Abstract
The main objective of this investigation is to replace calcined marl (0, 10, 20%) and condensed silica fume (SF, 0, 7, 10%) partially for ordinary Portland cement (OPC). Marl, a calcium-based supplementary cementitious material (SCM) calcines at a considerably lower temperature (750 °C) than OPC (up to 1480 °C). The calcined marl contributes in prolonged pozzolanic reactions and represents characteristics of latent cement chemistry. To approach the major conclusions, 27 mixes were designed based on the three ratios of water to the total binders (W/B) of 0.38, 0.42 and 0.45. The calcined marl and SF proportioned in mixes with “0, 10 and 20%” and “0, 7 and 10%”, respectively. After the ages of 7, 28 and 90 days all mixes improved, mechanically. In particular, the hardened concretes containing 10 and 20% of calcined marl show stronger reaction for substitution of OPC. In the lower limit of W/B ratio (0.38) mixes with 20% calcined marl exhibit a remarkable increase of some 2.4-fold strengths from 7 to 90 days. Also, the results obtained by tensile strength and modulus of rupture for concrete mixes containing 10 and 20% calcined marl highlight the mechanical progress of hardened concrete after 28 days. Collectively, both SCMs replaced OPC in a considerable amount (up to 30%). However, long-term enhancement in mechanical strength and durability indices are typically supported by calcined marl.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The OPC is the most widely used construction material for concrete production. However, for generating one ton of OPC about 0.7–1.1 ton of CO2 is emitted into the atmosphere, leading to a significant amount of contribution (~ 7%) in total global pollution [1,2,3]. A considerable amount of CO2 emission (~ 50%) produced by the cement industry is from the calcination of limestone, 40% from combustion of fuel in the kiln and 10% from transportation and manufacturing operations [4]. To reduce the amount of CO2 emission from cement production, improvement of manufacturing processes and generating new SCMs such as industrial and natural pozzolans are the main attempts in concrete technology. However, most industrial pozzolans are infrequent, limited in supplies and contribute mostly in early stages of cement reactions [5, 6]. Alternatively, natural pozzolanic resources are geologically available in varied areas and their application as partial substitution for OPC, results in higher improvement because of the close chemical affinity to the composition of OPC. This consequence has recently led to spread out the application of calcined clays for pozzolanic purposes [7,8,9,10]. But little contribution is reported about the dormant period of calcined marl. Hence, the major part of this investigation is concerned with a detailed study of long-term improvement and partial replacement of calcined marl with OPC. Also, the study focuses on the chemical specifications of calcined marl which are related to the enhancement of mechanical strengths and durability indices.
Marl, a natural calcareous-argillaceous clay, geologically occurs as marine sedimentary deposits belonging to the family of pelitic rocks (clay sizes < 0.02 mm) which are dominated by calcite and minor carbonates such as aragonite, dolomite and siderite minerals [11,12,13]. The textural and mineralogical characteristics of marl indicate both detrital-clastic and chemical-biogenic lime-rich mudstones that are consolidated in moderately deep basins (i.e., continental slope). The geological and Ca-based characteristics of marl significantly influence the nature and the amount of phases that can be formed during solid-state reactions under non-equilibrium conditions [12, 14].
In this study, the calcination temperature of marl is low (750 °C) that is substantially lower than the corresponding temperature for OPC (up to 1480 °C). The lower thermal processing and lower magnitude of CaCO3 in marl leads to a valuable advantage which is the lower amount of CO2 emission into the atmosphere. Furthermore, calcined marl has pozzolanic potential for chemical reactions and filler effects [15,16,17]. It shows a substantial effect on the hydration of the clinker phases and behaves as latent cement that in low water to total binders (W/B) ratio creates a very dense microstructure in the hardened paste [e.g. 18–20]. For this reason the calcined marl can be considered as an outstanding SCM. The reactivity of calcined marl is similar to slag and fly ash but with a higher Ca content. The combination of slag and fly ash with high Ca content shows strong pozzolanic and latent cement chemistry [21, 22]. However, the reactivity of calcined marl in combination with SF in OPC, remains clouded in uncertainty [23, 24].
