Abstract
In the present study, some parameters such as current density, reaction time, pH and the ratios of H2O2/Fe2+ and \(\frac{{{\text{ml}}\,{\text{H}}_{2} {\text{O}}_{2} }}{\text{Lit PW}}\) were investigated and the efficiency of them on the two different electro-Fenton processes using nanoiron particles and AAO electrode as catalyst was discussed. The efficiency of the first case was about 75.52%, and the other one had about 65.03% yield. The results showed that the efficiency of using nanoiron particles was more than that of the electrodes of aluminum.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Petrochemical wastewater (PCW) treatment is a challenge. Petrochemical wastewater is unavoidable and continuously generated in the petroleum refining and petrochemical processes such as liquid–liquid extraction, distillation, cooling systems and washing operations where the use of water is intensive, and this contains a large amount of highly suspended or dissolved toxic and poorly biodegradable organic matters and will result in serious environmental pollution (Mu et al. 2016; Chen et al. 2015; Azimi et al. 2017).
These wastewaters can contain significant amounts of organic and inorganic compounds such as phenols, aliphatic and polycyclic aromatic hydrocarbons (PAHs), oil, heavy metals and other chemicals commonly used in oil production (Cechinel et al. 2016). These are very toxic organisms that inhibit microbial activity and leads to a series of problems, such as poor wastewater quality and unstable performance (Guo et al. 2009). Petrochemical wastewater (PCW) discharge can cause important environmental pollutions and human health worries (Chen et al. 2007, 2015; Hansen et al. 2016; Mohammadi et al. 2005).
Regarding the undesirable effects of this wastewater, various methods for its purification are used: the use of hydrogen peroxide, permanganate, ozonation, chlorine, chlorine dioxide, UV, ozonation in the presence of UV, photo-catalyst method, electrochemical method, solvent extraction, activated carbon adsorption, membrane processes and biological treatment (Ma et al. 2009; Kujawski et al. 2004). Application of some of the purification processes of this industrial wastewater, due to the high cost of treatment, requiring of additional treatment, the formation of hazardous side products and low efficiency, sludge disposal and inefficiency in various concentrations of the pollutant, is limited; for example, its biological treatment is difficult at high concentrations (Bódalo et al. 2009; Bi et al. 2007). In recent years, advanced oxidation processes such as photo-catalyst oxidation, ozonation, ultrasound, Fenton oxidation and photo-Fenton have been proposed for the treatment of sewage containing non-biodegradable biomass or toxic substances and also in sewage with very high concentrations of pollutants (Liao et al. 2009; Palma et al. 2007).
Because advanced oxidation processes (AOPs) are capable of converting organic pollutants into harmless materials, recently they have emerged as powerful alternatives for the treatment of such sewage (Bedolla-Guzman et al. 2016). Generation of reactive oxygen species such as ·OH is the common feature of these methods, and because of hydroxyl radical high standard reduction potential (E° = 2.80 V/SHE), they can attack most organics and convert them into CO2 and inorganic ions. Electro-Fenton process is an AOP which includes the production of Fenton’s reagent in acidic environments (pH = 3), and one or both of the reagents Fe2+ and H2O2 are produced by electrochemical Fenton oxidation (Jaafarzadeh et al. 2017; Chmayssem et al. 2016; Flores et al. 2015; Rezakazemi et al. 2018).
The rapid production and use of nanoscale materials have increased concerns about the potential unwanted effects of nanoparticles (NPs) on the environment and human health (Zhang et al. 2017). Nanoparticles have a significant effect on the process of sewage treatment. The use of Fe3O4 and support of metal oxides such as CeO2, TiO2 and Al2O3 for the synthesis of Fenton such as heterogeneous catalyst have advantages including cost-effectiveness, high activity, simplicity and green perspective (Gogoi et al. 2016; Neyens and Baeyens 2003). In Patra et al.’s report, it is clear that c-Al2O3-a-Fe2O3–core–shell composite nanoparticles in the presence of H2O2 act as a heterogeneous Fenton catalyst to convert cyclohexanone into adipic acid in water, in one step with high catalytic activity (Li et al. 2014; Patra et al. 2013).
