. Very recently, Peng and co-workers have connected the fluorinated-thienyl side chains to the BDT central unit to obtain a new donor molecule 44 (Fig. 19) with unique physical and chemical properties such as low-lying HOMO energy level, high crystallinity and thermo-stability [107]. Using SVA method, the PCE was augmented up to 9.80%, which is the top efficiency of the SM-OPVs among the reported values so far. A number of additional structural variations has been explored for BDT molecules, which, unfortunately, provided moderate efficiencies, including DPP acceptor units, substitution of TT for thiophene along the backbone, various acceptor end-caps, conjugated and non-conjugated side chain structures, and D1–A–D2–A–D1 structures with FBT and BT acceptors [99]. Bin et al. have recently reported the excellent performance for the nonfullerene device constructed using compound 45 and IDIC acceptor (Fig. 19) [108]. Compound 45 possessing BDT-T-based donor and difluorobenzotriazole acceptor provided a V oc of 0.98 V, J sc of 15.21 mA cm−2 and FF of 0.65, consequently a PCE of 9.73%.
Recently, a novel A–D–A small molecule 46, based on thienyl substituted dithienobenzodithiophene as a donor group and rhodanine as an electron withdrawing unit, have been synthesized for BHJ OPVs by Kwon and co-workers (Fig. 20) [109]. Solution-processed BHJ OPVs of 46:PC71BM blend showed high PCE of 7.10%, without the use of additives or thermal treatments. The five-ring polycyclic moiety indacenedithiophene (IDT) comprising consecutive thiophene-phenylene-thiophene aromatics through sp3-carbon bridges, demonstrated a great development for small molecule OPV applications due to high degree of planarity and large π-conjugation [110]. IDT, as a widely used electron rich unit, possesses a rigid symmetrical structure and provides an ordered packing, thus, favoring high charge carrier mobility and an effective intermolecular interaction. Wang and co-workers synthesized molecule 47, having 2-ethylhexyl side chains and attached fluorine atoms on BT units (Fig. 20) [111]. OPV measurement yielded a relatively high PCE of 9.1% and high FF of 77%, which is the highest FF value reported in the literature for solution-processed SM-OPVs.
Optical, electrochemical and OPV parameters of some fused thiophene possessing small molecules are listed in Table 3.
4.2 Organic Field-Effect Transistors
Organic field-effect transistors are responsible for charge transport of active semiconductors between source and drain electrodes. To date, substantial progress has been made considering the first release of p-channel (hole-transporting) OFET in 1986 [112] with a mobility of 10−5 cm2 V−1 s−1 to the present organic single crystals with a mobility up to 15–40 cm2 V−1 s−1 [113]. Semiconductors with the highest performances in OFET applications are p-channel devices, which possess good hole-transporting materials. Thiophene and fused thiophene-based π-conjugated systems have been developed as promising p-channel organic semiconductors for OFET applications, owing to their chemical stabilities, and versatile chemical modifications to tune their electronic properties [114, 115]. In addition, sulfur atoms are able to produce S···S, S···H, and S···π intermolecular interactions emerged from their the high polarizability, which is not only more efficient charge carrier, but also largely changes the packing motifs of such systems, providing strong π–π stacking in the solid state [17].
Studies on fused thiophene-based oligomers for OFET applications are mostly focused on DTTs [57, 112]. Generally, they are either substituted by aryl or alkyl groups to extend their conjugation or enhance their solubilities and stabilities [57]. While compound 48-H with a cross-conjugated DTT demonstrated a mobility of 0.89 cm2 V−1 s−1 with an on/off ratio up to 107, compounds 48-Ar possessing phenyl end units showed mobilities even as large as 2.0 cm2 V−1 s−1 and on/off ratio up to 1 × 108, which were prepared by using a two-stage film deposition process (Fig. 21) [116]. Fusing octylphenyl units to the cross-conjugated DTT furnished 49 with an U-shaped configuration which had a hole mobility of 3.8 cm2 V−1 s−1 with an on/off current ratio of 106 emerging most probably from the induced anisotropic conduction properties [117]. New π-conjugated DTT derivatives acting as high-performance p-channel organic semiconductors reported by Adachi and co-workers [118]. High carrier mobilities of up to 10.2 cm2 V−1 s−1 and high on/off ratios of 107 were obtained in solution-processed organic single-crystal OFETs, fabricated using molecules 50a and 50b under ambient conditions (Fig. 21).
Dinaphtho[2,3-b:2′,3′-f]thieno[3,2-b]thiophene 51-H [119] with six fused aromatic rings gave high performance OFETs with mobilities up to 3 cm2 V−1 s−1 prepared by vapor processes (Fig. 22). Its dodecyl derivative 51-R (R=C10H21) [120] with an improved stable π-extended skeleton afforded OFETs with mobilities as high as 7.9 cm2 V−1 s−1 with vapor-deposited thin films and 11 cm2 V−1 s−1 with solution-processed single-crystalline films [121]. Recently, several groups have reported that careful controlling the above-mentioned experimental parameters rendered vertical phase separation in the semiconducting small molecule and polymer blend films, which significantly improved the device performance of OFETs [122]. A vertical phase separated structure of an organic semiconducting blend, consisting of thienoacene 52 with polystyrene, led to a considerably high field-effect mobility of up to 43 cm2 V−1 s−1, owing to the formation of highly aligned films, formed using an off-center spin coating method, which is the highest value reported to date for all organic molecules (Fig. 22) [123]. Fusing 2-phenylthiophene units to the both side of 52 gave rise to 53, of which recorded maximum mobility, however, was 6.32 cm2 V−1 s−1, which was much lower than that of 52 [124]. Recently, the chemistry of [1]benzothieno[1]benzothiophene (BTBT) for OFETs has been investigated and structural modifications have been performed to tune the properties such as solubility, crystallinity, processability and stability [57, 125,126,127,128].
The development of efficient n-channel (electron-transporting) semiconductors is more recent and relatively rare [35, 129, 130]. To improve the electron mobilities of fused thiophene-based semiconductors, a new series of molecules 54a,b,c, end-capped with diperfluorophenylthien-2-yl units, were synthesized for OTFT applications by Marks and co-workers (Fig. 23) [131]. Thin film of 54c exhibited a hypsochromic shift in optical absorption, while those of 54a and 54b demonstrated bathochromic shifts. Molecules 54a and 54c provided good n-channel TFT performances with mobilities up to 0.43 and 0.33 cm2 V−1 s−1, respectively, which are among the best performing n-channel materials of all fused thiophenes reported to date. Replacement of perfluorophenylthien-2-yl units with DTT in 54 led to 55 which demonstrated the relatively high p-type charge mobility of 0.81 cm2 V−1 s−1 [132]. At the same time, most of the researchers focused on ambipolar semiconductors, which are either n- or p-channel materials in OFETs. These materials had the typical D–A structures, consisting of electron-rich and electron poor heteroaromatic units. For ambipolar semiconductors, lower-lying LUMO and appropriate HOMO energy levels are required along with strong intermolecular association, making the overlap of neighboring orbitals feasible. Recently, Zhu and co-workers synthesized a A–D–A–D–A type molecule (56), which is a combination of π-conjugating spacers of planar electron rich TT with core unit DPP and electron deficient dicyanovinyl groups (Fig. 23) [133]. 56 indicated an ambipolar behaviour with both hole and electron mobilities as well as a low-lying LUMO level. The FETs fabricated using 56 films demonstrated ambipolar charge carrier properties with an electron mobility of 0.80 cm2 V−1 s−1 upon thermal annealing at 90 °C and a hole mobility of 0.024 cm2 V−1 s−1 at 150 °C.
The D–A type molecules 57 and 58, containing ladder type silaindacenodithiophene donors with DPP as an electron-accepting linker, and octylrhodanine as electron-accepting end groups were synthesized and used as active layers in solution-processable OFETs (Fig. 24) [134]. Molecule 58, having bulky siloxane-terminated hybrid alkyl chain, demonstrated a higher mobility of 3.04 cm2 V−1 s−1 with respect to 57, having 2-ethylhexyl substituted DPP, which is attributed to the strong interactions between siloxane side chains, excellent solubility and better crystallinity.
New promising classes of small molecules of fused thiophenes with naphthalenediimide as an electron acceptor for n-channel semiconductor have recently been reported by several research groups [129, 135].
4.3 Organic Light-Emitting Diodes
For OLED applications, organic materials must exhibit high luminescence efficiency, intense colour, good charge-carrier mobility and stability [136]. Operation of OLEDs relys on the recombination of electrons and holes, therefore, called double charge injection devices, whereas a single charge injection is involved in OFETs. Majority of OLED designs incorporate electron- and hole-transporting layers, the interface between them functions as a site for the recombination and light emission processes [134]. Currently, π-conjugated organic materials have been widely used in applications as active components in OLEDs [69]. Even though non-thiophene electroluminescent (EL) materials surpass them in many ways, thiophene-based materials supply good control of OLED colour [69] and can easily generate red colour, which is difficult to obtain with materials such as poly(phenylenevinylene)s, poly(p-phenylene)s and polyfluorenes [70]. Among organic semiconductors, fused oligothiophenes and their derivatives have attracted considerable attention owing to their chemical versatility, elongated π conjugation, rigid planarity and favourable electrical and optical properties, hence, resulting in high charge transporting properties and chemical stabilities [22, 116]. In solid state, fused thiophene-based materials exhibit a red shift in absorption spectra, typically ascribed to the increase in planarity of the conjugated backbone coupled with enhanced intermolecular interactions, such as π–π stackings, bringing about further electron delocalization [70]. As such, thiophene-based materials are still of important interest for emissive applications and this growing interest is the result of various approaches for overcoming the commonly reduced fluorescence efficiencies [71].
