Abstract
A simple, fast and one-pot synthesis procedure based on the bio-reduction ability of an algal extract solution has been used to produce silver nanoparticles (AgNPs). The obtained colloids have been characterized by UV–Vis, TEM and XRD. Then, the thermal conductivity of the nanofluids containing 0.2, 0.4, and 0.6 wt% AgNP synthesized using Sargassum Angostifolium in deionized water was measured in the range of 25–85 °C. The thermal conductivity increases with increasing the temperature and particle concentrations. A maximum detraction and enhancement of 33% at 0.2 wt% and 25 °C and 31% at 0.6 wt% and 85 °C were observed. Silver–water nanofluid for each concentration has critical temperature of 51, 49 and 47 °C for mass fraction percentage of 0.2, 0.4 and 0.6, respectively. There is a decrease in thermal conductivity value below critical point of temperature so adding nanoparticle for application below these points is not recommended. The measurement of the thermal conductivity of this new kind of nanofluid showed that it is an ideal fluid for heat transfer because of great value in synthesizing nanoparticles with well-controlled sizes.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The synthesis of nanomaterials, especially metal nanoparticles is of current interest due to their wide variety of applications, high specific surface area and high fraction of surface atoms (Lugli et al. 2010; Karni et al. 2012; Zalevsky et al. 2009; Taylor 2008; Kalidindi and Jagirdar 2012; Philippot and Serp 2013; Etheridge et al. 2013; Mata et al. 2012). Because of the unique physicochemical characteristics (Catauro et al. 2005; Krolikowska et al. 2003) they are gaining the interest of scientists for their novel methods of synthesis. Silver nanoparticles have become the focus of intensive research owing to their wide range of applications in different areas (Jiang et al. 2004; Babu and Prabu 2011; Duran et al. 2005; Becker 1999; Silver 2003; Tao et al. 2003; Shiraishi and Toshima 1999). The biosynthesis of nanoparticles is now established as a cost effective and environmentally friendly alternative to chemical and physical methods. A few publications exist for synthesis of nanoparticles from marine plants (Govindaraju et al. 2009; Nabikhan et al. 2010; Venkatpurwar and Pokharkar 2011; Kannan et al. 2013). Sargassum Angostifolium is a brown alga and is known to have beneficial properties such as antioxidants and antibacterial which encouraged us to carry out the present investigation on the synthesis of AgNPs using it (Hwang et al. 2010). However, it is very desirable to devise alternative, ‘green’ methods of nanomaterial preparation that use environmentally friendly reactants. The silver nanoparticles obtained by the green synthesis method are candidates to be used in thermal systems as nanofluid. The present work is part of this new line of research. To the best of our knowledge, there is no report in the literature on the thermal conductivity of silver nanoparticles using extract of the S. Angostifolium.
Many of fluids have poor thermal properties that restrict their use as coolants in industrial applications. Nowadays a number of methods are available to enhance the heat transfer rate of any conventional fluid. One such method may be the addition of small-sized solid particles (millimeter and micrometer) in conventional fluid that can improve its thermal properties. But use of these fluids has shown serious problems such as clogging, high erosion, pressure drop in pipelines and poor stability of suspension. About a decade ago, nanometer-sized particles replaced these milli-and micro-sized particles in the suspension, leading to the development of a new class of fluids called ‘nanofluids’. These nanofluids have a number of advantages, such as, better stability, greater thermal conductivity and lower pressure drop compared to the base fluid. Also, use of these nanofluids has shown a remarkable improvement of performance parameters in machining, such as, milling, grinding, drilling and turning of various metals and their alloys. Sharma reviewed the literature available on nanofluid application in various machining processes (Sharma et al. 2015). Most published studies have focused on the thermal conduction behavior of nanofluids. These studies indicated that addition of nanoparticles in conventional fluids remarkably enhanced their thermal conductivity (Murshed et al. 2008; Choi et al. 2001; Nasiri et al. 2012; Tiwari et al. 2014; Turgut et al. 2009; Yu et al. 2008; Tiwari et al. 2015). Saidur et al. observed that the thermal conductivity of nanofluids increased with the increase in particle volumetric concentration in base fluid (Saidur et al. 2011). The mixing of nanoparticles with base fluid may alter the thermophysical properties of fluids as the nanoparticles possess higher thermal conductivity than base fluids (Wang and Majumdar 2007). However, various experiments have shown that the increase in thermal conductivity might be offset by an increase in viscosity and a little penalty in pressure drop was noticed (Godson et al. 2010a, b; Daungthongsuk and Wongwises 2007; Chang et al. 2012; Sarkar 2011). Recent investigations on the enhancement of thermal conductivity have revealed an enhancement with the metallic nanoparticles and oxide nanoparticles (Xuan et al. 2003; Lee et al. 2008; Das et al. 2003; Masuda et al. 1993; Grimm 1993; Choi and Eastman 2001; Eastman et al. 1997, 2001; Aberoumand et al. 2016; Godson et al. 2010a, b).