In the present study, the removal of COD using heterogeneous EF with nanoiron particles and nanoporous aluminum electrode as catalyst was carried out and the results were compared. The influences of the main operating parameters such as current density, the ratios of H2O2/Fe2+ and \(\frac{{{\text{ml}}\,{\text{H}}_{2} {\text{O}}_{2} }}{\text{Lit PW}}\), pH and reaction time on COD removal were studied.
2 Experimental Section
2.1 Materials and Chemicals
Reagent grade chemicals without further purification were used here. We chose Shanghai Chemical Reagents Company to prepare all the analytical grade chemicals such as ferric chloride, reduced iron powder, sodium borohydride, sulfuric acid and sodium sulfate anhydrous. A Millipore Milli-Q system with resistivity > 18 MX cm at 25 °C was used to obtain ultrapure water for preparing all the solutions. The Merck Company was chosen as a supplier for sulfuric acid and heptahydrated ferrous sulfate preparation for pH adjusting and as a catalyst, respectively. The applied petrochemical wastewater had COD 1400–1700 mg/l, color 100 color unit, BOD/COD 0.4–0.6 and pH 6–6.7.
2.2 Fe@Fe2O3 Preparation
A ferric solution was prepared by dissolving 0.3 g amount of FeCl3.6H2O in 100 mL of distilled water, and for NaBH4 solution 0.6 g amount of NaBH4 was dissolved in 40 mL of distilled water. To reduce the ferric ions into metallic iron, the NaBH4 solution was dropped to solution. Finally, the deionized water was washed thoroughly and then dried in nitrogen (FathinejadJirandehi and Mohebbizadeh 2016).
2.3 Preparation of the Nanoporous Anodic Aluminum Oxide (AAO)
The AAO films were fabricated using the two-step anodization of 6063 aluminum alloy sheets (1 mm thickness). The aluminum sheet was initially cut into 1 cm × 5 cm pieces and degreased in acetone, without further thermal treatment or chemical polishing. The first anodization step was then carried out on the aluminum specimen, suspended in the electrolyte as an anode, under a constant current density of 5 mA/cm2 for 10 h. Another aluminum specimen was used as a cathode. Sulfuric acid solution (0.4 M concentration) was used as electrolyte, and the electrolyte temperature was ambient. The formed AAO film was chemically removed by immersing the specimen in 0.4 M phosphoric acid solution for 1 h. The second anodization step was subsequently conducted under the same condition mentioned before for the first step, to produce the final AAO film with a regular nanopore array. Some final samples were immersed in 0.2 M phosphoric acid to widen the pores. Finally, deionized water was used to rinse the specimens several times and then they were dried in air (Moghadam et al. 2013).
2.4 Electro-Fenton Method
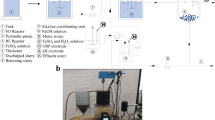
These experiments were carried out in a cylindrical electrochemical chamber of 6 cm diameter and a capacity of 400 ml, and a magnetic stirring was used to increase the mass transport toward the electrodes during the treatment. An acidic range was chosen for pH because a very high pH prevents the development of Fenton-based systems (FathinejadJirandehi and Mohebbizadeh 2016; Tsantaki et al. 2012). The pH was measured using a CyberScan pH 1500 pH meter from Eutech Instruments. A 250 ml of wastewater was used in a single run, and before starting the system certain amounts of hydrogen peroxide and iron (Fe2+) were added. Finally, placement of electrodes was done and the mixing of solutions was done at 350 rpm. Figure 1 shows the schematic of electro-Fenton setup. At the end of the run, the samples were allowed to stand for 30 min (for solids sedimentation) and the supernatant was then taken for wastewater quality measurements. The electrodes were washed thoroughly with water to remove any solid residues on the surfaces. COD was, respectively, measured at 475 nm and 605 nm wavelengths using a UV–Vis spectrophotometer.