Thiophene-based semiconductors are easily modified by introducing a variety of functional groups into the aromatic backbone, and the sulfur atom is easily oxidized allowing access to both sulfoxide (S-oxide) and sulfone (S,S-dioxide) derivatives [78]. The oxidation of sulfur also converts the electron donating nature of thienyl sulfur to an electron accepting fragment. It should be noted that thienyl-S,S-dioxide-based materials improved the photo- and electroluminescence (EL) efficiencies in the solid state; therefore, affirming them as excellent candidates for the development of OLEDs [78, 137]. The group of Barbarella reported that the presence of the rigid core, 3,5-dimethyldithieno[3,2-b;2′,3′-d]-thiophene-S,S-dioxide in 59 and 60, led to the increase in PL in solution and solid state (Fig. 25) [138]. In solid state, the quantum PL efficiencies of 59 and 60 were measured to be 48 and 24%, respectively. The quantum PL value measured for 59 is the highest recorded so far for oligothiophene-S,S-dioxides.
Fused thiophene-containing D–A π-systems have been investigated to enhance their OLED efficiencies. In most D–A systems, the HOMOs are localized on the electron rich donors, while the LUMOs are found on the electron deficient acceptors. Barbarella and co-workers reported OLED applications of A–D–A type molecule 61, having DTT as a strong electron rich with dimesitylboranes as electron acceptors (Fig. 25) [139]. In solid state, 61 displayed two emissions of a broad blue-green and a weak red with peaks at 480 and 680 nm, respectively, emerging from the cross-like dimer, which was confirmed by photophysical and theoretical investigations. Single layer spin-coated OLED device, based on 61, afforded white light EL, which is the superposition of both emissions, with an EQE of 0.35% and a maximum luminance of 3800 cd m−2 at 18 V. To investigate the effect of TT and TPA units on their photophysical and electrochemical properties and OLED performances, Ozturk and co-workers synthesized a series of D–A and D–A–D type molecules 62–64 in which mesitylboron acceptors were linked to TPA donors through a TT π-conjugated spacers (Fig. 25) [140]. These ambipolar molecules demonstrated high emission quantum efficiencies between 46 and 68% in solution with efficient intramolecular charge transfer properties. Their solid-state quantum yields were recorded up to 42%. Among these molecules, the fabricated EL device of 62 exhibited a sky-blue bright luminescence with an EQE of 0.61%, a maximum luminance of 1200 cd m−2 at 4.0 V and a highest luminescence efficiency of 1.13 cd A−1.
Another popular approach to enhance the fluorescence efficiency of thiophene-based materials is the application of fused thiophene rings, particularly fused 2,2′-bithiophenes such as CPDT, DTS, DTP and DTPh [69, 70]. The ground states of them adapt to the planar nature and they lead to an increased emission. Moreover, the side chains located on the central ring permits the use of fairly bulky groups, and introduction of small steric interactions can cause a reduced backbone planarity in the resulting materials. A detailed description of OLED devices and PL properties of organic semiconductors can be found in a comprehensive review over π-conjugated systems, fused thiophene-based oligomers and polymers [71].
DST-based materials, having carbazole units (65), displayed an emission quantum yield as high as 80% (Fig. 26) [141]. Although the DTP-based materials are very weak emitters, the most recent DTP units, containing N-acyl group (66) to provide stabilized HOMO levels and augmentation of fluorescence efficiency, have been shown to to double the Φ F afford the maximum value of 92% (Fig. 26) [142]. Other successful approach to fused thiophene-based materials to improve their fluorescence had the application of the phosphorous analogue as DTPh. Most of these DTPh-based materials achieved high fluorescence quantum efficiency of above 50% [71]. Moreover, Baumgartner and co-workers reported highly luminescent π-conjugated oligomer (67) having two dithienophosphole units linked via a bithiophene spacer (Fig. 26) [143]. Molecule 67 displayed red-shift emission features and an excellent PL quantum yield of 80%.
Light emitting materials possessing dibenzothiophene (DBT) derivatives exhibit many interesting features in OLED applications [144,145,146]. They are important electron donors, which may facilitate the electron injection and enhance the electron transport in the emitting layer. Lin and co-workers synthesized carbazole/DBT hybrid material 68 with a bipolar transporting property, in which the DBT unit was used as an electron-transporting moiety (Fig. 26) [147]. By using compound 68 as a host material, high-performance solution-processed blue and white phosphorescent organic light emitting devices with an EQE of 13.9%, 29.7 cd A−1, 18.6 lm W−1 and an EQE of 13.8%, 29.1 cd A−1, and 17.7 lm W−1, respectively, were realized. In contrast to thiophene, the sulfur atoms in DBT are easily oxidized giving both sulfoxide and sulfone derivatives, which are known as sulfur-containing acceptors [148]. He and co-workers reported a new D–A–D type fluorescent material 69, possessing 9,9-diphenyl-9,10-dihydroacridine as an electron donor and dibenzo[b,d]-thiophene-5,5-dioxide (DBT) as an electron acceptor, which showed excellent thermal stability and positive solvatochromic behaviour (Fig. 26) [149]. Molecule 69 exhibited nearly 100% solid-state efficiency and a high EQE of 13.1% in multi layered OLED.
5 Thiophene and Fused Thiophene-Based Polymers as Organic Semiconductors
Thiophene-based polymers have been involved in the syntheses of the most important conjugated polymers used in a broad spectrum of applications, such as conducting polymers, OPVs, OFETs and OLEDs, due to their promising optical and electrical properties, as well as excellent thermal and chemical stabilities. The high polarizability of sulfur atom in thiophene leads to a stabilization of the conjugated main chains and superb charge carrier properties [150]. Important features of thiophene-based polymers are: (i) optical properties of polythiophenes can easily be tuned via pre-functionalization of monomers and the band gaps can be altered from 1 to 3 eV; (ii) polythiophene derivatives can be processed from solution, which supplies cost-efficient the construction of thin films; (iii) higher stabilities and longer life time of polythiophene derivatives provide them with better photovoltaic properties [151]. The narrower bandwidth and high band gap in absorption spectra of polythiophenes are the main obstacles to accomplish high efficiency in polymer solar cells (PSCs). The focus has been on overcoming this manipulation of the band gaps towards lower values. Regarding PSCs, suitable functionalized polythiophenes such as preparation of thiophene monomers with proper side chains before polymerization was successful. In addition, there are several other factors influencing the band gaps of the conjugated polymers, such as (i) intra-chain charge transfer, (ii) bond-length alternation, (iii) aromaticity, (iv) substituent effects, (v) intermolecular interactions and (vi) π-conjugation length. Low band-gap semiconducting polymers can ensure properties, such as easy doping with possible intrinsic metallic conductivity, better photo-conductivity and large nonlinear optical coefficients [150].
Thiophene and its benzene and thiophene fused structures have been widely utilized as donor units to construct low band gap semiconducting D–A polymers for applications in organic electronics. Homopolymerization and copolymerization with other electron-rich units of thiophenes result in conjugated polymers possessing tunable optical and electronic properties due to the stabilized quinoid structures and extended π-conjugation systems [150]. Thiophene-based polymers demonstrate hole transport properties and they have been commonly applied as p-type semiconductors for OFETs and OPVs [149]. Generally, thiophene-based polymers contain fused thiophene building blocks including TT, DTT, bridged bithiophene, CPDT, BDT, naphthodithiophene (NDT), IDT and other complex fused thiophenes as donor monomers and BT, dithienyl-BT (DTBT), dithienyl-DPP (DTDPP), thieno[3,4-b]thiophene derivatives, TPD, isoindigo (IIG), as acceptor moieties [149]. These units have proper electron donating and accepting abilities, and they can be modified using side chains and functional groups. Moreover, they have close to linear molecular structures [152].
5.1 Organic Photovoltaics
Conjugated polymers have been extensively used for the construction of single, double, and triple heterojunction PSC devices, which afforded above 10.5, 11.0, and 11.5% of PCEs, respectively [153]. Thiophene-based π-conjugated molecules as an attractive alternative to the traditional polymer-based systems is the research topic of significant current interest, owing to their potential use as organic semiconductors in optoelectronic devices. Moreover, their strong electron-rich natures allow thiophene and its derivatives to be widely used as donors for the construction of conjugated polymers [154]. Regarding BHJ-OPV applications, π-conjugated molecules with A–D–A system are among the most promising semiconductor materials [150] as such A–D–A electronic structures can provide low band gaps through tuning FMOs [153], intense and broad light absorption and efficient charge transport [155]. Furthermore, HOMO, the extent of charge delocalization and charge separation process are significant factors influencing the performance of organic semiconductor devices. The length of charge delocalization directly affects the charge separation process on fast time scales, such as in OPVs [156].
In the early stage of OPV research, homopolymers, including poly(3-hexylthiophene) (P3HT) and poly(p-phenylenevinylene)s (PPVs), were the most studied materials [154]. Recently, Hou and co-workers reported a novel polythiophene derivative P1 as a wide band gap material for the fabrication of tandem OPV (Fig. 27) [157]. P1 is a highly crystalline polymer with strong π–π stacking interactions. The OPV device, fabricated using P1:PC71BM yielded a high PCE of 7.2%, with a high V OC of 0.91 V and a FF of 72%. Inherent polythiophenes are not the ideal candidates for organic optoelectronic applications due to their poor solubility and processability. Thus, incorporation of a strong electron-withdrawing group to produce a D–A type copolymer can usually increase the V OC [153]. Recently, Thompson and co-workers reported two families of copolymers, featuring varying percentages of hexylthiophene-3-carboxylate, namely poly(3-hexylthiophene-co-3-hexylesterthiophene) (P2) and poly(3-hexylthiophene thiophene 3-hexylesterthiophene-diketo-pyrrolopyrrole) (P3) (Fig. 27) [158]. Through the introduction of strong electron withdrawing DPP component in P3, FMOs were tuned, and V OCs of the resulting PSCs were significantly enhanced compared to P2.
