Abstract
This article evaluates the desorption of heavy metals from lime-stabilized, contaminated semi-arid soils under harsh environmental conditions involving chemical extractants, specifically, acetic acid (CH3COOH), nitric acid (HNO3), ethylene diamine tetra-acetic acid (EDTA), and diethylene triamine penta-acetic acid (DTPA). In this study, heavy metals, such as arsenic (As), chromium (Cr), mercury (Hg), and lead (Pb), were spiked with two semi-arid soils (exhibiting different plasticity characteristics) at different load ratios (10–250 mg/kg); they were later stabilized with lime to evaluate the reduction in their mobility. Extensive desorption tests were performed on these soil mixtures to study the efficacy of lime for the in situ fixation of heavy metals. This study revealed that irrespective of the nature and size of the heavy metal, the removal efficiency for the soils was greater than 80%, and EDTA at a concentration of 1 M yielded the maximum removal efficiency. It was found that the cationic metal ions Hg and Pb were desorbed effectively by EDTA, while As was desorbed by HNO3 and Cr by DTPA. The order of desorption for heavy metals was found to be as follows: As > Cr > Hg > Pb. The lime amended soils exhibited lower removal efficiencies (more than 48% retained) due to efficient retention of metals in the soil interstices, making them inert and immobile. There was an improvement of 40% in soils amended with lime compared with virgin soils in the case where soil washing by harsh washing agents was resisted; this proves that the contaminants were embedded in the interstices of the soil calcium interfaces. Therefore, this study asserts the notion that the mobility of heavy metal ions from brownfields can be effectively reduced by in situ lime treatment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Soil contamination is a major global issue, and the resulting improper waste disposal practices are a major environmental concern. There are thousands of known brownfields, most of which are contaminated with heavy metals such as arsenic (As), chromium (Cr), mercury (Hg), and lead (Pb). Being toxic, exposure to these contaminants may pose serious health concerns. Thus, a cost-effective and permanent method for treating contaminated sites is urgently needed. Several techniques, such as washing, solidification/stabilization, electrokinetics, bioremediation, and phytoremediation, are available. Soil washing has been a prospective technique used for a long time. However, it has been found to be too laborious and gives rise to liquid wastes that require treatment; this makes it uneconomical, especially in developing nations. Electrokinetics is another technique that involves the use of electricity to treat contaminated soils. Long-term supply of electrical energy for treatment makes this method nonviable on many contaminated sites. Bioremediation and phytoremediation are biological methods, but that are based on slow natural processes, and thus, they require a long remediation time (usually years). With the growing number of contaminated sites especially in industrial countries, the applicable and practical remediation methods are limited [1,2,3].
Stabilization/solidification has been found to be effective for the remediation of heavy metals in soils. The leachability of heavy metals is drastically reduced under stabilization or solidification of the soils, depending on the material used for stabilization. The stabilization of soils makes the heavy metals in the soil get immobilized due to a chemical process via chemical bonds forming an immobile matrix, thereby reducing vaporization into the environment. The advantage of stabilization is that it forms considerably stable and comparatively less leachable contaminant. The process of stabilization may only be effective in lowering the leachability of heavy metals. However, it reduces the complete effect of the heavy metal concentration by producing a byproduct that will remain dormant or inert, and some stabilizers, such as sulfur, chemically bonded phosphate, ceramic encapsulation, and polyethylene encapsulation, have been extensively studied. Still, the associated methods are sensitive in terms of application because pretreatment is required to fix the heavy metals chemically in an insoluble form before encapsulation. Instead, physico-chemical sorption can be achieved in other way using lime for soil stabilization; here, physico-chemical sorption process takes place via the chemical binding of soil matrices, thereby forming stable complexes [4,5,6,7,8]. The solidification/stabilization of contaminated soils relying on low-cost amendments, such as cement, bentonite, fly ash, and lime, along with innovative materials such as nano-compounds, has been found to be a proven technique to encapsulate metal ions.
Moghal et al. [9, 10] have extensively investigated the use of lime amended soil as a sorbent for retaining heavy metals (e.g. Cr, As, Hg, and Pb), conducting detailed batch adsorption and leaching studies on semi-arid and tropical soils. Their results indicated that heavy metal sorption in soils is a multi-step process (surface and chemisorption, redox reactions, ion exchange, precipitation, intra-particle diffusion, and surface complexation) due to the heterogeneity in soils. More importantly, their work inferred that sorption phenomena are effective in the short term, but in the long term, tropical soils desorb the metal ions with aging. Hence, the stability of sorption dependents on the age and surrounding environment.
Among the low-cost amendments, lime has been found to be the most commonly available, effective, and economical stabilizer. A salient feature of lime is that it remains stable over a period of time, whereas many sorbents work over the short term and desorb metal ions in the long term. Despite being amended with good materials [11,12,13]. The stability of sorbed metal ions on sorbents can be ascertained by subjecting them to chemical extraction techniques using a fluid comprising chemical reagents of acids/bases, chelating agents, redox agents, or salts; such reagents help transfer metal ions to an aqueous solution from soils. The soil texture and cation exchange capacity play an important role in the efficient removal of metal [1]. Generally, extractants in the form of acids rely on the phenomenon of ion exchange and dissolution of various components of soil for extracting the entrapped metals. Those desorbed from the soil interface are targeted by the surfactants and metal complexation which are dissolved by chelating agents. Multiple studies have been carried out on soil-washing techniques and applied to field problems [14]. It has been found that this approach is site and metal ion specific. Often, to remove some elements to fulfill the prescribed limits set by regulatory agencies, repeated washing cycles have to be undertaken. Considering these limitations, this study is aimed at working in converse to soil-washing techniques such that a stabilizer added as an amendment to soil must not only stabilize the heavy metals by making them inert and immobile but also perform satisfactorily when subjected to harsh chemical extraction processes.
The stability of lime-stabilized soils to attenuate sorbed heavy metals under harsh chemical environments is investigated. This study focused on targeting a proper stabilizer (for each metal contaminant), thereby minimizing the removal efficiency. Extractant was intended to assess and quantify the stabilizer dosage that would give the maximum removal efficiency and evaluate the efficacy of the selected stabilizer that would give the lowest removal efficiency with an aggressive extractant (higher molar); this can be judged as ideal to treat soils rich in the selected heavy metals. In other words, it means soil amendment is done in a way so that even if subjected to soil washing the encapsulated heavy metal should not be mobile and should stay with soil only.