3 Results and Discussion
3.1 Electro-Fenton Effective Parameters
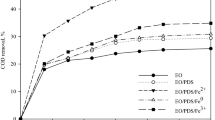
The simultaneous effect of five parameters such as volume fraction of H2O2 to petrochemical wastewater (ml/l), density, pH, time and H2O2/Fe2+ molar ratio on the electro-Fenton process in two separate experiments using nanoiron particles, as well as aluminum nanoporous electrodes, is shown in Figs. 1, 2, 3, 4 and 5, and their effects are compared. The effect of these parameters on the efficiency of COD removal is studied in all these cases. The blue line shows the effect of the iron nanoparticles, and the orange-colored line indicates the effect of the nanoporous aluminum electrode on the removal efficiency of COD. The effect of all parameters in the case of using iron nanoparticles is higher than nanoporous aluminum electrodes, and this effect is examined for each individual parameter. It is observed that the use of iron nanoparticles which simultaneously have the property of being nano and catalyst has a greater impact on the efficiency than that of the aluminum electrodes, which only have the nanoproperty.
In all charts, one peak is observed, where the degree of COD removal reaches its highest level, and then the elimination is reduced. In fact, it can be concluded that the purification operation is only useful to this point, and for the reasons that are described below for each individual parameter, the continuation of the operation is inappropriate and causes waste of energy, material and time, and the result is inverse.
The efficiency of time on the COD removal is shown in Fig. 2. In this diagram, the effect of the use of iron nanoparticles and aluminum nanoporous electrodes has been compared. As can be seen, the efficiency of the use of iron nanoparticles is higher than that of aluminum nanoporous electrodes, and this efficiency is more clearly defined at higher times. Over time, it seems that the impact of aluminum nanoporous will be reduced and this can be due to the reduction of the effective surface of electrodes over time due to corrosion; yet, the effect of the presence of nanoparticles is still high probably because the nanoparticles retain their catalytic role over time and keep the reaction yields high. The positive effect of reaction time on the electro-Fenton progress is clear. But its impact is reduced by the increase in the time so that after optimum time, the efficiency changes are not considerable. The approximately 2/3 of the total time is the optimum response time (Li et al. 2009). Over time, the amount of reactive substances in the reaction medium is reduced and it reduces the rate of removal of contaminants. This effect can be seen in the graphs of the interaction between time and mole ratio and volume ratio, and in low ratios, the increase in time does not have a specific performance improvement and only if the amount of the available materials for the production of antiseptics be sufficient this increasing will be effective. The system must also be given sufficient time to get the proper efficiency until OH ion can be produced sufficiently and purification of organic pollutants in the environment happens, and this is clearly evident in the interaction graphs, as the increase in molar and volume ratios without increasing time, it has a very small effect on the treatment of wastewater. As the time increases, the system appears to reach a chemical equilibrium and the maximum removal value occurs at the beginning of this equilibrium, and the passage of time does not have much effect on the improvement. Another reason for the reduction in the efficiency with the increase in time is the rising of solution temperature. It is noticeable that the increase in the temperature of the electrochemical cell will accelerate kinetic reactions, and as stated, reactants will be consumed more quickly; on the other hand, by increasing the temperature of the system to more than 25 °C, the occurrence of the H2O2 decomposition parasitic reaction according to reactions (1) and (2) to molecular oxygen and water will increase (Pereira and Zaiat 2009):
Figure 3 shows the current density efficiency on COD removal. It is clear that, in general, the efficiency of nanoparticles is much higher than that of nanoporous aluminum electrodes. But, can be seen, at the beginning of the reaction, the use of aluminum nanoporous has higher efficiency, which can be due to the better reaction due to the higher conductivity of aluminum in low currents. It is observed that increase in the flow rate increases the nanoparticles’ efficiency, but this increasing has an optimum point and after passing through the optimum point, the current density increasing efficiency is reversed and the efficiency of the reaction is reduced and we only see the waste of energy and capital. It is also observed that the slope of the efficiency variation versus current density changes in the use of iron nanoparticles is more than the slope changes in aluminum electrodes and this represents a better performance of nanoparticles with current density changes and both of these factors have a direct impact on the H2O2 decomposition and the production of hydroxyl ions. After the optimum point, the process efficiency is reduced. Based on reactions 3 and 4, H2O2 decomposition can explain this phenomenon at high currents in the anode and also Brillas et al. and Zhang et al. reported that (Flox et al. 2006; Brillas et al. 1998)
In addition, according to reaction 5, the recovery of four electrons from the O2 molecule and the production of two molecules of water can be in competition with the H2O2 production (Özcan et al. 2008). As a result, less H2O2 will be produced and the oxidation of the substances in the wastewater will be reduced.