2 Compositional Affinity of RC and Calcined Marl
According to the phase equilibria in the system CaO–SiO2–Al2O3, the following compounds would eventually form in an RC [11,12,13,14]: monocalcium silicate (CS); dicalcium silicate (C2S) or belite; tricalcium disilicate (C3S2); dicalcium aluminosilicate (C2AS) or gehlenite; tricalcium aluminate (C3A) or celite; and tetracalcium aluminoferrite (C4AF) or brownmillerite. In contrast to OPC, tricalcium silicate (C3S) or alite cannot form in RC because of the low-calcination temperatures [11, 13]. In an RC, all of the phases form by solid-state reactions between the constituents of marl, i.e., primarily calcite, quartz, and clay minerals particularly illite, the major minerals recorded in the XRD analysis (Fig. 1). This is the main reason that calcined marl is considered as an appropriate substitution for OPC by this study.
The lime saturation factors (LSF) are based on the quaternary diagram CaO–SiO2–Al2O3–Fe2O3. The one used mostly is: LSF = CaO/[(2.8 × SiO2)+(1.2 × Al2O3 + 0.65 × Fe2O3)]. This module gives LSF for OPCs typically ranging from 0.92 to 0.98 [25,26,27,28]. To obtain information about the ratio of alite to belite, we calculated the LSF for OPC and calcined marl which resulted in 94% and 47%, respectively (Table 1). The LSF value for OPC is within the above-mentioned standard range. The OPC with the higher LSF will have a higher proportion of belite to alite than will a low LSF. Higher LSF indicates that free lime is likely to be present in the OPC [28, 29]. This means that at LSF = 1.0 all the free lime should have combined with belite to form alite. If the LSF is higher than 1.0, the lime will remain freely in OPC [29]. However, the calculated LSF for calcined marl is considerably below 1.0 and falls within the RC values ranging from 46 to 54% (Table 1). Therefore, mixing calcined marl (as RC) with OPC can prevent the formation of free CaO crystals [30].
The LSF module yields a cementation index (CI) of 0.98–1.09 [12]. The CI is used historically to compare the chemical composition of SCMs. For RCs, the CI value ranges from 1.00 to 2.00. Based on the formula of [CI = (2.8 × SiO2 + 1.1 × Al2O3 + 0.7 × Fe2O3)/(CaO + 1.4 × MgO)] presented by [14, 16], the calculated CI values for SCMs (Table 1) are: 0.98 (for OPC) and 2.01 for calcined marl in which both values locate within the CI range reported by [14, 16]. In particular, the CI value of calcined marl (2.01) is very similar to the CI value of RC marls (Sample AT-L1, Table 1) and confirm the compositional feasibility of calcined marl to be used as high initial strength pozzolan and a dormant period SCM [12].
As shown in Table 1, the chemical composition of calcined marl is typically dominated by major oxides including CaO (38.50 wt%), SiO2 (26.41 wt%), Al2O3 (5.54 wt%) and Fe2O3 (2.20 wt%) contents. The amount of individual minor oxides is commonly less than two percent. The XRF data obtained for calcined marl is compositionally similar to the data for the RCs (Table 1). Overall, the calcined marl is moderately lower in loss on ignition (LOI = 23.70 wt%) compared to this value for RC samples (LOI = 29.29–31.49 wt%) which are shown in Table 1. This means that the calcined marl was initially low in organic compounds with no evidence of fossils or fossil traces in hand specimens. However, when RC samples are high in LOI content, extensive micro fossils are observed in their marlstone out crops [e.g. 12]. It is noteworthy that the CI and SiO2/CaO ratios obtained for OPC are usually about half of the corresponding ratios obtained for calcined marl and RCs (Table 1). This is due to higher content of CaO (62.02 wt%) in OPC. Therefore, the high amount of CaO in SCMs is not correlated with reactivity, but the mineralogy and the origin of carbonates are essential factors. The SiO2/CaO ratio for calcined marl is 0.69 which is almost correlated to the upper limit of this ratio when compared with RC samples (SiO2/CaO = 0.58–0.66).