2 Materials and Methods
2.1 Soils
Soil samples were procured from the ‘Al-Ghat’ area (a locality about 270 km northwest of the city of Riyadh; 26°32′42″N, 43°45′42″E) denoted as ‘Soil A’ and from ‘Al-Qatif’ (a coastal oasis near the western side of the Arabian Gulf, Eastern Saudi Arabia; 26°56′0″N, 50°1′0″E), denoted as ‘Soil B’. The physicochemical properties of these soils were examined and the mineralogy of soil A was found to be kaolinite, while that for soil B was montmorillonite (Table 1). Both the soils were identified as clay with high plasticity CH according to the Unified Soil Classification System [USCS]. The value of the specific gravity for soil A and soil B was found to be 2.84 and 2.71, respectively.
2.2 Contaminants
Analytical grade (AR) calcium hydroxide was used as lime. Nitrate salts (AR) of arsenic, lead, and mercury were used for preparing stock solutions. Potassium di-chromate (AR) was used to prepare hexavalent chromium stock solution. The stock solutions were used in predetermined concentrations to spike the soils to achieve the target levels of As, Cr, Hg, and Pb in the soils. All the chemicals procured for the study were sourced from Winlab Chemical UK. The lime dosages were selected as 2, 4, and 6% by weight of dry soil. The maximum lime dosage was fixed at 6% based on the consumption of lime in the initial stage and optimum lime content for the selected soils based on earlier studies [9, 15,16,17].
The soils were spiked (using salt solutions) with targeted load ratios (10–250 mg/kg) of the respective metal contaminant at an almost wet consistency in borosilicate glass containers. The glass containers were thoroughly rinsed with double-distilled deionized (DI) water and a known quantity of dried soil was added; this was spiked with heavy metal solution and mixed uniformly, maintaining the predetermined load ratios, for all four heavy metals individually. The containers were covered with an aluminum foil marked with small perforations to allow free flow of air and prevent dust and cross-contamination. The samples were kept on a dry platform 1 m above ground and left in a well-ventilated room with a temperature range of 22–25 °C and average relative humidity of 45%. In an earlier study, the authors evaluated the sorption of heavy metals onto lime-stabilized soil and found that the heavy metals initially sorb to the soil, but may partly desorb with time until stabilized conditions are reached during the optimum curing period of 30 days. In this study, lime-treated soils were spiked with metal contaminants in a similar manner and cured for 30 days. At the end of the curing period, the acid digestion technique [20] was conducted, and the concentrations of heavy metals were measured in all the soil samples using an atomic absorption spectrophotometer (AAS;PerkinElmer Model A Analyst 400). This allowed computation of the real contaminant load ratios achieved for each case (Table 2). Table 2 shows that for a target load ratio of 10 mg/kg, the real load ratios found for the different metal contaminants ranged from 1.2 to 7%; moreover, for a load ratio of 250 mg/kg, the real load ratio varied from 2.4 to 8%. Accordingly, the available or real load ratio was used as a benchmark for the calculation of removal efficiencies [18].
2.3 Extractants
The following four extracting agents comprising, two chelating agents and two acids (one strong and one weak) were selected for the desorption testing:
Ethylene diamine tetraacetic acid (EDTA): This is a widely used chelating agent that forms four or six bonds with a metal ion while developing chelates with transition-metal and main-group ion;
Diethylene triamine penta acetic acid (DTPA): This is an aminocarboxylic acid consisting of three nitrogens and five carboxylate groups as coordinating sites. It is an expanded (upgraded) version of EDTA. DTPA wraps across a metal ion as a chelating agent by forming up to eight bonds;
Nitric acid (HNO3): This is a highly corrosive, strong oxidizing agent with a planar molecule; it readily accepts electrons from another substance; and
Acetic acid (CH3COOH): This is the simplest form of carboxylic acid after formic acid, comprising a couple of smaller function groups (AcOH); it is even considered as a group linked by the methyl group and carboxyl group.
Four molar concentrations (0.01, 0.1, 0.5 and 1.0 M) and a solid to liquid ratio of 1:20 were selected for the desorption testing. Apart from these four extractants, double distilled water (DI) was also used for comparison purposes.
2.4 Desorption Testing Program
The soil samples spiked with heavy metals were cured for a period of 30 days; sub-samples of cured soils were used for desorption tests with different extracting agents. Extractants with concentrations of 0 (representing double distilled DI water), 0.1, 0.25, 0.5 and 1 M were taken in 500 mL polytetrafluoroethylene bottles, and subjected to shaking using an ASTM D3987 specified mechanical shaker for 24 h at 30 rpm, maintaining a ratio of liquid to solid (L/S) equal to 20 [19]. This was done until the slurry of soil and water were uniformly dispersed. Whatman 42 ash-less filter paper was used for filtering the slurry, and the residue that passed through the filter paper was tested for its metal contaminant concentrations using the AAS. The pH of the final solution was also measured. All the experiments were done in triplicate and the average value was taken, thereby satisfying the reproducibility and repeatability criterion [20, 21]. The removal efficiency in percentage was then calculated using the following equation:
where CL is the concentration of contaminant in supernatant in mg/L, CS is the concentration of contaminant in soil in mg/kg, VL is the volume of supernatant in liters, and MS is mass of soil (dry) in kg.
3 Results
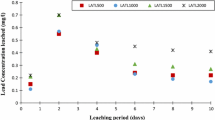
The experimental data obtained by conducting extensive desorption tests were plotted with the concentration of extractants on the X-axis and percentage removal efficiency on the Y-axis for all four heavy metals considered in this study, along with two load ratios of 10 mg/kg and 250 mg/kg, respectively. Soils A and B were amended with three percentages of lime, namely, 2, 4, and 6%. Typical results for unamended soil and lime-amended soil are shown in Figs. 1, 2, respectively. All the other results are shown in the Supplemental Data (Fig. S1–S8).