Also, when the current density is zero, the degradation of organic materials is very low, which indicates the importance of the current density applying as an effective parameter in the electro-Fenton process.
Figure 4 shows the pH efficiency on the COD removal. As can be seen, the effect of the presence of iron nanoparticles is more than that of the presence of nanoporous aluminum electrodes. This effect is higher in acidic levels, and at the optimum response point, it reaches its highest level and as it approaches the basic range, this effect is reduced and the effect of the presence of iron nanoparticles and nanoporous electrodes will be approximately equal and their impact is reduced. Reducing the effect of iron nanoparticles occurs with increasing levels of alkalinity, and this indicates that the catalytic effect of nanoparticles is very high in acidic range and with decrease in acidity, this effect is also reduced. The highest removal efficiency is seen at pH = 3. At this pH, the best conditions for hydroxyl radical formation are available in the Fenton process. Also, in acidic conditions, according to reaction 6, hydrogen peroxide is well formed by cathodic rebound. With increase in pH to the neutral range, it is observed that the electro-Fenton process has less efficiency in COD removal which results from the formation of ferric species that reduces ferrous ion reproduction, thereby preventing the production of more hydroxyl radicals (Sheng et al. 2011; Flox et al. 2006).
Figure 5 shows the volume fraction of H2O2 to petrochemical wastewater (\(\frac{{{\text{ml}}\,{\text{H}}_{2} {\text{O}}_{2} }}{\text{Lit PW}}\)) efficiency on the COD removal. As can be seen, the effect of iron nanoparticles is more than the nanoporous aluminum electrodes. This effect is low in both cases at low ratios, and the difference of these effects is approximately equal and as the ratio increases, the impact of these factors also increases and this increase continues to the optimum point, and then it will decrease and the most difference is observed at the optimum point of these two factors. Figure 6 shows the \(\frac{{{\text{mol}}\,{\text{H}}_{2} {\text{O}}_{2} }}{{{\text{mol}}\,{\text{Fe}}^{2 + } }}\) molar ratio efficiency on the COD removal. As can be seen, in this case, the effect of the presence of nanoparticles is more than that of the presence of nanoporous aluminum electrodes. And this effect is low at the beginning of the range and is almost equal in both cases, but as the ratio increases, the impact of the two factors increases and at the optimum point it reaches its maximum level and then it decreases. The slope of increasing and decreasing when using the iron nanoparticles is more than that when using the nanoporous aluminum electrodes, and this reflects the better reactivity of nanoparticles.
The \(\frac{{{\text{mol}}\,{\text{H}}_{2} {\text{O}}_{2} }}{{{\text{mol}}\,{\text{Fe}}^{2 + } }}\) and \(\frac{{{\text{ml}}\,{\text{H}}_{2} {\text{O}}_{2} }}{\text{Lit PW}}\) ratios are dependent on each other; therefore, the effects of both are considered together to study COD elimination. Increasing the \(\frac{{{\text{ml}}\,{\text{H}}_{2} {\text{O}}_{2} }}{\text{Lit PW}}\) ratio, due to the constant volume of wastewater, increases the amount of H2O2, and this increase leads to an increase in the ratio of \(\frac{{{\text{mol}}\,{\text{H}}_{2} {\text{O}}_{2} }}{{{\text{mol}}\,{\text{Fe}}^{2 + } }}\) and consequently an increase in reactive substances in the reaction medium.
As the graphs show, increasing the amount of reactant to the optimum point increases the amount of COD removal and when it passes through the optimum point, it wastes the reactants and also reduces the COD removal.
In general terms, the optimum point on the amount of reactants in the process can be explained by the fact that the excessive increase in ferrous ion can greatly reduce the process efficiency by using hydroxyl radicals. In addition, production of ferric ion can lead to the hydrogen peroxide decomposition in the reaction medium and the production of OOH˚ radical has a negative effect on the electro-Fenton progress (Pignatello et al. 2006; Bigda 1995; Casero et al. 1997; Tengrui et al. 2007).