The silica ratio (SR) determines the relationship between solid and liquid phases. Its value should not exceed the limits of 2.1 to 3.4 in raw material [29]. According to the usual formula of the SR modulus [SR = SiO2/(Al2O3 + Fe2O3)], this value is exactly 3.412 for calcined marl of this study. The SR ratio controls mainly the composition of clays and marls, i.e. the higher the SR the higher the amount of silicate phases (C2S and C3S) and the lower the aluminates (C3A and C4AF). Of particular interest is the alumina modulus (AM) that is described as the [AM = Al2O3/Fe2O3] formula which determines the viscosity of the liquid phase. The AM ranges between 0.64 and 2.5 in raw SCMs [31, 32]. The AM value of calcined marl is 2.518 and corresponds to the upper limit of the requirement. Furthermore, according to the ASTM C115 the equivalent alkali (EA) calculation based on the formula of Na2O + 0.658 × K2O should not exceed 0.6 for SCMs. Likewise, the calculated EA value for calcined marl is 0.599 which confirms the standard requirement. Collectively, the assessment and evaluation of all the above compositional features indicate that calcined marl is a proper activated RC for cement reactions.
3 Experimental Setup
To obtain meaningful results for testing compressive and tensile strengths, modulus of rupture, ultrasonic velocity (UV) and specific electrical resistant (ER) of concretes made with calcined marl, 27 mixes were designed (Table 2). For each mix, 15 cylindrical, two prismatic and two cubic specimens were prepared leading to a total of 513 samples, all cured by submerging into water until the testing ages which are commonly after 7, 28 and 90 days (Table 3). The curing temperature was retained at lower degree (20 °C) as the maximum amount of pozzolans (30%) is relatively high. The mixes are based on the three ratios of W/B of 0.38, 0.42 and 0.45. Accordingly, the proportions of calcined marl and SF for contribution in concrete mixes include ratios of “0, 10, 20%” and “0, 7, 10%”, respectively. The total SCMs was kept at 450 kg/m3 for all mixes (Table 2).
Compared to the calcined marl, the proportion of SF used is small. The exact addition rate of SF depends upon the specific performance characteristic to be improved. However, there is no “scientific” method for proportioning SCMs [32]. This means that there is no chart that can be used to derive the exact mixture ingredients to meet a specified level of performance. One caution when working with SF is to ensure that the mixing is adequate to break up the particle agglomerations (see Sect. 4). In this view, many variables such as the ratio of coarse aggregate, the moisture content of aggregate and the ratio of W/B must be taken in consideration. To develop the mixture proportions for a specific project, it is best to follow trial estimation and use SF judiciously. For example, when SF is the only additive for high-performance concrete, the proportion of SF ranges from 0 to 25% but the optimum replacement is 13–15% of the cement weight and then compressive strength decreases [33,34,35]. Also, higher SF demands more water and decreases flexural strength whereas at low W/B ratios, agglomerations and filler effects occur. For the above reasons and due to the relatively limited supplies of SF, the present work restricted the proportion of SF to 7 and 10%. It is notable that this study combines proportions of SF and calcined marl to evaluate and compare the pozzolanic activity of calcined marl with a SF as a commonly used additive in high-performance concretes.
4 Materials and Methods
The OPC used is a commercially available ASTM type (I-425), prepared from Tehran Cement Factory, Iran. The measured physical properties of Tehran OPC include: specific gravity of 3.18 gr/cm3; modulus of fineness of 290 m2/kg and particle size analysis (PSA) of (1–1.5) × 104 nm (Fig. 2). According to the Bogue calculation, the clinker mineral content of OPC determines C3S (alite 54.6 wt%), C2S (belite 20.0 wt%), C3A (aluminate 5.1 wt%) and C4AF (ferrite 9.06 wt%). Its cementation index (CI) is 0.98 which is a typical value for OPCs [30,31,32]. Its CI (0.98) and LOI (2.98) values confirm the standard requirement of Iran cements (ISIRI 389), one of the most used OPCs in the country.
The marlstone was taken from Mashhad cement quarry, crushed to a sandy gravel size. Perfect grinding continued using a Los Angeles mill at the SRTTU lab until the powder became micronized with 90% of particles less than 100 µm in size (Fig. 2). To determine the exact mineralogy of calcined marl, the powder was analysed by XRD after thermal processing. Based on the XRD analysis (Fig. 1) and the differential thermal gravity (DTG) diagram (Fig. 3), the micronized powder was gently calcined at 750 °C and remained in the kiln at that temperature for 3 hours. The process was carried out by the Iranian Mineral Processing Research Centre located in Karaj region, NE Tehran. The calcined marl is chemically similar to a Type Q SCM according to EN197-1:2000 designation. It possesses lower thermal conductivity than OPC.