3.1 Effect of Extractants on Untreated Soils
Four extractants were used for desorbing the four selected metal contaminants (As, Cr, Hg, and Pb); the obtained results are shown in Fig. 1 for unamended soils A and B and in Fig. 2 for soils A and B amended with 6% lime. It can be seen that, for the four different extractants and two soils, the contaminant removal increased proportionally with an increase in the extractant concentration. It was observed that, for different loading rates of 10 mg/kg and 250 mg/kg, there was only a minor difference of 12–15% in the removal efficiency, probably due to the saturation of metal ion species in the matrices of soil at this loading range. In addition, the concentration of extractant remained the same for both the load ratios; hence, the difference in removal efficiency was marginal (Table 3). As a kaolinitic soil, soil A desorbed more than did soil B which is indefinite as a montmorillonite soil. This may be due to the presence of more ligand-based organic matter in soil B. In addition, EDTA was found to desorb the maximum amount compared with the other extracting agents. It can be seen in Fig. 1 that the divalent cations, namely, Hg(II), and Pb(II), were maximally desorbed by EDTA, with desorption in the range of 71–93% for soil A and 69–86% for soil B at a molar concentration of 1 (see Fig. 1). It has been found that, for divalent cations, EDTA is a frequently used chelating agent that forms four or six bonds with a metal ion while forming chelates in terms of the transition-metal and main-group ions.
Similarly, for arsenic which is an anion, the maximum desorption levels were in the range of around 95% and 86% (see Fig. 1) for soil A and B, respectively, in the presence of strong nitric acid (HNO3) as the extractant. This was attributed to the presence of H+ ions in excess, triggering oxidation reactions that eventually resulted in the desorption of As from the soil matrices. In addition, since nitric acid is highly corrosive, it readily accepts electrons from other substances. The excess desorption of As was attributed to the planar molecular nature of nitric acid, in which two of the N–O bonds are relatively short and equivalent. Theories of resonance elucidates that canonical forms exhibit a double bond character that causes the reduction of dimensions compared with the usual N–O bonds; the elongation of the third N–O bond is caused because O is an attached proton [20,21,22,23,24,25,26,27,28,29,30].
3.1.1 pH Effect
The pH level plays an important role in the desorption process, as the pore fluid pH present in the soil has been found to reach the state at which the heavy metals start dissolving or reduce the charge to a minimum of clay minerals. The form of clay minerals and the quantity of H+ ions present in the pore fluid plays a vital role in the process of desorption, and this needs to be evaluated. In addition, the main aim of this work was reducing the desorption by amending the soil with lime so that the maximum sorption of heavy metals in the interstices of mineral phases of the soil would be achieved.
In the desorption of arsenic, the results showed a gradual decrease in the amount of desorbed arsenic with the increase in pH. This decrease in desorption can be attributed to the changes sustained in the sorbate, that is, the soils and the oxide surfaces on the soils, as well as hydroxides of metal complexes under different pH conditions. The protonation of the functional group is affected by the pH of the solution on the surface of the adsorbent of soil and the metal complexes formed from it. At lower pH values, proton bonds are formed between the metal complexes and surfaces due to adsorbed protons, which are exchanged on the surface areas of the soil particles. Metal complexes with positive and negative charges are attracted or repelled on the surfaces, depending on their nature, after they are generated as charges from sorbed protons.
Hexavalent chromium was desorbed to the maximum by DTPA, reaching 86% for un-amended soils and 82% for amended soils, respectively (Figs. 1, 2, and Table 3). The presence of chromate ion with 6 valency played a major role. DTPA is an amino carboxylic acid consisting of three nitrogen and five carboxylate groups as coordinating sites. It is an expanded version of EDTA. DTPA wraps across a metal ion as a chelating agent by forming up to eight bonds. The behavior of Cr in soils when it is desorbed or precipitated is due to a variety of factors, such as the oxidation state, redox potential, pH, competing ions, soil minerals, and complexing agents. The desorption is reduced due to the soil constituents of hydroxyl groups present on the surface of the soils such as Mn, Fe, and Al oxides. The solid oxide/hydroxide–water interface, ferrous hydroxide (Fe(OH)2), ferric chromate (FeCrO4−), and ferrous oxide (FeO) represented the iron-chromium complex. The interface of water and solid hydroxide represented the sorption taking place physically, and this phase is predominant at a pH of 3–10. The interaction of FeO and Fe(OH)2 is considerably different, and the point of zero surface charge occurred at a pH of 9; below this Ph level, Fe(OH)2 was predominant, while above 9 it, FeO was dominant. Fe(OH)2 formed a stable precipitate at pH 9 and remained constant. This demonstrated that the complexation of chromium took place from a pH of 4 to 8. Since Fe is a good reducing agent and Cr is a highly reactive element, the reduction of Cr took place effectively, and protonation of H+ ions occurred. Therefore, Fe(OH)2 was dominant at acidic pH levels and after reaching a neutral pH, FeO became dominant with the complexation of Cr taking place from pH 4 to 8. As a result, it could be concluded that reduction and complexation play an important role in reducing the Cr desorption at varying measures of pH. Fe oxides present in the soil exhibited the strongest affinity for Cr, followed by Al2O3, kaolinite, and montmorillonite.
The use of double distilled DI water, employed as a washing solution, resulted in little removal efficiency of the four metal ions. This indicates that the metal ions bound firmly and did not mobilize voluntarily in aqueous solutions. The use of DI water suggests that it reached the metal fraction, which was bound weakly to the soil particles and was susceptible to mobility. It was found that all the metal ions were strongly bound; hence, the removal efficiencies were quite low, and they were found to be negligible. The phenomenon involved may be attributed to the water clay systems, which are composed of multiple charge layers. The various surfaces resulted in a continuum of rotational and transitional diffusions. There were three main types of H bonded water, each with a different rotational and transitional diffusion rates because of the variation in energy between the water layers which bind them together with cations or interlayer water at the surfaces of external clay.
In a Ca montmorillonite soil balanced with water, it has a couple of layers that hydrate randomly with single layer hydrate. Ca montmorillonite is composed of stacks in multiple layers, with up to 100–400 layers in an unsaturated state. This results in the bi-modal distribution of pore sizes.
Water present between the particles occupies the pores of the micro scale, and the nanopores are filled by the interlayer water, which is called cationic water, with the bond is termed an H-bond. As dehydration of calcium montmorillonite takes place, the loss of the water (H-bond) between the layers, that is, from the micropores, takes place rapidly in the initial few hours. The loss of water is reduced with time, since the cationic water, which is tightly held, does not succumb to dehydration.