Also, excessive amounts of hydrogen peroxide in the wastewater lead to the absorption of active hydroxyl radicals and the production of OOH˚ radical. OOH˚ radical species is a much weaker oxidant compared to hydroxyl radical, whose production reduces the efficiency of the electro-Fenton process (Kim et al. 2004; Bigda 1995).
Hydrogen peroxide produced cannot oxidize organic compounds that are resistant to decomposition alone, and the presence of ferrous ion in very low amounts leads to the formation of radical hydroxyl. Increasing the amount of ferrous ion to the optimum amount will increase the efficiency, but after this amount of process efficiency will be reduced; according to Haber and Weiss’s proposed reactions, it is hypothesized that according to reaction 10, the excessive ferrous ion reacts with hydroxyl radical, which is subsequently associated with the reduction of oxidation of organic compounds. Consequently, it can be said that controlling of the concentration of ferrous ion in the electro-Fenton reaction is very important (Zhang et al. 2008; Haber and Weiss 1934).
4 Conclusions
In this work, the modified electro-Fenton process using the Fe@Fe2O3 and nanoporous anodic aluminum oxide (AAO) electrodes was applied to investigate the petrochemical wastewater COD removal. The efficiency of various parameters such as current density (25–80 mA/m2), pH (2–5) and reaction time (10–90 min) was investigated. 75.52% and 65.03% COD removal have been obtained during experiments. As can be seen, iron nanoparticles have a better impact on the reaction, and this is because nanoparticles provide more active surface for chemical reactants and also they can act as an auxiliary agent for iron catalyst.
References
Azimi A, Azari A, Rezakazemi M, Ansarpour M (2017) Removal of heavy metals from industrial wastewaters: a review. ChemBioEng Rev 4(1):37–59
Bedolla-Guzman A, Sirés I, Thiam A, Peralta-Hernández JM, Gutiérrez-Granados S, Brillas E (2016) Application of anodic oxidation, electro-Fenton and UVA photoelectro-Fenton to decolorize and mineralize acidic solutions of Reactive Yellow 160 azo dye. Electrochim Acta 206:307–316
Bi XY, Peng W, Jiang H, Xu HY, Shi SJ, Huang JL (2007) Treatment of phenol wastewater by microwave-induced ClO2-CuOx/Al2O3 catalytic oxidation process. J Environ Sci 19(12):1510–1515
Bigda RJ (1995) Consider Fentons chemistry for wastewater treatment. Chem Eng Prog 91(12):1–12
Bódalo A, Gómez E, Hidalgo AM, Gómez M, Murcia MD, López I (2009) Nanofiltration membranes to reduce phenol concentration in wastewater. Desalination 245(1–3):680–686
Brillas E, Mur E, Sauleda R, Sanchez L, Peral J, Domènech X, Casado J (1998) Aniline mineralization by AOP’s: anodic oxidation, photocatalysis, electro-Fenton and photoelectro-Fenton processes. Appl Catal B 16(1):31–42
Casero I, Sicilia D, Rubio S, Pérez-Bendito D (1997) Chemical degradation of aromatic amines by Fenton’s reagent. Water Res 31(8):1985–1995
Cechinel MA, Mayer DA, Pozdniakova TA, Mazur LP, Boaventura RA, de Souza AAU, Vilar VJ (2016) Removal of metal ions from a petrochemical wastewater using brown macro-algae as natural cation-exchangers. Chem Eng J 286:1–15
Chen S, Sun D, Chung JS (2007) Treatment of pesticide wastewater by moving-bed biofilm reactor combined with Fenton-coagulation pretreatment. J Hazard Mater 144(1–2):577–584
Chen J, Mu L, Yin H, Song X, Li A (2015a) Thermogravimetric analysis and kinetics of the combustion of refining and chemicals wastewater in different oxygen concentrations. J Therm Anal Calorim 119(3):2205–2219
Chen C, Yu J, Yoza BA, Li QX, Wang G (2015b) A novel “wastes-treat-wastes” technology: role and potential of spent fluid catalytic cracking catalyst assisted ozonation of petrochemical wastewater. J Environ Manag 152:58–65