In this study, we investigated experimentally the thermal conductivity of the water-based spherical Ag nanoparticle synthesized using Sargassum Angostifolium. Then, we compared the obtained experimental results with two analytical methods (Hamilton–Crosser and Timofeeva methods).
2 Materials and Methods
2.1 Synthesis of Silver Nanoparticles Using Sargassum Angostifolium
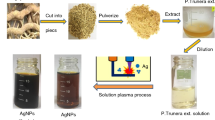
The AgNPs were synthesized according to the procedure suggested by Ghaemi and Gholamipour (2017). In brief, the alga extract used for the reduction of silver ions (Silver nitrate (AgNO3), Merck, analytical grade) to silver nanoparticles was prepared by placing 10 g of washed dried fine cut alga in 250 mL glass beaker along with 100 mL of distilled water. The mixture was then boiled for 15 min until the color of the aqueous solution changes from watery to light yellow color. Then the extract was centrifuged at 6000 rpm for 30 min to remove the heavy biomaterials before filtering with Whatman No. 1 filter paper. The filtrate was collected and stored at 4 °C in order to be used for further experiments. Subsequently, in the round-bottom flask, 50 mL of the extract was added to 50 mL of 1 mM aqueous silver nitrate solution and incubated in dark at 70 °C and pH 10 for 90 min. An InioLab WTW 730 pH meter was used for monitoring the pH values. The samples changed their visual appearance shortly after addition of the extract, indicating that a reduction reaction took place.
2.2 Characterization of AgNPs
The S. Angostifolium extract mediated silver nanoparticles were confirmed with UV–Vis spectrophotometer (Analytik-Jena) equipped with a 1-cm quartz cell. A transmission electron microscope (Zeiss-EM10C) was used for recording of TEM images. The obtained solution then centrifuged at 10,000 rpm for 30 min to isolate the silver nanoparticles from possible impurities. The obtained precipitation was washed three times with double-distilled water and freeze-dried. The freeze-drying process was performed on an Operon model freeze-dryer with the temperature maintained at − 55 °C (Seoul, Korea). The dried powder was used for XRD analysis and preparation of nanofluids. The XRD measurements were taken on the XRD Bruker D8 Advance.
2.3 Nanofluid Preparation
To prepare Ag-based nanofluids with various mass fractions, different masses of AgNPs were added to 25 ml of the base fluids (deionized water (DW). Then the mixtures were subjected to ultrasonic vibration for 30 min at room temperature and obtain uniform dispersions of AgNPs. Using mass fraction in preparing nanofluids seems to be more appropriate than using volume fraction because the precise density value of AgNPs is not available. The prepared fluid suspensions were 0.2, 0.4 and 0.6 wt% AgNP.
There are different ways to measure thermal conductivity by experiments, in this research we use a standard instrument that is used in different researches, KD2 pro a product of DECAGON Company of the United States which was based on the transient hot-wire method, to measure the conductivity of nanofluids. This probe has a heating element and a thermoresistor in side, which is connected to a microprocessor for controlling conducting measurements. In order to obtain accurate results, the experimental apparatus was initially calibrated by glycerin, which was provided by Decagon Devices Inc. for verification use only, with an estimated accuracy less than 2%. The vessel and probe were maintained at a constant temperature for 15 min to reach equilibration before each measurement. Table 1 shows the thermophysical properties of based fluid (DW) and nanoparticle (AgNP) at room temperature. In the present work, the effect of volume fractions on the thermal conductivity of DW/AgNP nanofluids was measured at room temperature (20 °C). In addition, the effect of temperature on the enhancement of thermal conductivity was also studied and the tested temperature range was 25–85 °C with 10 °C intervals.