At an acidic pH, sorption of cations (Hg and Pb) mostly occurs via ion exchange, and as the pH increases to the alkaline range, precipitation becomes dominant. A desorption study was done to ascertain the sorption capability of soils under harsh conditions in the presence of strong extracting agents. After the desorption test, the pH of the filtered liquid was determined. It was found that, as a strong acid, HNO3 maintained its low pH at all molar concentrations, with a range of 0–2. In contrast, as a weak acid, acetic acid had a pH range of 0–3. It should be recalled that acetic acid is a relatively economical, biodegradable, and environmentally friendly material. Acetates in contact with metals are highly soluble in water, and they can desorb heavy metals from soil particle surfaces. Acetic acid is widely used as a purging solution to enhance the extraction of heavy metals. Similarly, as EDTA and DTPA are chelating agents, the solution pH was in the range of 6–7.
It was found that the pH of EDTA increased with an increase in molarity. This was due to the addition of ammonium hydroxide to dissolve EDTA, which has a netural pH because it is a salt. As a weak acid, acetic acid being a weak acid maintains its pH at a low range, and as the molarity increases, the concentration also increases, resulting in decreased pH. In the case of HNO3, the pH value continues to reduce as the concentration increases. HNO3 maintains its pH at a very low range due to its high acidic property. In this study, desorption with unamended soils was of the order As > Cr > Hg > Pb. The silica surfaces were positively charged at lower pH values. An electron affinity developed in the central silicate ion causing the oxygen atoms to become less alkaline; this eventually generated a weaker acid, forming a silanol (SiOH) group because of the reaction reacts with water. Due to the increase in the pH, the total amount of positively charged entities decreased in relation to the negatively charged entities. The point of zero charge of silica is around 2; the soil surfaces of oxides such as alumina and iron also portray a similar process of developing a combination of positive or negative charges in terms of the pH. The point of zero charge of Fe2O3 is around 6–7, while that of Al2O3 is about 8–8.5. Therefore, a pH increase of solution increases the sorption of metal oxide sites.
The domination of metal hydroxides begins at greater values of pH because the soil surface acquires a negative charge a precipitation reaction starts. Due to a strong disposition of chemical bonding taking place among the oxide surface and metal groups, adsorption of metals occurs in the soil. Hence, sorption of cationic metals is enhanced with increasing pH values because of the attraction of electrostatic forces. Adsorption of metal cations to the surfaces of oxides is quite similar to cations undergoing hydrolysis. The hydroxyl complexes are distributed depending on the pH of the solution and corresponding constants of stability. The hydroxyl metals complexes undergo adsorption with greater inclination compared with the metals that are totally hydrated, since the OH group is formed over the metal, thereby reducing the requirement of free energy for sorption. Therefore, in this study, it was found that the metal ion sorption was linked to the variation in metal hydroxide availability. The inclination of soil to various metal ion sorption level depends on the availability of charged hydroxyl complex metals; the sorbents that were available were oxides of Al2O3, Si2O3, organic ligands, and Fe2O3. With the pH values increasing, a gradual decrease in hydroxide surfaces influenced by the hydronium ions was observed; and that is a result of greater metal removal from the soil solution.
3.2 Effect of Extractants on Lime Treated Soils
As stated above set of tests were conducted on soils amended with 2, 4, and 6% lime to study the effect of lime addition to these soils for desorption. The effects of the molar concentration and loading rate on the selected soils are hereby enumerated in Fig. S2 and S6 with 2% lime addition, Fig. S3 and S7 with 4% lime addition, and Fig. S4 and S8 with 6% lime addition for the four selected 4 metal ions.
In addition, Table 3 gives consolidated data on the best performance of the different extractants used in this study. It was found that the behaviors of the four extractants used on the metal ions were similar to the behavior as observed with untreated soils (Table 3), but the removal efficiency was reduced with the addition of different percentages of lime, and this occurred in proportion to the percentage of lime added. It can be seen in Fig. S2, S3, S4, S7, and S8 that the lime addition and removal efficiency were inversely proportional. There was a decrease in removal efficiency from 71% for untreated soil to 37% for soil amended with 6% lime. It can be inferred from the given data that 48% of metal ions were retained in the soil–lime mixtures and did not desorb despite being subjected to harsh extractants (Table 3). There are four explanations for this behavior by metals mobilized in soils can, which are as follows (a) a change in acidity, (b) a change in the strength of the solution’s ionic form, (c) redox potential change, and (d) complexes being formed. These explanations are discussed below.
It was found that, due to the change in acidity, the solution ionic strength for all four extracting agents for soil amended with lime varied, and behaved quite differently from those in untreated soil. It was observed that, at a low solution concentration of 0.01 M, the pH was in a neutral range for all the four extracting agents. This may have been due to the presence of very low strength acid at a molarity of 0.01, as well as because the addition of calcium may have increased the pH of the solution. Meanwhile, due to the increase in the solution concentration, the acidity of the solution increased, and hence, at a higher molar concentration, the pH moved toward the acidic range. For EDTA and DTPA, the pH values reduced from 7.27 to 6.76 as the concentration increased. As EDTA percolates through the soil, it extracts metal ions, and they become complexed. It has been reported that there is no significant adsorption of EDTA species on soil during its interaction with soil elements. For acetic acid, the pH changed from 7.35 to 3.05 as the concentration increased. The pH value for HNO3 was 6.81 for a concentration range of 0–0.01 M; then, it reduced to 0.58 at 0.1 M and 0.17 at a concentration of 1 M. It can be inferred that a higher extractant concentration correlates with a harsher environment for the retention of metal ions in soils.
4 Discussion
This study aimed to elucidate the desorption behavior of soils amended with lime and spiked with heavy metals. The samples were subjected to soil washing using distilled water, strong and weak acid, and two chelating agents A nonconventional method was used that involved obtaining a condition based on amendment and the concentration of desorbing agents to find the best combination for maximal desorption resistance. In the course of the study, the phenomena involved in the behavior of our samples were considered in relation to a wide range of studies in the literature available from various fields. The findings are related to previously published studies below.