Fused thiophene rings in the main chain can enhance the charge carrier mobility and diminish the band gaps of the resulting polymers, owing to strong intermolecular interactions rendering structural planarity and linearity of the polymer backbone. Generally, the TT-based oligomers and the polymers have lower solubilities at room temperature, which can be overcome by alkyl substituents and fluorine possessing aromatic units in the main chain [23, 159]. Moreover, the stabilities and lower HOMO levels are achieved on the account of larger resonance stabilization energy of the extended conjugated units [153].
The D–A interactions of such polymers in the main chain can increase the quinoid character between the repeating units. Impressively, the anticipated absorption and molecular energy properties can be obtained by controlling the intramolecular charge transfer from D to A. Also, the molecular properties including the dipole moment, planarity of the backbone and charge transport characteristics can be adjusted by modifying D and A interactions. In recent years, major developments in design of photovoltaic polymers have been attributed to the breakthroughs in D–A type polymers, which have led to steady improvements in the PCEs of BHJ OPVs [153]. McCulloch and co-workers synthesized a series of TT based copolymers with thiophene, selenophene and tellurophene π-spacers, applying DPP unit as an acceptor [160]. The band gaps of the polymers are diminished with the increased size of the chalcogen atoms owing to the reduced aromaticity. Among these polymers, with P4, a high PCE of 8.8% was achieved in the inverted P4:PC71BM device with a J SC of 23.5 mA cm−2 (Fig. 28).
Another important electron donor group for OPV applications is bithiophene unit. Because of the strong electron delocalization ability, bithiophene displays intense molecular orbital hybridization with electron acceptors, which effectively narrows the bandgaps of the resulting D–A copolymers [154]. In addition, the five-membered thiophene rings have less steric hindrance with the neighboring blocks compare with their six-membered benzene analogue, which is helpful for the production of a highly planar D–A systems [154]. Tao and co-workers reported a co-polymer P5 (DTS-TPD), which displayed a larger band gap of 1.73 eV with a lower HOMO energy level of − 5.57 eV [161]. Consequently, a higher PCE of 7.3% was reached with a large V OC of 0.88 V. Later, the same group reported even a higher PCE for dithienogermole (DTG) based polymer P6 as 8.5%, after careful device engineering (Fig. 28) [161]. Yang and co-workers embedded a strong electron donating oxygen atom into a donor unit in P7, which increased the HOMO (− 5.26 eV) slightly and, thereby, reduced the band gap (ca. 1.38 eV). Consequently, it brought about a broad absorption extending to NIR (900 nm) leading to a high J SC of 18 mA cm−2 and a relatively high PCE of 8.0% [162].
In the past few decades, thiophene-based building blocks have been developed by fusing them with hetero rings. In particular, lactam units have appealed attention owing to their strong electron-withdrawing abilities for fine-tuning energy levels and band gaps [153]. The imide group at the center of the bithiophene imide (BTI) unit endowed the BTI units with a less steric encumbrance, and this enabled BTI copolymerization with a number of comonomers rendering extended π-conjugations. The resultant polymers had excellent solution processability, high molecular weights and low HOMO levels, which led to a high V OC of over 0.9 V in PSCs [153]. The first BTI-based polymers developed for OPV applications exhibited encouraging PCEs up to 5.5% [163]. Recently, P8 was developed, using a novel pentacyclic lactam fused-thiophene-based acceptor unit (TPTI), which had a low-lying HOMO level of − 5.40 eV. OPVs, fabricated using P8:PC71BM blend, displayed an initial PCE of 7.80% along with an EQE of above 70% between 435 and 640 nm [164]. In a later work, P8:PC71BM devices achieved remarkable PCE of 9.2% with 1,8-diiodooctane (DIO) additive material [165]. Additionally, P9 was constructed with a new lactam acceptor unit, [7,7′-bidithieno[3,2-b:2′,3′-d]pyridine]-5,5′(4H,4′H)-dione, having a rigid planar structure and high hole mobility, and thiophene as the donor unit (Fig. 28). The low HOMO (− 5.52 eV) level increased the stability of P9 against oxidation resulting in a high V oc (0.96 V) in OPVs. The OPV devices based on P9 provided an outstanding PCE of 9.13%, which is among the highest performances observed for wide band gap polymers [166].
BDT, another fused thiophene based molecule, which was initially used in the synthesis of polymers, became one of the most successful building blocks in the synthesis of highly efficient photovoltaic materials [152, 167]. The rigid and coplanar conjugated structure of BDT and its analogues as building blocks have been attractive for achieving highly tunable molecular energy levels and band gaps, as well as high hole mobilities in device applications. Single junction OPV devices based on BDT gave rise to a PCE of 11.21% for polymers and 9.95% for small molecules; also, PCEs of all types of OPVs were recorded as 11.48% according to the National Renewable Energy Laboratory (NREL) data [152]. A detailed description of OPV devices and charge transport properties of organic semiconductor polymers possessing BDT are given in the comprehensive reviews [151,152,153].
The electron-donating side chains allow us to tune the optoelectronic properties of polymers, and thereby an enhancement of the device performances. Especially, well-accepted alkylthio and alkoxy side chains are utilized to fine-tune the absorptions and energy levels of conjugated polymers [168]. Yu et al. used the ester-substituted thieno[3,4-b]thiophene unit as an acceptor building block to copolymerize with BDT having alkoxy side chains [169]. Moreover, thieno[3,4-b]thiophene units [170], possessing fluorine atom have been utilized as an electron acceptor in high performance polymers (P10) (Fig. 29). After optimization of the devices, the device with an active layer of P10:PC71BM yielded a PCE of 7.4% with a V OC of 0.74 V, a J SC of 14.50 mA cm−2 and an FF of 69%. Very recently, Cao and coworkers have reported poly[(9,9-bis(30-(N,N-dimethylamino)propyl)-2,7-fluorene)-alt-2,7-(9,9-dioctyl-fluorene)] (PFN) as a cathode interlayer together with PC71BM in device, which exhibited a V OC of 0.75 V, a J SC of 17.46 mA cm−2, an FF of 0.7, leading to an impressive PCE of 9.21% [171]. When thienyl group was utilized instead of the alkoxy of P10, its absorption band was observed at a longer wavelength by almost 25 nm due to the improved coplanarity of P11 (Fig. 29) [172]. The P11-based inverted PSC device reached a high PCE of 9.35%, as one of the best results at that time, for which a new fullerene derivative-doped zinc oxide (ZnO-C60) nanofilm as the cathode interlayer was used. Another small modification came from Chen and He, who replaced 25% of F atoms with Cl atoms in P11 leading to the enhancement of device performance from 7.91 to 8.31% [173]. Liu et al. probed the effect of fluoroalkyl chain substituted at the end of the alkyl unit of ester functional group in P11 on surface energy of bulk materials. Based on their systematic investigation, fluorination of side alkyl chain increases the phase domain size but diminishes the PCE from 7.56 to 1.98% when going from P11 to P11-C6F13 [174]. He at al elegantly improved the performance up to PCE of 10.61% with a V OC of 0.83 V, a J SC of 17.43 mA cm−2, an FF of 0.74 using a single-junction device possessing 65% of PC71BM by weight [175]. The recorded EQE of the devices reached 80% at 500 nm (Fig. 30). Recently, Wei and coworkers reported the comparable PCE of 10.4% measured from the device based on the blend of P11, a derivative of 42, possessing a dimethoxythiophene in the central terthiophene fragment, and PC71BM in a ratio of 0.9:0.1:1.5 (w/w/w) as the active layer [176]. Cui and coworkers synthesized a co-polymer P12a with a linear alkylthio side chain having an optical band gap of 1.57 eV and a HOMO level of − 5.41 eV (Fig. 29). The device, fabricated using P12a, demonstrated a PCE of 8.42% [177]. Later, the same group maufactered inverted-structured PSCs using P12a, which provided a higher PCE of 9.58% with an interestingly high V OC of 0.89 V and a high FF of 71.0% [178]. Replacement of 2-ethylhexyl with n-octyl in P12b rendered an optical band gap of 1.51 eV and a HOMO level of − 5.33 eV [179]. Its PSC device provided a PCE of 9.48%, and further optimatization of the device enhanced its PCE above 10% [180].