The thermogravimetric analysis carried out with a Mettler Toledo TGA/SDTA 851. About 150 mg of the frozen sample was weighed into aluminium oxide crucibles. Prior to the thermal analysis, the samples were submitted to a drying step to avoid interference by the non-reacted free or adsorbed water. During the drying step, the sample was kept at 20 °C and dried in the instrument by the purge gas, N2, at a flow of 50 ml/min. This procedure lasted for 1–2 h. At that time the mass of the sample had become more or less constant. Immediately after the drying step, the thermal analysis was carried out. The sample was heated from 20 °C to 1200 °C with a heating rate of 10°C/min.
The thermogravimetric analysis of marl occurred at three stages. At low temperature (~ 110 °C), free or adsorbed water is released. At mid-range temperature (600–800 °C), the loss of bound water or dissociation of hydroxyls from the lattice induced amorphization of the lattice structure. At high temperature (800–1200 °C), the structure of residual layered mineral (clay) broke-down and recrystallization progressed towards the formation of new mineral and/or glass phases. The maximum pozzolanic activity of marl corresponds to the temperatures between the events identified as dehydroxylation and recrystallization [e.g. 35]. This temperature event is around 750 to 850 °C, illustrated in DTG diagram (Fig. 3). Because in this study SF is combined with calcined marl, the maximum degree of pozzolanic activity of calcined marl is not emphasised but its long-term strengthening (dormant period) is favoured which is attainable in minimum calcination temperature (750 °C).
The SF was added as a dispersive and complementary SCM for improving the pozzolanic reactions of calcined marl at early stages. Physical properties of condensed SF include amorphous spherical particles (size < 0.50 µm) with a density of 2.21 g/cm3 and specific surface area of 20 m2/g (Fig. 2; Table 1). To control the hydration and the slump of fresh concrete particularly at the lower W/B ratios, following the recommendations of ASTM C494 B.D.G and ISIRI 2930, a super plasticizer ZHIKAPLAST (ZP) based on ether carboxylic was added to the concrete mixes in a range of 80–120 mg. To disperse SF uniformly throughout the concrete and to break down agglomerations that make up by condensed SF, after all ingredients were in the mixer, the concrete was mixed for 3 min followed by a 3 min rest then followed by a 2 min final mix, confirming the ASTM C192 specification.
The fine and coarse aggregates used for concrete mixes are naturally occurring gravel and sands that were collected from Karaj River, north Tehran. The aggregates were washed and graded at the pit. The total amount of fine and coarse aggregates occupy 74 wt% of the fresh concrete. The coarse aggregates are mixtures of volcano-sedimentary rocks predominantly consolidated tuff, sandstone, siltstone, basalt and andesite which strongly influence the concrete’s freshly mixed and hardened properties, mixture proportions and economy. The coarse aggregates are quasi-regular in shape and rough in surface with a maximum size of 20 mm and an average of 2% water absorption (moisture content at SSD). The fine aggregates are a mixture of igneous rock forming minerals consisting mainly of quarts, feldspar and sporadic crystals of muscovite. They are hard, strong and durable against alkali silica reactions. Most of the fine aggregate particles are smaller than 5 mm which significantly decrease the size of micro-cracks in concrete. They show average water absorption of 3.66% and a fineness modulus of 4.06. To disperse SCMs uniformly throughout the concrete, the ratio of fine aggregate to coarse aggregate was retained at 2.33 for all mixing designs (Table 1).
5 Mechanical Properties and Durability Indices
5.1 Compressive Strength
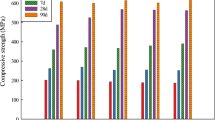
Improvement in the compressive strength of concrete samples by calcined marl and SF is clearly observed when comparing Figs. 4, 5 and 6 for the ages of 7, 28 and 90 days, respectively. In particular, Fig. 6 shows that the uppermost replacement of calcined marl (20%) occurs when W/B is low (0.38) and SF is absent (SF = 0%). In this set of design (W/B = 0.38), contribution of 7 and 10% SF slightly reduces the activity of calcined marl. These insights are the challenging part of this research and designate that calcined marl potentially contributes in pozzolanic and RC reactions, confirming the ASTM C618 requirement. The progressive substitution of calcined marl at the age of 90 days leads to a compressive strength of 60 MPa, indicating that while an OPC paste sets within several hours and attains most of its final strength at the early ages of concrete, the calcined marl develops its full strength within several months. For example, the compressive strength value of the phase with 20% calcined marl and W/B = 0.38, shows an improvement of some 2.4-fold from 7 to 90 days (Figs. 4, 6). This long-term in paste performance is due to the difference between calcined marl and OPC which is usually ascribed to the lack of alite (C3S) and the presence of belite (C2S) in calcined marl [e.g. 16, 32]. Consequently, significant exceeding in compressive strength of calcined marl that of even excellent contemporary OPC means that if calcined marl is used in concrete for aggressive environments or in constructions under water, its hardening will continue for a longer period. This scenario is consistent with the dormant period which is an interval between 1 and 26 weeks in which the hydration of calcined marl occurs slowly and after this time interval its hydration continues at a higher rate [14, 33].