Extraction efficiency is influenced by the following factors; the type of metal extracted-metal chelant ratio, soil physicochemical characteristics, and adsorption and biodegradability of the chelant. The formed soluble metal chelant complexes can dissolve soil hydroxides, and the solubilized metals can compete with the chelating agent. Fe, Mn, Al, and Ca are important competing agents for the ligand. The dissolution of soil mineral phases containing Fe, Mn, Al, and Ca reduced the amount of available ligands for the targeted metal.
X-ray diffraction (XRD) readings samples classified the different minerals available in the two soils under study. The minerals illite, dolomite, muscovite, halloysite, quartz, kaolinite, dickite, montmorillonite, and calcium sulphate hydroxide were found to be prominent. Major constituents of Al, Mg, Fe, and Si were identified.
The addition of lime contributes to Ca in the soil system, and it starts competing and thereby reducing the available ligands for complexation. In this study, statistical analysis was performed on a variety of soils using multiple regression and support vector machine models. The rankings of retention or resistance to desorption were determined for Quartz (Cu = Pb > Zn > Cr), Kaolinite (Cu > Cr > Pb > Zn), Al oxides (Cr > Zn > Pb > Cu), and Fe oxides (Zn > Pb > Cr = Cu). From the study, it was found that, since the soils belonged to the kaolinitic and montmorillonitic categories, the resistance to desorption was completely in accordance with the findings by Gonzalez et al. [35].
Changes in the redox potential were investigated for all four elements. It was found that the reduction potential for all the elements was of the order of Hg > Pb > As > Cr > Ca. Hg had the highest reduction potential and Ca had the lowest; hence, Hg has the highest affinity for acquiring electrons and being reduced, whereas calcium has the least tendency to acquire electrons, and thus, contributes strongly to the oxidation process.
Solubility products can play an important role to predict the precipitation in specific conditions. They are even effective for identifying a suitable condition for separating two substances of different chemical origin used in the solution by fractional precipitation. The removal efficiency is based on the rate of solubility of the metals present in the fluid used for washing, and this can be ascertained from the solubility product values. The solubility products of our elements were of the order Pb > Ca > Cr > Hg; it can be inferred that Ca and Pb had the highest affinity to form precipitates compared with the other elements, while Hg had the lowest affinity. Since two chelants were used, the concept of stability constants and their role in the formation of complexes was explored, (Kinetic and thermodynamic stabilities were conceived when the establishment of complexes in solution was analyzed). The terminology of thermodynamics indicated that the constants of equilibrium in a reaction depend on the heat liberated in the reaction and change in entropy during the reaction. The greater stability of products of the reaction could be seen due to the greater degree of heat liberated in the reaction. Furthermore, it was found that, with increase in entropy during the reaction, the stability of products also improves. The transformation of the products leading to the equilibrium and rate at which it takes place are referred, to as complexes in kinetic stability. Per the stability constants of different elements, the ranking of affinity of elements to form complexes is of the order Hg > Pb > Cr > Ca. It can be observed that divalent heavy metals have the highest stability constants for complex formation.
The reactivity of the chelating agent mainly depends on the stability constant of the complexes of metal chelants developed. Whenever a chelating agent is added to soil, it complexes the metal present in the soil. This relies on the concentration of metal ions, present in the soil, and their stability constants. Complexation occurs until an equilibrium is reached. This results in the solubilisation and mobilization of metal ions. Based on data available from the sequential extraction procedure, it was found that the complexation occurs mainly in the non-residual fractions namely exchangeable metals and metal bound to hydroxides and organic matter in soil. Elements that are present in the residual fractions are permanently embedded in the interstices of soil and they are non-reactive to external agents. Here, the combination that gave the least removal efficiency proved that the element had embedded in the residual fraction of the soil, and hence, was not washed with the strong chelating agent [16].
The role of the atomic radius (atom size, generally taken as the average of the distance between the boundary of the electron and its nucleus) should also be considered. Because the periphery is not clearly defined in practical terms, the understanding of an atom’s radius is conceived in different ways. The atoms are considered individually, as a mass of many atoms in which molecules are bonded covalently, or in an excited state; their value is found by conducting experiments. This was also explored in this study to elucidate the desorption process. The order of atomic radii for our elements was As < Cr < Hg < Pb ~ Ca. It can be inferred that the elements with the smallest radius had a higher affinity for mobility (desorption). In our study, it was found that all four elements considered desorbed in the same order as given above, that is, As was highly mobile and Pb and Ca were the least mobile [28].
After forming complexes with metals, chelants come in contact with metal hydroxides in soil. They are absorbed on the mineral surfaces. This is an important process for the dissolution of oxides incited by the chelants. Soil destabilization is caused when the amorphous oxides dissolve and the process of dissolution may not be effective due to the presence of crystalline phases.
It was found that, for a soil system when there is gain in ionic strength with reduced adsorption, the system makes it to becomes sorbed over the outer sphere surface complexes and when gain in adsorption and ionic strength makes it becomes sorbed over the inner sphere complexes. The higher sorption with a gain in ionic strength is due to the greater activity of available counter ions in the test solution, which nullifies the charge over the surface developed by the specific ion adsorption.
For divalent cations, adsorption of heavy metals can be understood according to inner sphere complexes. Outer surface complexes are described for the adsorption of earth metals of an alkaline nature. The pH level influences adsorption; that is, at a pH of 3.5 ions are adsorbed by inner-sphere complexes, and at a pH of 6, they are adsorbed by outer sphere complexes. In this study, it was observed that as the concentration of EDTA was increased, the desorption efficiency increased. This shows that most of the ions were present in the outer sphere complex, and hence, the maximum desorption for EDTA took place. [31,32,33,34,35,36].
Divalent ions are precipitated due to lime, which is used as an amendment in soil and thereby increasing the pH of the solution. The presence of Fe, Al and Mn from the hydroxyl groups on the soil surface which are also the constituents of soil, plays a vital role in sorption.