3 Results and Discussion
3.1 Synthesis and Characterization of the Silver Nanoparticles
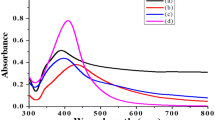
The addition of alga extract to 1 mM AgNO3 solution resulted in color change of the solution from light yellow to brown due to the production of silver nanoparticles. As apparent from Fig. 1, the absorption peak appeared at about 428 nm corresponds to the characteristic surface plasmon resonance of the resulting AgNPs (Ghaemi and Gholamipour 2017). The sample TEM images are provided in Fig. 2. According to the TEM images, the AgNPs are spherical in shape and the average size of the AgNPs was 32 ± 10 nm.
Analysis through XRD was carried out to confirm the crystalline nature of the silver nanoparticles (Fig. 3). A reflection appears in the XRD pattern of Ag NPs at 2θ values of 38.45°, 44.80°, 68.25° and 77.25° corresponding to (111), (200), (220) and (311) Bragg reflections, respectively, which may be indexed based on the face–centered cubic structure of silver (Ghaemi and Gholamipour 2017). X-ray diffraction results clearly show that the silver nanoparticles formed by the reduction of Ag+ ions by the Sargassum extract are crystalline in nature.
3.2 Thermal Conductivity Analysis of AgNPs Nanofluids
3.2.1 Thermal Conductivity Measurement
The Ag nanoparticles suspended in DW with different mass fractions (0.20, 0.40 and 0.6%) were tested to measure the thermal conductivity. We used ultrasonic bath after each experiment to have a smooth and uniform nanofluid to increase accuracy of our experiments. The thermal conductivity of the DW-based AgNPs nanofluids as a function of AgNPs concentration was measured at room temperature (25 °C). The measured thermal conductivity values are shown in Fig. 4. The results show that the thermal conductivity of dispersed nanoparticles in suspension, in this temperature, is less than the base fluid. But, with increasing the concentration of nanoparticles, the thermal conductivity of nanofluids increases very slowly due to thermal contact resistance (Wei et al. 2017). The same results were obtained at 35 and 45 °C. At higher temperature, the thermal conductivity of dispersed nanoparticles in suspension was more than the base fluid and with increasing the concentration of nanoparticles, the thermal conductivity of nanofluids increases with higher slope rather than lower temperature. On the other hand, thermal conductivity of AgNPs nanofluids as a function of tested temperature (in the range of 25–85 °C with 10 °C intervals) is exhibited in Fig. 5 and Table 2. From Table 2, for each concentration, regardless of other samples, thermal conductivity increases by increasing temperature, but there is a conflict that thermal conductivity of all samples were below DI water in temperatures below a specific temperature which was named critical temperature. We showed this fact in Fig. 5. Also, as shown in Fig. 5, with the increase in temperature, the thermal conductivity of deionized water was almost constant compared with AgNPs nanofluids, while the thermal conductivity of nanofluids was increasing. Furthermore, below the critical point, as the temperatures increased, the thermal conductivity of more concentrated AgNPs fluids is increased in a sharper manner than that of less concentration AgNPs fluids. The critical temperature for 0.6% of mass fraction is about 47 °C and for 0.2 and 0.4% is 51 and 49 °C, respectively. It means that adding nanoparticle for applications that the working temperature is under critical temperature not only is not useful, but also reduces performance and productivity. There is a high slope on thermal conductivity increasing around critical temperature for all of the three samples and after that we have moderate slope of enhancing thermal conductivity for each concentration.
As we showed in Fig. 5, addition of nanoparticle to DW has enhanced thermal conductivity for 0.2, 0.4 and 0.6% mass concentration, about 4.6, 10.7 and 17.03%, respectively, at 55 °C. For temperatures 65, 75 and 85 °C increase in thermal conductivity of samples in comparison with DW is about values at 55 °C temperature.
3.2.2 Theoretical Models
In this research, we use different mass fraction percentages but all analytical models are based on volume fraction so Eq. 1 should be used to obtain volume fraction of nanofluids that are reported in Table 3, we calculate the volume fractions at room temperature (Li et al. 2016 and Li et al. 2017).
where φ means the volume fraction of nanofluids, m stands for mass and ρ determines the density. The subscripts n and b represent nanoparticle and base liquid, respectively.