(Reprinted (adapted) with permission from He et al. Copyright 2015 Nature Publishing Group [175])
a Absorption coefficients of pristine spectra of P11 and P11:PC71BM blends in various ratios by weight. Purple filled triangles: pure polymer film. b J–V characteristics of the best performing PSCs with 60 wt % PC71BM in the active layer. c EQE/IPCE spectra of the corresponding best-performing PSCs (red circles). The integrated product of the IPCE spectrum with AM 1.5G photon flux is also shown (blue squares)
Since co-polymers of BDT and BT demonstrated outstanding photovoltaic performances [152], Jones and coworkers examined the influence of the molecular weight on the photovoltaic performance of polymer P13 (Fig. 29) [181]. It had an optical band gap of 1.75 eV with an absorption edge of around 710 nm, and HOMO and LUMO levels of − 5.45 and − 3.65 eV, respectively. Inverse OPV device constructed using ZnO as an interface layer provided a high PCE of 8.5% with a V OC of 0.92 V, a J SC of 14.5 mA cm−2 and an FF of 64%. The modification of ZnO layer with fullerene resulted in a higher PCE of 9.4%. Duan et al. linked BDT to difluoro-BT through a thiophene unit which was aimed to enhance the FF and subsequently efficiency of solar cell. However, the recorded PCE was 7.7% with V OC of 0.84 V, a J SC of 14.9 mA cm−2 and a low FF of 61% [182]. TPD unit has a symmetric planar structure with an alkyl-substituted strong electron withdrawing imide fragment fused to thiophene unit. Beaujuge and co-workers reported a series of D–A conjugated systems with different side-chains on BDT and TPD due to their important effects on photovoltaic properties [183]. Polymer P14 with a branched 2-ethylhexyl unit on BDT and linear n-heptyl chain on TPD ensured a good PCE of 8.5%, with a V OC of 0.97 V, a J SC of 12.6 mA cm−2 and a FF of 70% (Fig. 29). For further improvement of the performance over the 10% efficiency threshold, three copolymers with different alkyl side chains on BDT electron donor and benzodithiophene-4,8-dione electron acceptor units were probed [184]. Among them, P15 featured the highest PCE of 10.3% accompanied by fullerene, which represented the record efficiency in wide band gap OSCs (Fig. 29). Inspired by this high-performance, P16a was synthesized, which demonstrated a significant role in various types of organic photovoltaic devices, rendering even higher PCE of 10.6%, when a highly absorbing non-fullerene acceptor was utilized with P16a (Fig. 29). The influence of 2,2-bithiophene and TT spacers on the photovoltaic performance of BDT possessing polymers was disclosed by Pan et al., who investigated the performance of devices fabricated using P16b and P16c. Their photovoltaic measurements provided that P16b was superior to P16c, and their PCEs were reported to be 6.93 and 3.92%, respectively, owing to the a deeper HOMO level and a more planar backbone structure characterized for 16b with a bithiophene spacer [185]. Li and co-workers realized the synthesis of the polymer P17-S composed of thienyl-substituted BDT and difluorinated benzotriazole, the fabricated device of which gave rise to an excellent PCE of 9.53% in the absence of fullerene acceptor (Fig. 29) [186]. Recently, Bin et al. replaced fused thiophenes on BDT and thioalkyls on thiophenes in P17-S with furan units and tripropylsilyl groups, respectively, resulting in P17-O having benzodifuran (BDF). It brought about an enhancement of the efficiency up to a PCE of 11.05% with a V OC of 0.96 V, a J SC of 16.48 mA cm−2 and a FF of 70% [187].
As potential candidates for OPVs, the planar multi ring fused ladder-type aromatics favor π-electron delocalization, reduce the energetic disorder of the polymer and elongate effective π-conjugation, leading to a broader and more intense absorption. Compared to the line-type structure, the ladder-type is probably more suitable for the construction of high-performance D–A co-polymers due to its lower HOMO level, higher charge carrier mobility and higher space occupancy of its chemical structure [153]. Yu and co-workers reported co-polymer P18, comprising pentacyclic dithieno[2,3-d:2′,3′-d]benzo[1,2-b:4,5-b′]dithiophene (DTBDT) and fluorinated thieno[3,4-b]thiophene units (Fig. 31) [188]. Consequently, the device using P18:PC71BM (1:1.2) delivered a relatively high PCE of 7.6% with a J sc of 13 mA cm−2 and a V oc of 0.87 V. Very recently, Yang and co-workers disclosed two ladder-type polymers (P19 and P20) combining IDT and indacenodithieno[3,2-b]thiophene (IDTT) units with 4-(2-octyldodecyl)-dithieno[3,2-b:2′,3′-d]pyridin-5(4H)-one (DTPO), which endowed the resulting polymers with deep HOMO energy levels (Fig. 31) [189]. While the fabricated device of P19 exhibited a PCE of 5.47%, P20-based device achieved a comparatively high PCE of 7.33%, ascribed to a better hole mobility, small torsion angle and more uniform morphology of P20.
5.2 Organic Field-Effect Transistors
It has been recorded that D–A polymers consisting of altering electron-rich D, such as oligothiophenes, and electron-deficient A, such as DPP, isoindigo (IIG) and naphthalenedicarboximide yielded high carrier mobilities exceeding 14 and 7 cm2 V−1 s−1 for holes and electrons, respectively, in OFETs [190].
To increase the mobility of semiconducting conjugated polymers (SCPs), fused thiophene rings (e.g., π-conjugated heteroarenes: TT, DTT, BDT, naphtho[1,2-b:5,6-b′]dithiophene, and IDT) have been introduced into the conjugated polymer backbone, which have been reported as stable organic semiconducting materials with high mobility of > 1.0 cm2 V−1 s−1 [190, 191]. Since the π-conjugated thiophene-based structure has a highly planar and strong π–π interactions with a large overlapping through polymer chains, their charge carriers are expected to be transported effectively not only along the polymer chain, but also through intermolecular hopping in the π–π direction [192]. Extention of the π-conjugation of partly planarized fused thiophene based units in the polymer backbone is an alternative approach for improving conjugation and optimizing solid-state properties. In a recent study by Yi et al., two new D–A type thiophene-based copolymers P21 and P22 incorporating sextetthiophene (T 6 ) and heptetthiophene (T 7 ) units as donor groups and DPP as an acceptor unit were synthesized (Fig. 32) [193]. Introduction of extented unsubstituted oliogothiophene units of DPP-oligothiophene copolymers was observed to enhance the solubility and thin-film morphology. Consequently, ambipolar polymers P21 and P22 displayed considerable high hole mobilities of 3.94 and 2.82 cm2 V−1 s−1, respectively, after thermal annealing at 180–200 °C. Two series of bithiophene-DPP polymers with or without fluorine atoms were used in OFET applications [194]. While fluorinated DPP polymer provided hole mobilities below 1.80 cm2 V−1 s−1, nonfluorinated DPP polymer exhibited high hole mobilities up to 11.16 cm2 V−1 s−1, which is among the highest reported hole mobility. Ong and coworkers reported the synthesis of a new D–A alternating copolymer P23 consisting of TT as an electron donor and DPP as an acceptor units (Fig. 32) [195], of which bottom-gate, bottom-contact (BGBC) device demonstrated a high field-effect mobility of 10.5 cm2 V−1 s−1 for both p- and n-channel operations (Fig. 33) owing to the fact that the unsubstituted planar TT reduced conformational energy disorder, facilitated intermolecular packing, and consequently promoted molecular orbital delocalization along the π framework.

(Reprinted (adapted) with permission from Li et al. Copyright 2012 Nature Publishing Group [195])
a BGBC devices on Si wafer. b Output curves and transfer characteristics of device fabricated using P23
Although the studies on n-channel polymers have increased recently, their electron mobility and device stability are still low in comparison to p-channel devices [184, 196]. Bronstein et al. developed a new co-polymer P24 by replacing thiophenes with larger TT units, which provided a more delocalized HOMO distribution along the backbone and, thereby, enhanced the intermolecular charge transport (Fig. 32) [197]. P24 exhibited a low hole mobility of 0.037 cm2 V−1 s−1, but a high electron mobility of 0.30 cm2 V−1 s−1. The high electron mobility might be attributed to the low LUMO level of P24. Donors based on bridged bithiophene are important building blocks for construction of D–A polymers, since they are highly planar and the five membered thiophene rings cause less steric hindrance with their neighboring acceptor units [149]. Among them, CPDT and BT possessing D–A polymer (P25) has become one of the most successful examples, demonstrating excellent performances in OFET applications (Fig. 32). Although the polymer P25 initially exhibited a hole mobility of 0.17 cm2 V−1 s−1 in FET devices [198], recently, an improved mobility of 5.5 cm2 V−1 s−1 has been realized by achieving high molecular order and pronounced alignment in single fibers within a short OFET channel by solvent vapor enhanced drop casting [199]. Noh and co-workers reported electrical characterization of a highly planar co-polymer P26, synthesized using DTS as an electron donor and difluoro-BT as an electron acceptor [189]. The optimized OTFT device exhibited an excellent p-type properties with the highest hole mobility of 9.0 cm2 V−1 s−1 (Fig. 32).
5.3 Organic Light-Emitting Diodes
Although thiophene-based materials are among the most commonly studied conjugated materials for optoelectronic applications, emissive conjugated materials have been produced using other systems, such as boron and nitrogen containing compounds [200, 201], owing to the low emission quantum yields of thiophene-based systems. Thiophene-based materials were first applied to OLEDs in 1991 by Yoshino and coworkers who fabricated red–orange emitting devices by utilizing poly(3-dodecylthiophene) and poly(3-alkylthiophene) analogous, along with polythiophenes having longer side chains [69, 71, 202]. However, this has been changing with the improvement of new highly fluorescent thiophene-based materials over the last decade [71]. Because of the higher molecular weight of polythiophenes, the nature of unfunctionalized polythiophene materials has low solubility, which hinders significant thin film investigations. As a consequence of the steric effect in functionalized polythiophenes, the emission is lower than that observed for the less substituted oligothiophenes with maximum Φ F values of ~ 40%. Therefore, a widely used method to enhance the fluorescence and electronic properties of materials consisting of thiophene is the introduction of thiophene as an ingredient of copolymeric materials. For the copolymer approach, the use of thiophene-based comonomer is very attractive owing to the versatility in its structure modification and hence excellent facility in electronic tunability [20]. Also, fused thiophene-based polymers, which have more rigid and planar structures in the backbone, are known to possess interesting and unique chemical and physical properties. In addition, the fused ring in polymeric backbone also reduces the reorganization energy of the polymer and easies intermolecular hopping and charge carrier mobility. Fused thiophene containing polymers are predominantly hole-transporting materials with low electron affinities. As accepted, thiophene copolymers, such as thiophene–phenylene and thiophene–fluorene copolymers, have improved colour tunability and efficacy [71]. Chen and co-workers synthesized a copolymer (P27) of 9,9-dihexylfluorene with unsubstituted TT and examined its potential applications for polymeric light-emitting diodes (PLEDs) [203]. Its device displayed pure green emission with a maximum brightness of 6000 cd m−2 and current efficiency of 2.7 cd A−1 in PLEDs (Fig. 34). Ozturk and coworkers reported a series of conjugated polymers, containing different ratios of DTT-S,S-dioxide, which displayed emission colours ranging from light blue to green, yellow and red [204]. Polymers with either fluorene bonded to the peripheral thiophenes of the DTT-S,S-dioxide unit or featuring a thiophene between them were reported to exhibit a red shift in solid state emission (up to 585 and 646 nm, respectively) with an increase in DTT-S,S-dioxide content. The increase in fluorine content, i.e. diminishing DTT-S,S-dioxide content, resulted, however, in a hypsochromic shift. Among these polymers, OLED fabrication of P28 exhibited green emission with a luminous efficiency of 0.129 cd A−1 and EQE of 0.042% (Fig. 34).