By increasing the age from 7 to 90 days (Figs. 4, 5, 6), substitution of SF in all specimens typically in low W/B ratio of 0.38 shows an increase in compressive strength of marl (up to 55 MPa). Although, by increasing the age all mixes have been improved mechanically, but there are some discrepancies and scattered results regarding to the admixture of SF with calcined marl. For example, the appropriate proportion of SF and calcined marl in low W/B ratio is 10 and 20%, respectively. In higher W/B ratios (0.42 and 0.45), a remarkable trend for reactions of 7 and 10% SF is not observed. Commonly, in a certain age, mixes with higher W/B ratios show lower compressive strength because of decreasing viscosity and development of pores. In other words, by increasing SF and W/B ratio, samples containing 10% calcined marl show better improvement than those with 20%.
It is noteworthy that for most mixes at the age of 7 days (Fig. 4), OPC mixes with/without SF show better improvement compared with calcined marl. At this age, the obtained strengths for all samples are nearly similar and considerable (up to 30 MPa). This implies that at this age, the strength of concrete mainly developed by OPC reactions. For most mix designs at the age of 28 days (Fig. 5), development of compressive strength particularly for samples containing 10 and 20% calcined marl, is preferably observed. This development is explained as the result of pozzolanic reactions and micro-filler effects in concrete microstructures, produced by calcined marl and SF [e.g. 13, 29, 34]. After the age of 28 days, the prolonged hydration time of belite phase in the calcined marl produced C-S-H phases, leading to a significant improvement in compressive strength of mixes (Fig. 6). In these mixes for high W/B ratios, the optimum replacement is 10% for both SF and calcined marl. Collectively, differences in the time of reaction, finer particle size of SF and variation in quality of SCMs produced slightly scattered results for mechanical strength.
5.2 Tensile Strength
The procedure for obtaining the tensile strength results is by breaking cylinders measuring 300 mm by 150 mm (Table 3), following the recommendation of ASTM designation C496. Figure 7 displays the results of splitting tensile strength tests for concrete mixes in three W/B ratios of 0.38, 0.42 and 0.45% all were cured until the age of 28 days. The higher tensile strengths (up to ~ 4.7 MPa) are observed when W/B ratio is 0.38, especially for samples containing 10% calcined marl when combined with 7 and 10% SF. The higher splitting tensile strength indicates that at low W/B ratio, appropriate viscosity and pozzolanic activity together with filler effects of SCMs are essential physio-chemical factors preventing crack or micro-crack development. For mixes designed at higher W/B ratios (0.42 and 0.45), increasing the W/B ratio lowered the viscosity of concrete, leading to a deficiency for enhancement of tensile strength to some extent. Regardless of the SCM proportioning, the mixes that were designed at higher water level represent almost similar tensile strengths (Fig. 7). This similarity implies that the hydrophilicity of calcined marl possibly entrapped more water molecules on its framework layers before entering water to hydration reactions [e.g. 34–36].
5.3 Modulus of Rupture
Figure 8 displays the results of modulus of rupture based on the third-point loading test in accordance with the ASTM C78 designation. The test results were obtained for concrete mixes with three W/B ratios of 0.38, 0.42 and 0.45% at the age of 28 days. For all W/B ratios, mixes containing “10% calcined marl and 7 to 10% SF”; and “20% calcined marl with 10% SF” show slightly higher modulus of ruptures (up to 13.5 MPa) compared to the control samples (SF 0% and Marl 0%). The higher performance of these samples is the result of pozzolanic reactions. At low W/B ratio (0.38) the obtained modulus of ruptures for different mix designs are high. However, in a certain mix design, significant difference between performing the SCMs is not observed. The similarity in the degree of development for OPC and calcined marl in the age of 28 days is responsible for SF because in the absence of SF, contribution of 20% calcined marl is always recognised. By increasing the water ratio, slight scattering data and lowering the strength are observed that may be related to inhomogeneity of concrete. For all mixing designs there is not significant different between the quality performance of SCM proportions. This is attributable to the young age of concretes which is 28 days and at this time long-term progress of calcined marl has not been involved.