There are various ways to obtain thermal conductivity of nanofluids theoretically, but we used the two models most commonly used in previous works, Hamilton and Crosser equilibrium and Timofeeva model (Hamilton and Crosser 1962; Timofeeva et al. 2007).
Hamilton and Crosser equilibrium is one of the equations that considers the particle shapes in calculating of nanofluid thermal conductivity (Hamilton and Crosser 1962) which is expressed in the following form:
where \(k_{\text{nf}}\), \(k_{\text{w}}\) and \(k_{\text{p}}\) are thermal conductivities of nanofluid, water and particle, respectively, and n is a constant that is six for cylindrical shape of nanoparticles and three for spherical shapes. Hence, we used n = 3 for this research because TEM images demonstrated that the synthesized Ag nanoparticles were spherical.
Timofeeva et al. (2007) used effective medium theory to calculate the thermal conductivity of nanofluids that is very simple compared to previous equation which is expressed as follows:
Table 4 shows calculated thermal conductivity by these two models at different temperature. Also, in this table we represent thermal conductivity of water obtained experimentally at different temperatures. It was noted that, the temperatures below 50 °C were used due to absence of equations for nanofluid thermal conductivity under critical temperature. Hence, we compared experimental data and theoretical data for temperature 55 °C and above. The comparison between experimental data, Timofeeva model and Hamilton–Crosser model with 0.2, 0.4 and 0.6% of mass fraction is shown in Figs. 6, 7 and 8, respectively. According to these figures, there is an obvious agreement between experimental data and theoretical data in high temperatures. As can be seen from Figs. 6, 7 and 8, with increasing concentrations of AgNPs, experimental data and theoretical data are closer together. The maximum difference of experimental data and analytical data does not exceed 2.2% at 55 °C at 0.2% of concentration sample. In all three samples, the slope of increasing thermal conductivity in experimental data and analytical data are approximately the same.
4 Conclusions
Nowadays, scientists and engineers are using the nanoparticles to enhance performance of the fluids in their applications but in some ways there are some conditions that we should consider to obtain optimum results. By this research, we can conclude some results which are categorized below:
-
1.
For thermal conductivity, critical temperature by which enhancement in thermal conductivity of nanofluids can be obtained. Silver–water nanofluid for each concentration has critical temperature of 51, 49 and 47 °C for mass fraction percentage of 0.2, 0.4 and 0.6, respectively.
-
2.
Increasing temperature will increase the thermal conductivity for each concentration
-
3.
Increasing mass fraction (volume fraction) can enhance thermal conductivity.
-
4.
There is a decrease in thermal conductivity value below critical point of temperature so adding nanoparticle for application below these points is not recommended
There is a field of research to consider size of green synthesis nanoparticle effects on nanofluid properties.
References
Aberoumand S, Javaherdeh K, Jafarimoghaddam A, Aberoumand H (2016) A complete experimental investigation on the rheological behavior of silver nanofluid. Heat Transf Asian Res. https://doi.org/10.1002/htj.21212
Babu SA, Prabu HG (2011) Synthesis of AgNPs using the extract of Calotropis procera flower at room temperature. Mater Lett 65:1675–1677
Becker RO (1999) Silver ions in the treatment of local infections. Met Based Drugs 6:297–300
Catauro M, Raucci MG, De Gaaetano FD, Marotta A (2005) Sol–gel processing of drug delivery materials and release kinetics. J Mater Sci Mater Med 16:261–265
Chang TB, Syu SC, Yang YK (2012) Effects of particle volume fraction on spray heat transfer performance of Al2O3-water nanofluid. Int J Heat Mass Transf 55:1014–1021