Another well-known fused bithiophene used as an emitting layer in OLEDs is DTS [205]. Their devices successfully afforded red emission, but the recorded luminance was quite low (< 6 cd m−2). Luminance of copolymer of DTS with 3,4-dibutylthiophene (P29) was improved up to 75 cd m−2 by addition of an electron-transporting tris(8-quinolinolato)-aluminium(III) (Alq3) layer (Fig. 34). Devices constructed using random copolymers of DTS and fluorene have also been reported giving quite successful results [206]. Their OLEDs emitted green light with a luminance of 13,100 cd m−2 and an efficiency of 0.41%. It should be noted that DTS-based materials have not generally produced very good performances in OLEDs. However, they were characterized to be good electron-transport materials for such device applications [71]. The use of fused thiophene derivatives and DTP-based emitters in OLEDs was first reported by Patri and co-workers [207], who synthesized copolymers providing red emission in OLED devices. Later, Rasmussen and co-workers reported devices with emissive colours that ranged from yellow-green to red–purple by using DTP-based homopolymer and copolymers [208], among which DTP–fluorene material P30 gave higher threshold voltages, nearly four times the maximum luminance (197 cd m−2) with a maximum efficiency of 0.8 Lm W−1 (Fig. 34) [206]. More recently, OLEDs, constructed using the CPDT-based copolymer P31 as an emitting layer, displayed an orange emission with threshold voltages of only 2.0 V, luminance up to 20,000 cd m−2 and a maximum current efficiency of 2 cd A−1 (Fig. 34) [209]. Further reading on OLEDs, concerning structure and emission properties of thiophene-based conjugated polymers can be found in the literature [71].
To achieve high quantum efficiency in OLEDs using polymers, it is also necessary to have balanced charge injection and transport of both holes and electrons in the emissive materials [210]. As a result, to improve the electron transport property, a common strategy of D–A type structure was employed to incorporate an electron rich (D) and electron deficient (A) groups into the polymeric backbone. Jäkle and co-workers reported a variety of fluorescent copolymers (for example, P32) containing 3-hexylthiophene as an electron donor and different boron units as electron acceptors in the main chain (Fig. 35) [211]. By increasing the length of the thiophene linker, the same group reported a series of copolymers P33 (e.g., Tn with n = 1–5) possessing different numbers of thiophene units, which were able to efficiently modulate the optical bandgaps of the polymers by preferentially altering their HOMO levels (Fig. 35) [212]. Similar to the absorption spectrum, strong emission bands of these polymers also comprise a wide-ranging wavelength in both solution and solid state. Hence, these new polymers based on borane and thiophenes are promising for applications in visualization and light emitting devices. P3HT was reported to be not a highly emissive polymer, however, incorporation of borane functional groups by considering regioregularity (rr) rendered fluorescent materials P34 and P35 (Fig. 35) [213]. Thiophenes in rr-poly(3-alkynylthiophene)s adopt a coplanar conformation favouring planarization of the polymer main chain and therefore leading to changes in the electronic structure of these polymers. Hence, polymers of higher regioregularity displayed a red shifted fluorescence bands.
Patri and co-workers reported a series of new low bandgap D–A type copolymers derived from various alkyl thiophenes containing TT as an electron donor and thiazolo[5,4-d]thiazole as an electron acceptor in the main chain for OLED applications [214]. All the polymers indicated strong fluorescence emissions both in solution and solid state. Thiazolo[5,4-d]thiazole also increased the air stability of the polymer by reducing the HOMO level. Among these copolymers, P36 exhibited a bright red emission with a maximum luminance of 224 cd m−2 and a maximum current efficiency of 0.036 cd A−1 when used as an active layer in EL devices (Fig. 36). Ozturk and co-workers reported a series of copolymers possessing TT donor and mesitylboron acceptor for light-emitting efficiencies [215]. Especially EL spectra of PLEDs fabricated using P37 and P38 displayed red shift emission bands from 620 to 677 nm and their devices exhibited pure white color according to the CIE (Figs. 36 and 37). Although their quantum efficiencies were relatively low, with this study, use of TT based D–A copolymers to obtain white light emitting polymers was demonstrated for the first time [215].
The use of thiophenes and fused thiophenes in OLED, OFET and OPV applications was provided in this concise chapter. However, in some cases thiophene containing materials unfortunately did not show high efficiency. It has been already mentioned that P17-S having BDT resulted in PCE of 9.53% [186], whereas replacement of sulfur atoms with oxygen gave BDF possessing P17-O, of which device delivered a PCE of 11.05% [187]. The discrepancy between recorded PCEs might emerge from the energy levels, self-assembly behavior and intermolecular interactions of BDF containing D–A polymers. Since sulfur atom is more polarizable and less electronegative than oxygen, P17-O has a lower HOMO energy level and thereby higher V oc. Therefore, thiophene and thienothiophene containing materials can be substituted by proper unit which diminishes HOMO level or they can be utilized together with strong electron acceptor, which overall lowers the energy of HOMO. Another point is FF parameter which can be improved by using different methodologies such as solution-process or direct evaporation of the compound. The intermolecular interaction or packing is well known as a drawback in case of OLEDs. To hamper the intermolecular interactions and keep the layers away from each other, appropriate steric substitutents (TPE, etc.) could be good candidates. Nonetheless, thiophenes and fused thiophenes have been appealing to scientists owing to their promising future applications in the field of semiconductors.
6 Conclusions
In this chapter, we addressed several of the best thiophene and fused thiophene based small molecules and polymers used for electronic and optoelectronic applications, such as OLED, OFET and OPV, and their properties were surveyed to give an overview on the current research in material chemistry. Because of their unique properties, thiophene-based materials are promising donors and π-spacer building blocks for future designs.
The D–A strategy has appeared to be the most promising method for obtaining high-performance conjugated small molecules and polymers for electronic applications. Donor and acceptor units, which rendered encouraging performances in D–A systems, have been listed and separately discussed. For example, rhodanine acceptor and its derivatives led to the enhancement of OPV performance above PCE of 9%. Other acceptors were also reported to increase the efficiency; DPP (8.8%), TPD (8.0%), fused lactam unit (> 9.1%). Donors like BDT are also crucial in determining efficiency. With BDT around 11.5% of PCE was recorded. The structural parameters do also play an important role. Axisymmetric molecules provided higher PCE than centrosymmetric molecules. Both TT-based sensitizers and other heteroatoms containing fragments like DTS were also demonstrated to improve the efficiency. The method considered to be most important is the solution-process method, which enhances device performance up to 10%.
For high OFET performances, tetracyclic and hexacyclic thiophene containing aromatic structures are promising materials to achieve high charge mobility as high as 43 cm2 V−1 s−1. While DPP acceptor together with TT recorded the charge mobility of 10.5 cm2 V−1 s−1, difluoro-BT acceptor with DTS provided a hole mobility of 9.0 cm2 V−1 s−1.
For high OLED performances, high luminescence quantum yield in solid state, high carrier mobility (both of p- and n-type), good quality film-forming properties, high thermal and oxidative stabilities and perfect color purity are inevitable assets. Extension of conjugation, for example replacement of bithiophene with terthiophene, the use of AIE active units, i.e. TPE (EQE = 4.49%) and incorporation of mesitylboron unit as an acceptor were briefly highlighted as some of strategies to elevate the device performance. Another popular approach to enhance the fluorescence efficiency of thiophene-based materials is the application of fused thiophene rings, particularly fused 2,2′-bithiophenes such as CPDT, DTS, DTP and DTPh. Hybrid materials, such as carbazole/DBT hybrid material, in which the DBT unit was used as an electron-transporting moiety, gave high-performance solution-processed blue and white phosphorescent organic light emitting devices with an EQE of 13.9%.
All these results provided in this chapter clearly demonstrate that thiophene based organic semiconductors will be part of a new generation of widely employed products for organic electronics.