5.4 Ultrasonic Velocity (UV)
This test method was performed according to the ASTM C597-16 for pulse velocity through hardened concretes and is applicable to assess the uniformity and relative quality of concrete, to indicate the presence of voids and cracks, and to evaluate the effectiveness of crack repairs. It is also applicable to indicate changes in the properties of concrete, and in the survey of structures, to estimate the severity of deterioration or cracking.
The results of UV tests for concrete mixes with three W/B ratios of 0.38, 0.42 and 0.45%, all at the age of 28 days, are presented in Fig. 9. Compared to control samples (SF0% and marl 0%), a considerable increase in bulk UV (up to 4750 m/s) is observed for mixes at the W/B ratio of 0.38% especially when the amount of calcined marl is 20%. Also, the most important credit for calcined marl is observed in all sets of W/B ratios when SF is absent (SF = 0%), it rather shows higher UV than control specimens (Fig. 9). This is a particular evidence for calcined marl to be likely durable, self-sufficient and can produce high-quality concretes, independently. However, at higher W/B ratios (0.42 and 0.45) all mixes show lower UV and scattered results. When SF proportions are 7 and 10%, a comparable development in UV (equal to control samples) is observed that may be related to the increase of density of mixes and filling pores, all of which are characteristics of total SCMs [12, 13, 15].
5.5 Specific Electrical Resistance (ER)
The bulk electrical conductivity of hardened concretes was determined according to the ASTM C1760-12 and illustrated in Fig. 10. The ER for three sets of W/B ratios and different amounts of SCMs at the age of 28 days shows distinct improvement for all mixes containing calcined marl in the presence or absence of SF, compared to control samples. The higher progress of calcined marl is also incredible even by increasing the water content (e.g. W/B = 0.45). This means that calcined marl developed its pozzolanic reactions exceedingly and can perform a higher durability index than SF and OPC. A significant increase in ER (nearby 200 Ωm) is pronounced in samples containing 20 and 10% calcined marl, and 10% SF at low W/B ratio (0.38). It seems that at lower W/B ratios, finer pore size distribution and less ionic concentrations may be formed [30, 34] and possibly the combination of calcined marl and SF beneficially increased the ER results at 28 days [31]. The obtained ER data for calcined marl designates new application of this material for lowering the rate of corrosion of embedded reinforcing steel after the break down of passivity [30, 31]. For this reason, the present study emphasises that calcined marl is a useful SCM for sustainable development purposes.
6 Summary and Conclusions
The main conclusions of this experiment can be summarised as follows:
-
Marl, a calcareous clay containing CaCO3 as one of the minerals, produces poorly crystalline silicate and aluminate minerals, when burnt at relatively low temperature (750 °C). The similarity in chemical composition of calcined marl and RC indicates that calcined marl is a suitable activated RC for both pozzolanic and long-term reactions in concrete hardening. The long-term in paste performance of calcined marl is due to the lack of alite (C3S) in OPC and the presence of belite (C2S) in calcined marl.
-
Replacement of 10 and 20% of calcined marl for OPC progressively improves the compressive strength (up to 60 MPa) of mixes designed at lower W/B ratio (0.38) in a long-term period. The exceeding strength of 20% calcined marl started after 28 days and continued to higher amount of some 2.4-fold strength at the age of 90 days. Therefore, two strength development profiles have identified, one characterises the pozzolanic reactions until the age of 28 days and the other by a dormant period which lasted for 90 days.
-
By increasing SF and W/B ratios, at the age of 7 days mixes with proportions of 10 and 20% calcined marl show a decrease in development (Fig. 4). At this age, the OPC shows a better performance even in the absence of SF. However, at the age of 28 days in this set of design the trend reversely changed towards the progressive replacement for mixes containing 10 and 20% calcined marl (Fig. 5). This consequence implies that calcined marl performs its pozzolanic reactions, independently.