Choi SUS, Eastman JA (2001) Enhanced heat transfer using nanofluids. US Patent 6,221,275
Choi SUS, Zhang ZG, Yu W, Lockwood FE, Grulke EA (2001) Anomalous thermal conductivity enhancement in nanotube suspensions. Appl Phys Lett 79(14):2252–2254
Das S, Putra N, Thiesen P, Roetzel W (2003) Temperature dependence of thermal conductivity enhancement for nanofluids. J Heat Transf 125:567–574
Daungthongsuk W, Wongwises S (2007) A critical review of convective heat transfer of nanofluids. Renew Sustain Energy Rev 11:797–817
Duran N, Marcato PD, Alves OL, De Souza GIH, Esposito E (2005) Mechanistic aspects of biosynthesis of silver nanoparticles by several Fusarium oxysporum strains. J Nanobiotechnol 3:8–14
Eastman JA, Choi SUS, Li S, Thompson LJ (1997) Enhanced thermal conductivity through the development of nanofluids. Mater Res Soc 457:3–11
Eastman JA, Choi SUS, Li S, Thompson LJ (2001) Anomalously increased effective thermal conductivities of ethylene glycol based nanofluids containing copper nanoparticles. Appl Phys Lett 78:718–720
Etheridge ML, Campbell SA, Erdman AG, Haynes CL, Wolf SM, McCullough J (2013) The big picture on nanomedicine: the state of investigational and approved nanomedicine products. Nanomed Nanotechnol Biol Med 9:1–14
Ghaemi M, Gholamipour S (2017) Controllable synthesis and characterization of silver nanoparticles using Sargassum Angostifolium. Iran J Chem Chem Eng IJCCE 36:1–10
Godson L, Raja B, Mohan Lal D, Wongwises S (2010a) Experimental investigation on the thermal conductivity and viscosity of silver-deionized water nanofluid. Exp Heat Transf 23(4):317–332. https://doi.org/10.1080/08916150903564796
Godson L, Raja B, Lal DM, Wongwises S (2010b) Enhancement of heat transfer using nanofluids—an overview. Renew Sustain Energy Rev 14:629–641
Govindaraju K, Kiruthiga V, Kumar VG, Singaravelu G (2009) Extracellular synthesis of silver nanoparticles by a marine alga, Sargassum wightii Grevilli and their antibacterial effects. J Nanosci Nanotechnol 9:5497–5501
Grimm A (1993) Powdered aluminum-containing heat transfer fluids. German Patent DE 4131516 A1
Hamilton RL, Crosser OK (1962) Thermal conductivity of heterogeneous two-component systems. Ind Eng Chem Fundam 1:187–191
Hwang PA, Wu C, Gau S, Chien SU, Hwang DF (2010) Antioxidant and immune-stimulating activities of hot-water extracts from seaweed Sargassum Hemiphyllum. J Mar Sci Technol 18:41–46
Jiang H, Manolache S, Wong ACL, Denes FS (2004) Plasma enhanced deposition of silver nanoparticles onto polymer and metal surfaces for the generation of antimicrobial characteristics. J Appl Polym Sci 93:1411–1422
Kalidindi SB, Jagirdar BR (2012) Nanocatalysis and prospects of green chemistry. Chem Sus Chem 5:65–75
Kannan RRR, Arumugam R, Ramya D, Manivannan K, Anantharaman P (2013) Green synthesis of silver nanoparticles using marine macroalga Chaetomorpha linum. Appl Nanosci 3:229–233
Karni TC, Langer R, Kohane DS (2012) The smartest materials: the future of nanoelectronics in medicine. ACS Nano 6:6541–6545
Krolikowska A, Kudelski A, Michota A, Bukowska J (2003) SERS studies on the structure of thioglycolic acid mono-layers on silver and gold. Surf Sci 532:227–232
Lee JH, Hwang KS, Jang SP, Lee BH, Kim JH, Choi SUS, Choi CJ (2008) Effective viscosities and thermal conductivities of aqueous nanofluids containing low volume concentrations of Al2O3 nanoparticles. Int J Heat Mass Transf 51:2651–2656
Li X, Zou C, Chen W, Lei X (2016) Experimental investigation of β-cyclodextrin modified carbon nanotubes nanofluids for solar energy systems: stability, optical properties and thermal conductivity. Sol Energy Mater Sol Cells 157:572–579
Li W, Zou C, Li X (2017) Thermo-physical properties of waste cooking oil-based nanofluids. Appl Therm Eng 112:784–792
Lugli P, Locci S, Erlen C, Csaba G (2010) Challenges and Perspectives. In: Korkin A, Krstic PS, Wells JC (eds) Nanotechnology for electronics, photonics, and renewable energy molecular electronics, 1st edn. Springer, New York, pp 1–40