References
Okamato Y, Brenner W (1964) Organic semiconductors. Reinhold Publishing Corporation, New York
Zaumseil J, Sirringhaus H (2007) Chem Rev 107:1296
Facchetti A (2013) Mater Today 16:123
Sirringhaus H (2014) Adv Mater 26:1319
Coropceanu V, Cornil J, da Silva DA, Olivier Y, Silbey R, Bredas JL (2007) Chem Rev 107:926
Murphy AR, Fréchet JMJ (2007) Chem Rev 107:1066
Facchetti A (2007) Mater Today 10:28
Torruellas WE, Neher D, Zanoni R, Stegeman GI, Kajzar F, Leclerc M (1990) Chem Phys Lett 175:11
Bäuerle P (1998) In: Wegner G, Mullen K (eds) Electronic materials: the oligomer approach. Wiley-VCH, Weinheim
Tour J (1996) Chem Rev 96:537
Shirota Y (2000) J Mater Chem 10:1
Musher JI (1969) Angew Chem Int Ed Engl 8:54
Gillespie RJ, Silvi B (2002) Coord Chem Rev 233–234:53
Dell EJ, Capozzi B, Xia J, Venkataraman L, Campos LM (2015) Nat Chem 7:209
Ghofraniha N, Viola I, Di Maria F, Barbarella G, Gigli G, Leuzzi L, Conti C (2015) Nat Commun 6:6058
Kitamura C, Tanaka S, Yamashita Y (1996) Chem Mater 8:570
Barbarella G, Zambianchi M, Bongini A, Antolini L (1993) Adv Mater 5:834
Barbarella G, Di Maria F (2015) Acc Chem Res 48:2230
Perepichka IF, Perepichka DF (2009) Handbook of thiophene-based materials: applications in organic electronics and photonics. Wiley, Chichester
Roncali J (1992) Chem Rev 92:711
McCullough RD (1998) Adv Mater 10:93
Mishra A, Ma CQ, Bauerle P (2009) Chem Rev 109:1141
Cinar ME, Ozturk T (2015) Chem Rev 115:3036 (and references therein)
Lin Y, Li Y, Zhan X (2012) Chem Soc Rev 41:4245
Lin Y, Zhan X (2016) Acc Chem Res 49:175
Sun Y, Welch GC, Leong WL, Takacs CJ, Bazan GC, Heeger AJ (2012) Nat Mater 11:44
Mazzeo M, Pisignano D, Favaretto L, Barbarella G, Cingolani R, Gigli G (2003) Synth Met 139:671
Hains AW, Ramanan C, Irwin MD, Liu J, Wasielewski MR, Marks TJ (2010) ACS Appl Mater Interfaces 2:175
Kunugi Y, Takimiya K, Negishi N, Otsubo T, Aso Y (2004) J Mater Chem 14:2840
Marseglia EA, Grepioni F, Tedesco E, Braga D (2000) Mol Cryst Liq Cryst Sci Technol Sect A 348:137
Zhou N, Prabakaran K, Lee B, Chang SH, Harutyunyan B, Guo P, Butler MR, Timalsina A, Bedzyk MJ, Ratner MA, Vegiraju S, Yau S, Wu C-G, Chang RPH, Facchetti A, Chen M-C, Marks TJ (2015) J Am Chem Soc 137:4414
Nicolas Y, Blanchard P, Levillain E, Allain M, Mercier N, Roncali J (2004) Org Lett 6:273
Sun X, Liu Y, Chen S, Qiu W, Yu G, Ma Y, Qi T, Zhang H, Xu X, Zhu D (2006) Adv Funct Mater 16:917
Ma C-Q, Mena-Osteritz E, Debaerdemaeker T, Wienk MM, Janssen RA, Bäuerle P (2007) Angew Chem Int Ed 46:1679
Li X-C, Sirringhaus H, Garnier F, Holmes AB, Moratti SC, Feeder N, Clegg W, Teat SJ, Friend RH (1998) J Am Chem Soc 120:2206
Gao JH, Li RJ, Li LQ, Meng Q, Jiang H, Li HX, Hu WP (2007) Adv Mater 19:3008
Mishra A, Bauerle P (2012) Angew Chem Int Ed 51:2020
Noma N, Tsuzuki T, Shirota Y (1995) Adv Mater 7:647
Sakai J, Taima T, Saito K (2008) Org Electron 9:582
Zhang F, Wu D, Xua Y, Feng X (2011) J Mater Chem 21:17590
Zhou J, Wan X, Liu Y, Zuo Y, Li Z, He G, Long G, Ni W, Li C, Su X, Chen Y (2012) J Am Chem Soc 134:16345
Shen S, Jiang P, He C, Zhang J, Shen P, Zhang Y, Yi Y, Zhang Z, Li Z, Li Y (2013) Chem Mater 25:2274
Wessendorf CD, Schulz GL, Mishra A, Kar P, Ata I, Weidelener M, Urdanpilleta M, Hanisch J, Mena-Osteritz E, Lindén M, Ahlswede E, Bäuerle P (2014) Adv Energy Mater 4:1400266
Fitzner R, Mena-Osteritz E, Mishra A, Schulz G, Reinold E, Weil M, Körner C, Ziehlke H, Elschner C, Leo K, Riede M, Pfeiffer M, Uhrich C, Bäuerle P (2012) J Am Chem Soc 134:11064
Liu Y, Wan X, Wang F, Zhou J, Long G, Tian J, You J, Yang Y, Chen Y (2011) Adv Energy Mater 1:771
Li Z, He G, Wan X, Liu Y, Zhou J, Long G, Zuo Y, Zhang M, Chen Y (2012) Adv Energy Mater 2:74
Ni W, Wan X, Li M, Wang Y, Chen Y (2015) Chem Commun 51:4936
Zhang Q, Kan B, Liu F, Long G, Wan X, Chen X, Zuo Y, Ni W, Zhang H, Li M, Hu Z, Huang F, Cao Y, Liang Z, Zhang M, Russell TP, Chen Y (2015) Nat Photon 9:35
Kan B, Li M, Zhang Q, Liu F, Wan X, Wang Y, Ni W, Long G, Yang X, Feng H, Zuo Y, Zhang M, Huang F, Cao Y, Russell TP, Chen Y (2015) J Am Chem Soc 137:3886
Wang Z, Li Z, Liu J, Mei J, Li K, Li Y, Peng Q (2016) ACS Appl Mater Interfaces 8:11639
Steinberger S, Mishra A, Reinold E, Levichkov J, Uhrich C, Pfeiffer M, Bäuerle P (2011) Chem Commun 47:1982
Tamayo AB, Dang X-D, Walker B, Seo JH, Kent T, Nguyen T-Q (2009) Appl Phys Lett 94:103301
Ko EY, Choi S, Park GE, Lee DH, Cho MJ, Choi DH (2016) Synth Met 221:39
Hong MH, Ahn J-S, Nguyen DN, Ngo TT, Lee DH, Cho MJ, Choi DH (2016) J Polym Sci Polym Chem 54:1339
Warnan J, Cabanetos C, Labban AE, Hansen MR, Tassone C, Toney MF, Beaujuge PM (2014) Adv Mater 26:4357
Chen H-Y, Hou J, Zhang S, Liang Y, Yang G, Yang Y, Yu L, Wu Y, Li G (2009) Nat Photon 3:649
Wang C, Dong H, Hu W, Liu Y, Zhu D (2012) Chem Rev 112:2208
Garnier F, Horowitz G, Peng XZ, Fichou D (1991) Synth Met 45:163
Horowitz G, Hajlaoui ME (2000) Adv Mater 12:1046
Halik M, Klauk H, Zschieschang U, Schmid G, Ponomarenko S, Kirchmeyer S, Weber W (2003) Adv Mater 15:917
Deman AL, Tardy J, Nicolas Y, Blanchard P, Roncali J (2004) Synth Met 146:365
Katz HE, Torsi L, Dodabalapur A (1995) Chem Mater 7:2235
Halik M, Klauk H, Zschieschang U, Schmid G, Radlik W, Ponomarenko S, Kirchmeyer S, Weber W (2003) J Appl Phys 93:2977
Usta H, Facchetti A, Marks TJ (2011) Acc Chem Res 44:501
Facchetti A, Mushrush M, Yoon MH, Hutchison GR, Ratner MA, Marks TJ (2004) J Am Chem Soc 126:13859
Yoon MH, DiBenedetto SA, Russell MT, Facchetti A, Marks TJ (2007) Chem Mater 19:4864
Ostroverkhova O (2016) Chem Rev 116:13279
Perepichka IF, Perepichka DF, Meng H, Wudl F (2005) Adv Mater 17:2281
Grimsdale AC, Chan KL, Martin RE, Jokisz PG, Holmes AB (2009) Chem Rev 109:897
Perepichka IF, Perepichka DF, Meng H (2009) In: Perepichka IF, Perepichka DF (eds) Handbook of thiophene-based materials. Wiley, Hoboken
Rasmussen SC, Evenson SJ, McCausland CB (2015) Chem Commun 51:4528
Rasmussen SC (2015) In: Mullen K, Kobayashi S (eds) The encyclopedia of polymeric nanomaterials. Springer, Heidelberg
Becker RS, de Melo JS, Macanita AL, Elisei F (1995) Pure Appl Chem 67:9
Pina J, Burrows HD, Becker RS, Dias FB, Maçanita AL, Seixas de Melo J (2006) J Phys Chem B 110:6499
Egelhaaf H-J, Oelkrug D, Oeter D, Ziegler Ch, Göpel W (1995) J Mol Struct 348:405
Zen A, Bilge A, Galbrecht F, Alle R, Meerholz K, Grenzer J, Neher D, Scherf U, Farrell T (2006) J Am Chem Soc 128:3914
Wu H-C, Lee W-Y, Lin C-J, Chen W-C (2013) Mat Chem Phys 138:542
Barbarella G, Pudova O, Arbizzani C, Mastragostino M, Bongini A (1998) J Org Chem 63:1742
Barbarella G, Favaretto L, Sotgiu G, Zambianchi M, Bongini A, Arbizzani C, Mastragostino M, Anni M, Gigli G, Cingolani R (2000) J Am Chem Soc 122:11971