-
The results of splitting tensile strength tests (up to 4.7 MPa) and modulus of rupture (up to 13.5 MPa) for concrete mixes with 10 and 20% calcined marl are mostly higher than or hardly equal to the control mixes after 28 days.
-
Data obtained for ultrasonic velocity and specific electrical resistance show that at the age of 28 days, pozzolanic reactions of calcined marl probably created very dense microstructures. In particular, the result of specific ER (Fig. 10) records higher development for all samples of calcined marl, compared with OPC. In this view, mixes made by calcined marl are highly compacted.
-
As a final point, clear identification of the novel findings for calcined marl include its higher development in pozzolanic reactions than SF, its long-term enhancement in mechanical strengths and durability indices, and better performance in low water contents when compared to OPC and SF. If the calcined marl would be used in aggressive environments, the rate of cement corrosion and deterioration of embedded reinforcing steel would be reduced.
References
Gartner E (2004) Industrially interesting approaches to “low–CO2” cements”. Cem Concr Res 34:1489–1498
Sata V, Sathonsaowaphak A, Chindaprasirt P (2012) Resistance of lignite bottom ash geopolymer mortar to sulphate and sulfuric acid attack. Cement Concrete Composite 34:700–708
Hughes DC, Jaglin D, Kozlowski R, Mayr N, Mucha D, Weber J (2007) Calcination of marls to produce Roman cement. J ASTM Int 4(1):1–12. https://doi.org/10.1520/JAI100661. https://www.astm.org
Bosoaga A, Masek O, Oakey JE (2009) CO2 capture technologies for cement industry. Energy Proc 1(1):133–140
Zhang MH, Lastra R, Malhotra VM (1996) Rice-Husk ash paste and concrete: some aspects of hydration and the microstructure of the interfacial zone between aggregate and paste. Cem Concr Res 26(6):963–977
Kiattikomol K, Jaturapitakkul C, Songpiriyakij S, Chutubtim S (2001) A study of ground coarse fly ashes with different fineness from various sources as pozzolanic materials. Cem Concr Compos 23:335–343
Justnes H, Østnor T, De Weerdt K, Vikan H, Calcined Marl and Clay as Mineral Addition for More Sustainable Concrete Structures. In: Proceedings of the 36th International Conference on Our World in Concrete & Structures, 14–16 August 2011; Singapore, ISBN 978-981-08-9528-0. http://cipermier.com/100036010
Papadakis VG, Tsimas S (2002) Supplementary cementing materials in concrete part I: efficiency and design. Cem Concr Res 32:1525–1532
Wang Q, Yan P, Mi G (2012) Effect of blended steel slag–GBFS mineral admixture on hydration and strength of cement. Constr Build Mater 35:8–14
Antiohos S, Maganari K, Tsimas S (2005) Evaluation of blends of high and low calcium fly ashes for use as supplementary cementing materials. Cem Concr Compos 27:349–356
Hughes DC, Jaglin D, Kozlowski R, Mucha D (2009) Roman cements-Belite cements calcined at low temperature. Cem Concr Res 39:77–89
Weber J, Gadermayr N, Kozlowski R, Mucha D, Hughes DC, Jaglin D (2007) Microstructure and mineral composition of roman cements produced at defined calcination conditions. Mater Charact 58:1217–1228
Hughes DC, Sugden DB, Jaglin D, Mucha D (2008) Calcination of roman cement: a pilot study using cement-stones from whitby. Constr Build Mater 22:1446–1455
Varas MJ, Alvarez de Buergo M, Fort R (2005) Natural cement as the precursor of Portland cement: methodology for its identification. Cem Concr Res 35:2055–2065
Gutteridge WA, Dalziel JA (1990) Filler Cement: the effect of the secondary component on the hydration of Portland cement: part I: a fine non-hydraulic filler. Cem Concr Res 20(5):778–782
Gutteridge WA, Dalziel JA (1990) Filler cement: the effect of the secondary component on the hydration of Portland cement: part II: fine hydraulic binders. Cem Concr Res 20(6):853–861
Lothenbach B, Scrivener K, Hooton RD (2011) Supplementary cementitious materials. Cem Concr Res 41:1244–1256
Kolani B, Buffo-Lacarrière L, Sellier A, Escadeillas G, Boutillon L, Linger L (2012) Hydration of slag-blended cements. Cem Concr Compos 34:1009–1018