Different levels of pH influence their corresponding speciation of soil due because the hydrous ferric oxides creating an interface between the solid oxide/hydroxide and water. This results in the development of ferrous oxide (FeO), ferrous hydroxide (Fe(OH)2), and the water interphases, and formation of complexes due to the iron zinc (FeOZn+), iron lead (FeOPb+), and iron copper complexes (FeOCu+). When the pH is between 4 and 1, physical adsorption is paramount; the process of adsorption is initiated by the solid hydroxide water interphase. FeO and Fe(OH)2 show distinct behavior in the process of desorption; that is, when the pH happens to be less than 6, Fe(OH)2 is dominant, and when the value of pH increases and surpasses 6, FeO dominates; furthermore, the former develops precipitates when the pH is exactly 6. This phenomenon is observed because the H+ ions undergo protonation. Studies have shown that the divalent metal ion complexation comes into existence when a suitable pH that is, between 4 and 11 is observed [11]. The presence of calcium is one of the factors that provide a suitable situation and maintain pH from 4 to 11; this pH range provides a suitable state for all the reactions to exist. The cause of the range of suitable pH is hypothesized to be the availability of more calcium, which helps divalent ions, be effectively precipitated and even undergo hydrolysis. When the range of pH is between 5 and 8, aluminum tri hydroxide Al(OH)3) is distributed, paving the way for divalent metal ions to undergo sorption and form a stable complex.
Pozzolanic reactions and the process of carbonation caused the lime used as an additive to be consumed in the soil when the curing takes place; it is also assumed that during the curing time, the formation of crystalline calcites takes place because of lime carbonation reactions. Clay changes its form to a noncrystalline state because the clay lattice is delaminated. The carbonation of calcium silicate hydrate (CSH) leads to the development of calcium carbonate and may be due to the formation of amorphous phases which detach themselves at a lesser temperature. Carbon dioxide (CO2) from the atmosphere enters and is dissolved in the voids of the soil-lime solution when carbonation takes place; the state of the pH in this situation is near 12.4. Eventually, the interaction between the free lime and dissolved CO2 illustrates the precipitation of calcite, which neutralizes the pH of the solution present in the voids. This halts the pozzolanic reactions that developed when the reactive silica and alumina were dissolved. However, the pH of soil pore solutions at the end of a 28-day curing period has been measured to be above pH 12.4, which confirms that the carbonation levels become minimal in lime-treated soil. This enhances the ability of the soil-lime mixture to retain heavy metals even if subjected to chelating agents [37,38,39,40].
In the case of As and Cr, it was found that calcium present in lime has enormous importance in the process of sorption, since it precipitates As ions by increasing the solution pH. It was found that effective sorption, took place when the pH was from 4.5 to 8.5; this was due to the formation of As–Ca and iron chromate complexes in this pH range, which stabilized As and Cr. The addition of calcium in soil immediately increases the pH, and this process leads to the possibility of XOH+ species (X = Pb and Hg) formation in the voids as well as on the surface of the soil. XOH+ species tend to be sorbed along with the metal ions on the surface of the soil; the soil then develops the capability to make more metal ions to remain in the soil. The surface of the soil grain maintains a pH of 0.5–1, which is higher than the usual. Therefore, the metal ion precipitation still has a scope. When the pH is low, the H+ ion concentration is higher and these H+ ions also compete with the metal ions for possibly swapping positions and sites of adsorption, thereby paving the way for lesser retention of metal ions when the pH is low. It was found that the metal adsorption improves with pH in the soil-lime system and attains equilibrium conditions. The possibility of XOH+ formation also exists and the hydrolyzed species are effectively adsorbed on the surfaces of the soil [2, 4].
Immobilization of the metal ions is assumed to occur when the insoluble precipitates are formed and incorporated into the crystalline structure of the metal oxides and clays or when they are entrapped physically in the stagnant water present in the micro and macro scale pores in the soil. Anionic surfactants also show effective results in the removal of Cr and Pb from the soil because of the formation of colloidal micelles, which dissolve the metals. Similarly, metals that are the main source of contamination are differentiated into two components, which are reversible and irreversible metal components. This may be due to the intraparticle movement of particles via leaching and molecular diffusion according to the mechanism of metal binding and within the bounds of soil particles. Components of soil being dissolved due to controlled metal ion motion with the use of acid washes and chelation represents the leading mechanism for EDTA washes during the removal of metal ions by calcium carbonate (CaCO3), which exchanged with complexes of calcium along with the calcium species. When the amounts of heavy metals of interest exceed the limit of their hydroxides’ and carbonates’ solubility, and/or phases of mineral hydroxy–carbonate at a specified pH reading, the precipitation of metals in solid form takes place. Eventually, these minerals in the solid form will become entrapped in soil or the matrix of sediments. Furthermore, the soil comprises humic constituents and minerals that take carboxylic and hydroxyl groups. The acid–base characteristics of these groups bestow the formation of electric charges on the surface of soils, and this plays an important role in metal ions retention. The pH of the solution incites the acid–base balancing reactions of the surface groups; this influences the soil’s metal retention by adsorption with metal ions to various pH-dependent levels. Along with the physical entrapment of hydroxides of metal or solids carbonates, the soil accommodates metals via direct interactions that include complexation.
Stronger chelators demonstrate the absolute solubility of metals with chelators. In addition, chelating agents will interact with potential partitions over the surfaces of soil, depending on the clay content, waste characteristics, metals, mineralogy, humic acid, soil texture, particle size distribution, and pH. In addition, the effectiveness of the chemical process for soil washing depends on high quantities of clay or silt content, high Fe and Ca elements and humic content. The presence of high calcite content increases the buffering capacity and reduces the acid leaching efficiency. The extraction of metals can be inhibited by the humic content in greater quantities due to the –COOH group’s influence on humic substances with more affinity toward heavy metals. The formulation of fluids for extraction can be affected by higher heterogeneity in soils that require multiple processes. This may be why soils amended with lime resist washing from strong chelating agents, and especially, why montmorillonitic soils amended with lime perform better than kaolinitic soils do. Recently, Lu and Zhang [41] demonstrated that the properties of pore fluid have a drastic effect on the sorptive and pressure potentials. They indicated that pore water pressure under unsaturated conditions can rise to a maximum of 0.6 GPA (giga pascal) creating a situation similar to suction; this is induced due to the sorptive potential, making the soil highly dense. It may be noted that both soils A and B amended with 6% lime generally resisted the desorption of heavy metals under harsh washing conditions due to the densification of soil as propounded by Lu and Zhang [11, 39, 41,42,43,44,45,46,47,48,49,50,51].