Maria FD, Zangoli M, Palamá IE, Fabiano E, Zanelli A, Monari M, Perinot A, Caironi M, Maiorano V, Maggiore A, Pugliese M, Salatelli E, Gigli G, Viola I, Barbarella G (2016) Adv Funct Mater 26:6970
Jou J-H, Kumar S, Agrawal A, Lia T-H, Sahooa S (2015) J Mater Chem C 3:2974
Noda T, Shirota Y (1998) J Am Chem Soc 120:9714
Noda T, Ogawa H, Shirota Y (1999) Adv Mater 11:283
Li J, Shan T, Yao M, Gao Y, Han X, Yang B, Lu P (2017) J Mater Chem C 5:2552
Mei J, Leung NLC, Kwok RTK, Lam JWY, Tang BZ (2015) Chem Rev 115:11718
Dikcal F, Ozturk T, Cinar ME (2017) Org Commun 10:56
Kumaresan P, Vegiraju S, Ezhumalai Y, Yau SL, Kim C, Lee W-H, Chen M-C (2014) Polymers 6:2645
Wang K, Liang R-Z, Wolf J, Saleem Q, Babics M, Wucher P, Abdelsamie M, Amassian A, Hansen MR, Beaujuge PM (2016) Adv Funct Mater 26:7103
Cui C, Guo X, Min J, Guo B, Cheng X, Zhang M, Brabec CJ, Li Y (2015) Adv Mater 27:7469
Kan B, Zhang Q, Li M, Wan X, Ni W, Long G, Wang Y, Yang X, Feng H, Chen Y (2014) J Am Chem Soc 136:15529
Walker B, Kim C, Nguyen T-Q (2011) Chem Mater 23:470
Roncali J (2009) Acc Chem Res 42:1719
Coughlin JE, Henson ZB, Welch GC, Bazan GC (2014) Acc Chem Res 47:257
Chen Y, Wan X, Long G (2013) Acc Chem Res 46:2645
Kim J, Shim HS, Lee H, Choi MS, Kim JJ, Seo Y (2014) J Phys Chem C 118:11559
Skabara PJ (2009) In: Perepichka IF, Perepichka DF (eds) Handbook of thiophene-based materials. Wiley, Chichester
Fernandes SSM, Castro MCR, Mesquita I, Andrade L, Mendes A, Raposo MMM (2017) Dyes Pigments 136:46
Collins SD, Ran NA, Heiber MC, Nguyen T-Q (2017) Adv Energy Mater 7:1602242
Zhou J, Wan X, Liu Y, Long G, Wang F, Li Z, Zuo Y, Li C, Chen Y (2011) Chem Mater 23:4666
van der Poll TS, Love JA, Nguyen T-Q, Bazan GC (2012) Adv Mater 24:3646
Huang Y, Wen W, Mukherjee S, Ade H, Kramer EJ, Bazan GC (2014) Adv Mater 26:4168
Ni W, Li M, Liu F, Wan X, Feng H, Kan B, Zhang Q, Zhang H, Chen Y (2015) Chem Mater 27:6077
Zhou Z, Xu S, Liu W, Zhang C, Hu Q, Liu F, Russell TP, Zhu X (2017) J Mater Chem A 5:3425
Zhang Y, Zhou Z, Hu Q, Liu F, Russell TP, Zhu X (2017) ACS Appl Mater Interfaces 9:6213
Li M, Liu F, Wan X, Ni W, Kan B, Feng H, Zhang Q, Yang X, Wang Y, Zhang Y, Shen Y, Russell TP, Chen Y (2015) Adv Mater 27:6296
Sun K, Xiao Z, Lu S, Zajaczkowski W, Pisula W, Hanssen E, White JM, Williamson RM, Subbiah J, Ouyang J, Holmes AB, Wong WWH, Jones DJ (2015) Nat Commun 6:6013
Wang Z, Xu X, Li Z, Feng K, Li K, Li Y, Peng Q (2016) Adv Electron Mater 2:1600061
Bin H, Yang Y, Zhang Z-G, Ye L, Ghasemi M, Chen S, Zhang Y, Zhang C, Sun C, Xue L, Yang C, Ade H, Li Y (2017) J Am Chem Soc 139:5085
Song HG, Kim YJ, Lee JS, Kim Y-H, Park CE, Kwon S-K (2016) ACS Appl Mater Interfaces 8:34353
Yong W, Zhang M, Xin X, Li Z, Wu Y, Guo X, Yang Z, Hou J (2013) J Mater Chem A 1:14214
Wang J-L, Liu K-K, Yan J, Wu Z, Liu F, Xiao F, Chang Z-F, Wu H-B, Cao Y, Russell TP (2016) J Am Chem Soc 138:7687
Tsumura A, Koezuka H, Ando T (1986) Appl Phys Lett 49:1210
Jiang W, Li Y, Wang Z (2013) Chem Soc Rev 42:6113
Ponomarenko SA, Kirchmeyer S, Elschner A, Alpatova NM, Halik M, Klauk H, Zschieschang U, Schmid G (2006) Chem Mater 18:579
Xiao K, Liu Y, Qi T, Zhang W, Wang F, Gao J, Qiu W, Ma Y, Cui G, Chen S, Zhan X, Yu G, Qin J, Hu W, Zhu D (2005) J Am Chem Soc 127:13281
Sun YM, Ma YW, Liu YQ, Lin YY, Wang ZY, Wang Y, Di CG, Xiao K, Chen XM, Qiu WF, Zhang B, Yu G, Hu WP, Zhu DB (2006) Adv Funct Mater 16:426
Mori T, Oyama T, Komiyama H, Yasuda T (2017) J Mater Chem C 5:5872
Yang YS, Yasuda T, Kakizoe H, Mieno H, Kino H, Tateyama Y, Adachi C (2013) Chem Commun 49:6483
Yamamoto T, Takimiya K (2007) J Am Chem Soc 129:2224
Kang MJ, Doi I, Mori H, Miyazaki E, Takimiya K, Ikeda M, Kuwabara H (2011) Adv Mater 23:1222
Nakayama K, Hirose Y, Soeda J, Yoshizumi M, Uemura T, Uno M, Li W, Kang MJ, Yamagishi M, Okada Y, Miyazaki E, Nakazawa Y, Nakao A, Takimiya K, Takeya J (2011) Adv Mater 23:1626
Kang M, Hwang H, Park W-T, Khim D, Yeo J-S, Kim Y, Kim Y-J, Noh Y-Y, Kim D-Y (2017) ACS Appl Mater Interfaces 9:2686
Yuan Y, Giri G, Ayzner AL, Zoombelt AP, Mannsfeld SC, Chen J, Nordlund D, Toney MF, Huang J, Bao Z (2014) Nat Commun 5:3005
Lee E-K, Lee MY, Choi A, Kim J-Y, Kweon OY, Kim J-H, Jung JY, Shin T-J, Oh JH, Park J-I, Lee SY (2017) Adv Electron Mater 1700142
Roche GH, Tsai Y-T, Clevers S, Thuau D, Castet F, Geerts YH, Moreau JJE, Wantz G, Dautel OJ (2016) J Mater Chem C 4:6742
Illig S, Eggeman AS, Troisi A, Jiang L, Warwick C, Nikolka M, Schweicher G, Yeates SG, Geerts YH, Anthony JE, Sirringhaus H (2016) Nat Commun 7:10736
Iino H, Usui T, Hanna J (2015) Nat Commun 6:6828
Takimiya K, Nakano M, Sugino H, Osaka I (2016) Synth Met 217:68
Polander LE, Tiwari SP, Pandey L, Seifried BM, Zhang Q, Barlow S, Risko C, Bredas J-L, Kippelen B, Marder SR (2011) Chem Mater 23:3408
Chen M-C, Vegiraju S, Huang C-M, Huang P-Y, Prabakaran K, Yau SL, Chen W-C, Peng W-T, Chao I, Kim C, Tao Y-T (2014) J Mater Chem C 2:8892
Youn J, Vegiraju S, Emery JD, Leever BJ, Kewalramani S, Lou SJ, Zhang S, Prabakaran K, Ezhumalai Y, Kim C, Huang P-Y, Stern C, Chang W-C, Bedzyk MJ, Chen LX, Chen M-C, Facchetti A, Marks TJ (2015) Adv Electron Mater 1:1500098
Vegiraju S, Huang D-Y, Priyanka P, Li Y-S, Luo X-L, Hong S-H, Ni J-S, Tung J-S, Wang C-L, Lien W-C, Yau SL, Liu C-L, Chen M-C (2017) Chem Commun 53:5898
Lin G, Qin Y, Zhang J, Guan Y-S, Xu H, Xu W, Zhu D (2016) J Mater Chem C 4:4470
Lim B, Sun H, Lee J, Noh Y-Y (2017) Sci Rep 7:164
Fukutomi Y, Nakano M, Hu J-Y, Osaka I, Takimiya K (2013) J Am Chem Soc 135:11445
Jarosz T, Lapkowski M, Ledwon P (2014) Macromol Rapid Commun 35:1006
Li P, Cui Y, Song C, Zhang H (2016) J Phys Chem C 120:14484
Barbarella G, Favaretto L, Sotgiu G, Antolini L, Gigli G, Cingolani R, Bongini A (2001) Chem Mater 13:4112
Mazzeo M, Vitale V, Sala FD, Anni M, Barbarella G, Fabaretto L, Sotgiu G, Cingolani R, Gigli G (2005) Adv Mater 17:34
Turkoglu G, Cinar ME, Buyruk A, Tekin E, Mucur SP, Kaya K, Ozturk T (2016) J Mater Chem C 4:6045
Park H, Rao Y, Varlan M, Kim J, Ko S-B, Wang S, Kang Y (2012) Tetrahedron 68:9278
Evenson SJ, Pappenfus TM, Delgado MCR, Radke-Wohlers KR, Navarrete JTL, Rasmussen SC (2012) Phys Chem Chem Phys 14:6101
Durben S, Linder T, Baumgartner T (2010) New J Chem 34:1585
Zhan X, Wu Z, Lin Y, Tang S, Yang J, Hu J, Peng Q, Ma D, Li Q, Li Z (2015) J Mater Chem C 3:5903
Yang Z, Mao Z, Xie Z, Zhang Y, Liu S, Zhao J, Xu J, Chi Z, Aldred MP (2017) Chem Soc Rev 46:915
Yook KS, Lee JY (2012) Adv Mater 24:3169
Lin W-C, Huang W-C, Huang M-H, Fan C-C, Lin H-W, Chen L-Y, Liu Y-W, Lin J-S, Chao T-C, Tseng M-R (2013) J Mater Chem C 1:6835