Aiban SA (1995) Strength and compressibility of Abqaiq Marl, Saudi Arabia. Eng Geol 39:203–215
Fookes PG, Higginbottom IE (1975) The classification and description of near-shore carbonate sediments for engineering purposes. Geotechnique 2:406–411
Hughes D, Swann S, Gardner A (2007) Roman cements part I: its origins and properties. J Archit Conserv 13(1):21–37
Hughes D, Swann S, Gardner A (2007) Roman cements part II: stucco and decorative elements, a conservation strategy. J Archit Conserv 13(3):41–58
Bagheri A, Zanganeh H, Moalemi MM (2012) Mechanical and durability properties of ternary concretes containing silica fume and low reactivity blast furnace slag. Cem Concr Compos 34(5):663–670
Lee KM, Lee HK, Lee SH, Kim GY (2006) Autogenous shrinkage of concrete containing granulated blast-furnace slag. Cem Concr Res 36:1279–1285
Aldea C, Young F, Wang K, Shah SP (2000) Effects of curing conditions on properties of concrete using slag replacement. Cem Concr Res 30:465–472
Chidiac SE, Panesar DK (2008) Evolution of mechanical properties of concrete containing ground granulated blast furnace slag and effects on the scaling resistance test at 28 days. Cem Concr Compos 30:63–71
Lübeck A, Gastaldini ALG, Barin DS, Siqueira HC (2012) Compressive strength and electrical properties of concrete with white Portland cement and blast-furnace slag. Cem Concr Compos 34:392–399
Smarzewski P, Barnat-Hunek D (2017) Property assessment of hybrid fiber-reinforcement ultra-high-performance concrete. Int J Civil Eng. https://doi.org/10.1007/s40999-017-0145-3
Biernacki JJ, Bullard JW, Sant G, Brown K, Glasser FP, Jones S, Ley T, Livingston R, Nicoleau L, Olek J, Sanchez F, Shahsavari R, Stutzman PE, Sobolev K, Prater T (2017) Cements in the 21st century: challenges, perspectives, and opportunities. J Am Ceram Soc 100(7):2746–2773
Avdic N, Delic S, Merdic N (2014) Interpretation of results obtained from analyses of some raw materials for cement production, from Ribnica and Grabovica deposits. Glasnik Hemicara I Tehnologa Bosne I Hercegovine. http://www.pmf.unsa.ba/hemija/glasnik/files/Issue%2042/42-17-20-Avdic.pdf
Mounika RR, Kiran RB, Krishna AV (2017) A study on replacement of cement with fly ash, silica fume and addition of steel slag in concrete. SSRG Int J Civil Eng (SSRG-IJCE) 4(5):82–88 http://www.internationaljournalssrg.org
Bernal SA, Juenger MCG, Xinyuan K, Matthes W, Lothenbach B, De Belie N (2017) Characterization of supplementary cementitious materials by thermal analysis. Mater Struct 50:26.https://doi.org/10.1617/s11527-016-0909-2
Ng S, Jelle BP (2017) Incorporation of polymers into calcined clays as improved thermal insulating materials for construction. Adv Mater Sci Eng. https://doi.org/10.1155/2017/6478236 (Hindawi, Article ID 6478236, 6 pages)
Shi C, Wu Z, Xiao J, Wang D, Huang Z, Fang Z (2015) A review on ultra high performance concrete: part I. Raw materials and mixture design. Constr Build Mater 101:741–751. https://doi.org/10.1016/j.conbuildmat.2015.10.088
Zhang Y, Lv W, Peng H (2016) Shear resistance evaluation of strain-hardening cementitious composites member. Int J Civil Eng. https://doi.org/10.1007/s40999-016-0123-1
Nili M, Azarioon A, Danesh A, Deihimi A (2016) Experimental study and modeling of fiber volume effects on frost resistance of fiber reinforced concrete. Int J Civil Eng. https://doi.org/10.1007/s40999-016-0122-2
Acknowledgements
The authors would like to acknowledge Shahid Rajaee Teacher Training University (SRTTU), Tehran, Iran, for technical laboratory equipment and experimental materials provided for performing this research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Soltani, A., Tarighat, A. & Varmazyari, M. Calcined Marl and Condensed Silica Fume as Partial Replacement for Ordinary Portland Cement. Int J Civ Eng 16, 1549–1559 (2018). https://doi.org/10.1007/s40999-018-0289-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40999-018-0289-9