Masuda H, Ebata A, Teramae K, Hishinuma N (1993) Alteration of thermal conductivity and viscosity of liquid by dispersion of ultra-fine particles. Netsu Bussei Jpn 4:227–233
Mata A, Palmer L, Tejeda-Montes E, Stupp SI (2012) Design of biomolecules for nanoengineered biomaterials for regenerative medicine. In: Navarro M, Planell JA (eds) Nanotechnology in regenerative medicine, 1st edn. Springer, Barcelona, pp 39–49
Murshed SMS, Leong KC, Yang C (2008) Investigation of thermal conductivity and viscosity of nanofluids. Int J Therm Sci 47:560–568
Nabikhan A, Kandasamy K, Raj A, Alikunhi AN (2010) Synthesis of silver nanoparticles by callus and leaf extracts from saltmarsh plant Sesuvium portulacastrum L. Colloids Surf B 79:488–493
Nasiri A, Niasar MS, Rashidi AM, Khodafarin R (2012) Effect of CNT structures on thermal conductivity and stability of nanofluid. Int J Heat Mass Transf 55:1529–1535
Philippot K, Serp P (2013) Concepts in nanocatalysis. In: Serp P, Philippot K (eds) Nanomaterials in catalysis, 1st edn. Wiley, Weinheim, pp 1–54
Saidur R, Leong KY, Mohammad HA (2011) A review on applications and challenges of nanofluids. Renew Sustain Energy Rev 15:1646–1668
Sarkar J (2011) A critical review on convective heat transfer correlations of nano-fluids. Renew Sustain Energy Rev 15:3271–3277
Sharma AK, Tiwari AK, Dixit AR (2015) Progress of nanofluid application in machining: a review. Mater Manuf Process 30(7):813–828
Shiraishi Y, Toshima N (1999) Colloidal silver catalysts for oxidation of ethylene. Mol Catal A Chem 141:187–192
Silver S (2003) Bacterial silver resistance: molecular biology and uses and misuses of silver compounds. FEMS Microbiol Rev 27:341–353
Tao A, Kim F, Hess C, Goldberger J, He R, Sun Y, Xia Y, Yang P (2003) Langmuir-blodgett silver nanowire monolayers for molecular sensing using surface-enhanced Raman spectroscopy. Nano Lett 3:1229–1233
Taylor A (2008) Nanophotonics: accessibility and applicability. The National Academies Press, Washington
Timofeeva EV, Gavrilov AN, McCloskey JM, Tolmachev YV (2007) Thermal conductivity and particle agglomeration in alumina nanofluids: experiment and theory. Phys Rev 76:061203
Tiwari AK, Ghosh P, Sarkar J, Dahiya H, Parekh J (2014) Numerical investigation of heat transfer and fluid flow in plate heat exchanger using nanofluids. Int J Therm Sci 85:93–103
Tiwari AK, Ghosh P, Sarkar J (2015) Particle concentration levels of various nano-fluids in plate heat exchanger for best performance. Int J Heat Mass Transf 89:1110–1118
Turgut A, Tavman I, Chirtoc M, Schuchmann HP, Sauter C, Tavman S (2009) Thermal conductivity and viscosity measurements of water based TiO2 nanofluids. Int J Therm phys 30:1213–1226
Venkatpurwar V, Pokharkar V (2011) Green synthesis of silver nanoparticles using marine polysaccharide: study of in vitro antibacterial activity. Mater Lett 65:999–1002
Wang XQ, Majumdar SA (2007) Heat transfer characteristics of nanofluids: a review. Int J Therm Sci 46:1–19
Wei B, Zou C, Yuan X, Li X (2017) Thermo-physical property evaluation of diathermic oil based hybrid nanofluids for heat transfer applications. Int J Heat Mass Transf 107:281–287
Xuan Y, Li Q, Hu W (2003) Aggregation structure and thermal conductivity of nanofluids. AIChE J 49:1038–1043
Yu W, France DM, Routbort JL, Choi SUS (2008) Review and comparison of nano-fluid thermal conductivity and heat transfer enhancements. Heat Transf Eng 29(5):432–460
Zalevsky Z, Mico V, Garcia J (2009) Nanophotonics for optical super resolution from information theoretical perspective: a review. J Nanophoton 3:1–18
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Bakhshan, Y., Samari, F., Ghaemi, M. et al. Experimental Study on the Thermal Conductivity of Silver Nanoparticles Synthesized Using Sargassum Angostifolium. Iran J Sci Technol Trans Mech Eng 43 (Suppl 1), 251–257 (2019). https://doi.org/10.1007/s40997-018-0153-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40997-018-0153-1