Pahlavanlu P, Christensen PR, Therrien JA, Wolf MO (2016) J Phys Chem C 120:70
He X, Shan T, Tang X, Gao Y, Li J, Yang B, Lu P (2016) J Mater Chem C 4:10205
Guo X, Baumgarten M, Müllen K (2013) Prog Polym Sci 38:1832
Mehmood U, Al-Ahmed A, Hussein IA (2016) Renew Sustainable Energy Rev 57:550
Lu L, Zheng T, Wu Q, Schneider AM, Zhao D, Yu L (2015) Chem Rev 115:12666
Yao H, Ye L, Zhang H, Li S, Zhang S, Hou J (2016) Chem Rev 116:7397
Cai Y, Huo L, Sun Y (2017) Adv Mater 29:1605437
Liu C, Wang K, Gong X, Heeger AJ (2016) Chem Soc Rev 45:4848
Gélinas S, Rao A, Kumar A, Smith SL, Chin AW, Clark J, van der Poll TS, Bazan GC, Friend RH (2014) Science 343:512
Zhang M, Guo X, Ma W, Ade H, Hou J (2014) Adv Mater 26:5880
Noh S, Gobalasingham NS, Thompson BC (2016) Macromolecules 49:6835
Albrecht S, Janietz S, Schindler W, Frisch J, Kurpiers J, Kniepert J, Inal S, Pingel P, Fostiropoulos K, Koch N, Neher D (2012) J Am Chem Soc 134:14932
Ashraf RS, Meager I, Nikolka M, Kirkus M, Planells M, Schroeder BC, Holliday S, Hurhangee M, Nielsen CB, Sirringhaus H, Mcculloch I (2015) J Am Chem Soc 137:1314
Su M-S, Kuo C-Y, Yuan M-C, Jeng US, Su C-J, Wei K-H (2011) Adv Mater 23:3315
Dou L, Chen C-C, Yoshimura K, Ohya K, Chang W-H, Gao J, Liu Y, Richard E, Yang Y (2013) Macromolecules 46:3384
Zhou N, Guo X, Ortiz RP, Li S, Zhang S, Chang RP, Facchetti A, Marks TJ (2012) Adv Mater 24:2242
Cao J, Liao Q, Du X, Chen J, Xiao Z, Zuo Q, Ding L (2013) Energy Environ Sci 6:3224
Li H, Cao J, Zhou Q, Ding L, Wang J (2015) Nano Energy 15:125
Cao JM, Qian L, Lu FT, Zhang JQ, Feng YQ, Qiu XH, Yip HL, Ding LM (2015) Chem Commun 51:11830
Hou JH, Park MH, Zhang SQ, Yao Y, Chen LM, Li JH, Yang Y (2008) Macromolecules 41:6012
Cui C, Wong W-Y (2016) Macromol Rapid Commun 37:287
Liang Y, Xu Z, Xia J, Tsai S-T, Wu Y, Li G, Ray C, Yu L (2010) Adv Mater 22:E135
Zhang C, Zhu X (2017) Acc Chem Res 50:1342
He Z, Zhong C, Su S, Xu M, Wu H, Cao Y (2012) Nat Photon 6:591
Liao S-H, Jhuo H-J, Cheng Y-S, Chen S-A (2013) Adv Mater 25:4766
Qu S, Wang H, Mo D, Chao P, Yang Z, Li L, Tian L, Chen W, He F (2017) Macromolecules 50:4962
Liu Y, Tang D, Zhang K, Huang P, Wang Z, Zhu K, Li Z, Yuan L, Fan J, Zhou Y, Song B (2017) ACS Omega 2:2489
He Z, Xiao B, Liu F, Wu H, Yang Y, Xiao S, Wang C, Russell TP, Cao Y (2015) Nat Photon 9:174
Lin Y-C, Su Y-W, Li Y-W, Lin B-H, Chen C-H, Chen H-C, Wu K-H, Yang Y, Wei K-H (2017) J Mater Chem A. doi:10.1039/C7TA04144F
Cui C, Wong W-Y, Li Y (2014) Energy Environ Sci 7:2276
Cui C, He Z, Wu Y, Cheng X, Wu H, Li Y, Cao Y, Wong W-Y (2016) Energy Environ Sci 9:885
Ye L, Zhang S, Zhao W, Yao H, Hou J (2014) Chem Mater 26:3603
Zhang SQ, Ye L, Zhao WC, Yang B, Wang Q, Hou JH (2015) Sci China Chem 58:248
Subbiah J, Purushothaman B, Chen M, Qin T, Gao M, Vak D, Scholes FH, Chen X, Watkins SE, Wilson GJ, Holmes AB, Wong WW, Jones DJ (2015) Adv Mater 27:702
Duan C, Gao K, Colberts FJM, Liu F, Meskers SCJ, Wienk MM, Janssen RAJ (2017) Adv Energy Mater 1700519
Cabanetos C, El Labban A, Bartelt JA, Douglas JD, Mateker WR, Fréchet JMJ, McGehee MD, Beaujuge PM (2013) J Am Chem Soc 135:4656
Liu T, Pan X, Meng X, Liu Y, Wei D, Ma W, Huo L, Sun X, Lee TH, Huang M, Choi H, Kim JY, Choy WCH, Sun Y (2017) Adv Mater 29:1604251
Pan X, Xiong W, Liu T, Sun X, Huo L, Wei D, Yu M, Han M, Sun Y (2017) J Mater Chem C 5:4471
Bin H, Zhang ZG, Gao L, Chen S, Zhong L, Xue L, Yang C, Li Y (2016) J Am Chem Soc 138:4657
Bin H, Zhong L, Yang Y, Gao L, Huang H, Sun C, Li X, Xue L, Zhang Z-G, Zhang Z, Li Y (2017) Adv Energy Mater 1700746
Son HJ, Lu L, Chen W, Xu T, Zheng T, Carsten B, Strzalka J, Darling SB, Chen LX, Yu L (2013) Adv Mater 25:838
Gao W, Liu T, Hao M, Wu K, Zhang C, Sun Y, Yang C (2016) Chem Sci 7:6167
Nketia-Yawson B, Lee H-S, Seo D, Yoon Y, Park W-T, Kwak K, Son HJ, Kim BS, Noh Y-Y (2015) Adv Mater 27:3045
Truong MA, Nakano K (2016) Bull Chem Soc Jpn 89:1034
He M, Li J, Sorensen M, Zhang F, Hancock R, Fong J, Pozdin V, Smilgies D, Malliaras G (2009) J Am Chem Soc 131:11930
Yi Z, Ma L, Chen B, Chen D, Chen X, Qin J, Zhan X, Liu Y, Ong WJ, Li J (2013) Chem Mater 25:4290
Zhang A, Xiao C, Wu Y, Li C, Ji Y, Li L, Hu W, Wang Z, Ma W, Li W (2016) Macromolecules 49:6431
Li J, Zhao Y, Tan HS, Guo Y, Di C-A, Yu G, Liu Y, Lin M, Lim SH, Zhou Y, Su H, Ong BS (2012) Sci Rep 2:754
Chen H-J, Liu Z, Zhao Z, Zheng L, Tan S, Yin Z, Zhu C, Liu Y (2016) ACS Appl Mater Interfaces 8:33051
Bronstein H, Chen Z, Ashraf RS, Zhang W, Du J, Durrant JR, Tuladhar PS, Song K, Watkins SE, Geerts Y, Wienk MM, Janssen RAJ, Anthopoulos T, Sirringhaus H, Heeney M, McCulloch I (2011) J Am Chem Soc 133:3272
Perzon E, Zhang FL, Andersson M, Mammo W, Inganas O, Andersson MR (2007) Adv Mater 19:3308
Wang S, Kappl M, Liebewirth I, Müller M, Kirchhoff K, Pisula W, Müllen K (2012) Adv Mater 24:417
Lee YH, Park S, Oh S, Shin JW, Jung J, Yoo S, Lee MH (2017) ACS Appl Mater Interfaces. doi:10.1021/acsami.7b05615
Ban X, Zhu A, Zhang T, Tong Z, Jiang W, Sun Y (2017) ACS Appl Mater Interfaces. doi:10.1021/acsami.7b04146
Ohmori Y, Uchida M, Muro K, Yoshino K (1991) Solid State Commun 80:605
Tang W, Ke L, Tan L, Lin T, Kietzke T, Chen Z-K (2007) Macromolecules 40:6164
Osken I, Gundogan AS, Tekin E, Eroglu MS, Ozturk T (2013) Macromolecules 46:9202
Ohshita J, Kimura K, Lee K-H, Kunai A, Kwak Y-W, Son E-C, Kunugi Y (2007) J Polym Sci Part A Polym Chem 45:4588
Liu MS, Luo J, Jen AK-Y (2003) Chem Mater 15:3496
Mishra SP, Palai AK, Srivastava R, Kamalasanan MN, Patri M (2009) J Polym Sci Part A Polym Chem 47:6514
Evenson SJ, Mumm MJ, Pokhodnya KI, Rasmussen SC (2011) Macromolecules 44:835
Kostyanovsky VA, Susarova DK, Adam G, Lyubovskaya RN, Troshin PA (2013) Mendeleev Commun 23:26
Zhu Y, Gibbons KM, Kulkarni AP, Jenekhe SA (2007) Macromolecules 40:804
Sundararaman A, Victor M, Varughese R, Jäkle F (2005) J Am Chem Soc 127:13748
Yin X, Guo F, Lalancette RA, Jäkle F (2016) Macromolecules 49:537
Guo F, Yin X, Pammer F, Cheng F, Fernandez D, Lalancette RA, Jäkle F (2014) Macromolecules 47:7831
Mishra SP, Palai AK, Kumar A, Srivastava R, Kamalasanan MN, Patri M (2010) Macromol Chem Phys 211:1890
Sahin O, Cinar ME, Tekin E, Mucur SP, Topal S, Suna G, Eroglu MS, Ozturk T (2017) ChemistrySelect 2:2889
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection “Sulfur Chemistry”; edited by Xuefeng Jiang.
Rights and permissions
About this article
Cite this article
Turkoglu, G., Cinar, M.E. & Ozturk, T. Thiophene-Based Organic Semiconductors. Top Curr Chem (Z) 375, 84 (2017). https://doi.org/10.1007/s41061-017-0174-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41061-017-0174-z