5 Conclusions
The desorption of metal ions by soils and soils amended by lime was studied for four extractants including a weak acid, strong acid, and two chelating agents on four metal ions, namely as As, Cr, Hg, and Pb. The removal efficiency of the metal ions was found to be highest for As with 1 M HNO3 and Cr with 1 M DTPA, whereas 1 M EDTA desorbed Hg and Pb. There was a drastic reduction in the removal efficiency when the soils were amended with 6% lime, although the worst case scenario was considered with harsh extractants there was an improvement of 48% in the retention of contaminants. It can be concluded that the addition of lime entrapped the metal ions in the interstices of the clay lime mixture, making them immobile and inert to chemical reactions, especially complexations. The classical method of soil washing with different extractants is used worldwide as a viable treatment for brownfields, but it is tedious, time consuming and lacks cost effectiveness. The amendment of soils with a low-cost stabilizer, such as lime, would go a long way toward revolutionizing the treatment options for cleaning up brownfields. In addition, lime can be used as an amendment with soils for landfill liner material, which would effectively attenuate the selected heavy metals.
The desorption experiments tested the stability of soils and lime amended soils for the retention of heavy metals under harsh environments. The following conclusions can be identified from this study;
- 1.
Lime amended soil exhibited greater efficacy levels in retaining heavy metals compared with natural soil, which was attributed to the existence of colloids in the liquid state of the soil–metal–lime complex.
- 2.
Heavy metal retention depends highly on pH. The domination of surface sorption was observed at pH values lower than usual, and precipitation was accommodated at higher pH values. The formation of stable precipitates was mainly due to the presence of Ca in lime.
- 3.
Soil A, as a kaolinitic soil, desorbed more than did soil B, a montmorillonite soil. This may have been due to the presence of more ligand based organic matter in soil B, as well as that EDTA was found to be desorbing the maximum amount when compared with the other extracting agents for metal ions Hg and Pb.
- 4.
For As, the maximum desorption occurred at values of 95% and 86% for soil A and B, respectively, with nitric acid (HNO3) as the extractant.
- 5.
Cr6+ exhibited the maximum desorption with DTPA, with extraction levels of 86% and 82%. DTPA is a strong chelating agent that wraps around the metal ion by forming up to eight bonds.
- 6.
The desorption with soils and lime amended soils was of the order: As > Cr > Hg > Pb.
- 7.
The solubility product of elements was of the order Pb > Ca > Cr > Hg which further corroborates that Ca and Pb exhibit the highest affinity to form precipitates.
- 8.
It was found that the reduction potential for all the elements was in the order of Hg > Pb > As > Cr > Ca. This order reveals that Hg has the highest affinity for acquiring electrons and being reduced, whereas calcium has the least tendency to acquire electrons contributing to a stronger oxidation process.
- 9.
Based on the stability constants of the selected metal ions, their affinity to form complexes is of the order Hg > Pb > Cr > Ca. It can be observed that divalent heavy metals form stable complexes owing to greater stability constants.
References
Peters RW (1999) Chelant extraction of heavy metals from contaminated soils. J Hazard Mater 66:151–210
Reddy KR, Chinthamreddy S (2000) Comparison of extractants for removing heavy metals from contaminated clayey soils. J Soil Sediment Contam 9:449–461
Dermont G, Bergeron M, Mercier G, Lafleche MR (2008) Metal contaminated soils: remediation practices and treatment technologies. J Pract Period Hazard Toxic Radioact Waste Manag 12(3):188–209
Reddy KR, Danda S, Yükselen-Aksoy Y, Al-Hamdan AZ (2010) Sequestration of heavy metals in soils from two polluted industrial sites: implications on remediation. Land Contam Reclama J 18:13–23
Ghosh S, Mukherjee S, Al-Hamdan AZ, Reddy KR (2013) Efficacy of fine-grained soil as landfill liner material for containment of chrome tannery sludge. J Geotech Geol Eng 31:493–500
Igberase E, Osifo P, Ofomaja A (2014) The adsorption of copper (II) ions by polyaniline graft chitosan beads from aqueous solution: equilibrium, kinetic and desorption studies. J Environ Chem Eng 2:362–369
Jayakumar R, Rajasimman M, Karthikeyan C (2014) Sorption of hexavalent chromium from aqueous solution using marine green algae Halimeda gracilis: optimization, equilibrium, kinetic, thermodynamic and desorption studies. J Environ Chem Eng 2:1261–1274
Trazzi PA, Leahy JJ, Hayes MHB, Kwapinski W (2016) Adsorption and desorption of phosphate on biochars. J Environ Chem Eng 4:37–46
Moghal AAB, Reddy KR, Mohammed SAS, Al- Shamrani MA, Zahid WM (2016) Lime amended semi arid soils in retaining copper, lead and zinc from aqueous solutions. Water Air Soil Pollut 227:372
Moghal AAB, Reddy KR, Mohammed SAS, Shamrani MAA, Zahid WM (2017) Sorptive response of chromium and mercury form aqueous solutions using chemically modified soils. ASTM J Test Eval 45(1):104–119
Ghosh A, Saez AE, Ela W (2006) Effect of pH, competitive anions and NOM on the leaching of arsenic from solid residuals. Sci Total Environ 363:46–59
Li N, Li X, Wang C, Shi X, Liu J (2016) Desorption of Cd(II) from tourmaline 46. At acidic conditions: kinetics, equilibrium and thermodynamics. J Environ Chem Eng 4:30–36
Zhu Z, Li W (2013) Efficient adsorption and desorption of Pb2+ from aqueous solution. J Environ Chem Eng 1:838–843
Dermont G, Bergeron M, Mercier G, Lafleche RM (2008) Soil washing for metal removal: a review of physical/chemical technologies and field applications. J Hazard Mater 152:1–31
Moghal AAB, Al-Obaid AK, Al-Refeai TO, Al-Shamrani MA (2015) Compressibility and durability characteristics of lime treated expansive semiarid soils. J Test Eval 43:255–263
Mohammed SAS, Moghal AAB (2016) “Efficacy of nano calcium silicate (NCS) treatment on tropical soils in encapsulating heavy metal ions: leaching studies validation” Innov. Infrastruct Solut 1:21
Mohammed SAS, Sanaulla PF, Moghal AAB (2016) Sustainable use of locally available red earth and black cotton soils to retain Cd2 + and Ni2 + from aqueous solutions. Int J Civil Eng 14(7):491–505
. EPA. 1996. Method 3050B: Acid digestion of sediments, sludges and soils. Revision 2
ASTM D3987-12: standard practice for shake extraction of solid waste with water. ASTM International, West Conshohocken
Harter RD (1983) Effect of soil pH on adsorption of lead, copper, zinc, and nickel. Soil Sci Soc Am J 47:47–51
Reemtsma T, Mehrtens J (1997) Determination of polycyclic aromatic hydrocarbon (PAH) leaching from contaminated soil by a column test with on-line solid phase extraction. Chemosphere 35:2491–2501
Ho TC, Ghai AR, Guo F, Wang KS, Hopper JR (1998) Adsorption and desorption of mercury on sorbents at elevated temperatures. Combust Sci Technol 134:263–289
Stanforth R, Qiu J (2001) Effect of phosphate treatment on the solubility of lead in contaminated soil. Environ Geol 41:1–10
Lenoble V, Bouras O, Deluchat V, Serpaud B, Bollinger JC (2002) Arsenic adsorption onto pillared clays and iron oxides. J Colloid Interface Sci 255:52–58
Tokunaga S, Hakuta T (2002) Acid washing and stabilization of an artificial arsenic-contaminated soil. Chemosphere 46:31–38
Cao X, Ma LQ, Rhue DR, Appel CS (2004) Mechanisms of lead, copper, and zinc retention by phosphate rock. Environ Pollut 131:435–444
Enell A, Reichenberg F, Warfvinge P, Ewald G (2004) A column method for determination of leaching of polycyclic aromatic hydrocarbons from aged contaminated soil. Chemosphere 54:707–715
Kleiv RA, Thornhill M (2004) Adsorptive retention of copper from acidic mine water at the disused sulphide mine at Løkken, central Norway—initial experiments using olivine. Miner Eng 17:195–203
Ladeira ACQ, Ciminelli VST (2004) Adsorption and desorption of arsenic on an oxisol and its constituents. Water Res 38:2087–2094
Lenoble V, Laclautre C, Serpaud B, Deluchat V, Bollinger JC (2004) As(V) retention and As(III) simultaneous oxidation and removal on a MnO2-loaded polystyrene resin. Sci Total Environ 326:197–207
Maturi K, Reddy KR (2008) Extractants for the removal of mixed contaminants from soils. J Soil Sediment Contam 17:586–608
Bhatnagar A, Vilar VJP, Botelho CMS, Boaventura RAR (2011) A review of the use of red mud as adsorbent for the removal of toxic pollutants from water and wastewater. J Environ Technol 32(3):231–249
Mishra SP (2014) Adsorption and desorption of heavy metal ions. J Curr Sci 104(4):601–612
Gates WP, Bordallo HN, Aldridge LP, Seydel T, Jacobsen H, Marry V, Churchman GJ (2012) Neutron time-of-flight quantification of water desorption isotherms of montmorillonite. J Phys Chem 116:5558–5570
Gonzalez CJ, Costaj J, Matias JM, Covelo EF (2017) Soil Cd, Cr, Cu, Ni, Pb and Zn sorption and retention models using SVM: variable selection and competitive model. J Sci Total Environ 593–594:508–522
Karna RR, Luxton T, Bronstein KE, Redmon JH, Scheckel KG (2017) State of the science review: potential for beneficial use of waste by-products for in situ remediation of metal-contaminated soil and sediment. J Crit Rev Environ Sci Technol 47(2):65–129
Goldberg S, Crisenti LJ, Turner DR, Davis JA, Cantrell KA (2007) Adsorption–desorption processes in subsurface reactive transport modelling. J Vadose Zone 6(3):407–435
Blais JF, Djedidi Z, Bencheikh R, Tyagi RD, Mercier G (2008) Metals precipitation from effluents: review. J Pract Period Hazard Toxic Radioact waste Manag 12:135–149
Potysz A, Grybos M, Kierczak J, Guiband G, Fondaneche P, Lens PNL, Van Hullebusch ED (2017) Metal mobilization from metallurgical wastes by soil organic acids. J Chemosphere 178:197–211
Santos NM, Nascimento CWA, Accioly AM (2017) Guideline values and metal contamination in soils of an environmentally impacted bay. J Water Air Soil Pollution 228(88):1–12
Lu N, Zhang C (2019) Soil sorptive potential: concept, theory and verification. J Geotech Geoenviron Eng 145(4):04019006
Bergendahl J (2005) Batch leaching tests: colloid release and PAH leachability. soil and sediment contamination. Int J 14:527–543
Jing C, Liu S, Patel M, Meng X (2005) Arsenic leachability in water treatment adsorbents. Environ Sci Technol 39:5481–5487
Larous S, Meniai AH, Lehocine MB (2005) Experimental study of the removal of copper from aqueous solutions by adsorption using sawdust. Desalination 185:483–490
Luo Z, Hu C, Zhou J, Cen K (2006) Stability of mercury on three activated carbon sorbents. Fuel Process Technol 87:679–685
Covelo EF, Vega FA, Andrade ML (2007) Heavy metal sorption and desorption capacity of soils containing endogenous contaminants. J Hazard Mater 143:419–430
Jegadeesan G, Al-Abed SR, Pinto P (2008) Influence of trace metal distribution on its leachability from coal fly ash. Fuel 87:1887–1893
Buchireddy PR, Bricka RM, Gent DB (2009) Electro kinetic remediation of wood preservative contaminated soil containing copper, chromium, and arsenic. J Hazard Mater 162:490–497
Cerqueira B, Covelo EF, Andrade-Couce ML, Vega FA (2011) Retention and mobility of copper and lead in soils as influenced by soil horizon properties. Pedosphere. 21:603–614
Baranimotlagh M, Gholami M (2013) Time-dependent zinc desorption in some calcareous soils of Iran. Pedosphere 23:185–193
Yang G-X, Jiang H (2014) Amino modification of biochar for enhanced adsorption of copper ions from synthetic wastewater. Water Res 48:396–405
Acknowledgements
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for funding this work through Research Group no. RG-1440-073. The authors thank the reviewers for their constructive comments which helped the cause of manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Moghal, A.A.B., Mohammed, S.A.S., Almajed, A. et al. Desorption of Heavy Metals from Lime-Stabilized Arid-Soils using Different Extractants. Int J Civ Eng 18, 449–461 (2020). https://doi.org/10.1007/s40999-019-00453-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40999-019-00453-y