Chmayssem A, Taha S, Hauchard D (2016) Scaled-up electrochemical reactor with a fixed bed three-dimensional cathode for electro-Fenton process: application to the treatment of bisphenol A. Electrochim Acta 225:435–442
FathinejadJirandehi H, Mohebbizadeh M (2016) Petrochemical wastewater treatment by modified electro-Fenton process with Nano Iron particles. J Part Sci Technol 1:215–223
Flores N, Sirés I, Garrido JA, Centellas F, Rodríguez RM, Cabot PL, Brillas E (2015) Degradation of trans-ferulic acid in acidic aqueous medium by anodic oxidation, electro-Fenton and photoelectro-Fenton. J Hazard Mater 319:3–12
Flox C, Ammar S, Arias C, Brillas E, Vargas-Zavala AV, Abdelhedi R (2006) Electro-Fenton and photoelectro-Fenton degradation of indigo carmine in acidic aqueous medium. Appl Catal B 67(1–2):93–104
Gogoi A, Navgire M, Sarma KC, Gogoi P (2016) Fe3O4–CeO2 metal oxide nanocomposite as a Fenton-like heterogeneous catalyst for degradation of catechol. Chem Eng J 311:153–162
Guo J, Ma F, Chang CC, Cui D, Wang L, Yang J, Wang L (2009) Start-up of a two-stage bioaugmented anoxic–oxic (A/O) biofilm process treating petrochemical wastewater under different DO concentrations. Bioresour Technol 100(14):3483–3488
Haber F, Weiss J (1934) The catalytic decomposition of hydrogen peroxide by iron salts. Proc R Soc Lond A 147(861):332–351
Hansen E, Rodrigues MAS, de Aquim PM (2016) Wastewater reuse in a cascade based system of a petrochemical industry for the replacement of losses in cooling towers. J Environ Manag 181:157–162
Jaafarzadeh N, Ghanbari F, Ahmadi M, Omidinasab M (2017) Efficient integrated processes for pulp and paper wastewater treatment and phytotoxicity reduction: permanganate, electro-Fenton and Co3O4/UV/peroxymonosulfate. Chem Eng J 308:142–150
Kim YO, Nam HU, Park YR, Lee JH, Park TJ, Lee TH (2004) Fenton oxidation process control using oxidation-reduction potential measurement for pigment wastewater treatment. Kor J Chem Eng 21(4):801–805
Kujawski W, Warszawski A, Ratajczak W, Porebski T, Capała W, Ostrowska I (2004) Application of pervaporation and adsorption to the phenol removal from wastewater. Sep Purif Technol 40(2):123–132
Li J, Ai Z, Zhang L (2009) Design of a neutral electro-Fenton system with Fe@Fe2O3/ACF composite cathode for wastewater treatment. J Hazard Mater 164(1):18–25
Li W, Wang Y, Irini A (2014) Effect of pH and H2O2 dosage on catechol oxidation in nano-Fe3O4 catalyzing UV–Fenton and identification of reactive oxygen species. Chem Eng J 244:1–8
Liao Q, Sun J, Gao L (2009) Degradation of phenol by heterogeneous Fenton reaction using multi-walled carbon nanotube supported Fe2O3 catalysts. Colloids Surf A 345(1–3):95–100
Ma H, Zhang X, Ma Q, Wang B (2009) Electrochemical catalytic treatment of phenol wastewater. J Hazard Mater 165(1–3):475–480
Moghadam H, Samimi A, Behzadmehr A (2013) Effect of nanoporous anodic aluminum oxide (AAO) characteristics on solar absorptivity. Transp Phenom Nano Micro Scales 1(2):110–116
Mohammadi T, Moheb A, Sadrzadeh M, Razmi A (2005) Modeling of metal ion removal from wastewater by electrodialysis. Sep Purif Technol 41(1):73–82
Mu L, Chen J, Yao P, Zhou D, Zhao L, Yin H (2016) Evaluation of co-pyrolysis petrochemical wastewater sludge with lignite in a thermogravimetric analyzer and a packed-bed reactor: pyrolysis characteristics, kinetics, and products analysis. Bioresour Technol 221:147–156
Neyens E, Baeyens J (2003) A review of classic Fenton’s peroxidation as an advanced oxidation technique. J Hazard Mater 98(1–3):33–50
Özcan A, Şahin Y, Koparal AS, Oturan MA (2008) Carbon sponge as a new cathode material for the electro-Fenton process: comparison with carbon felt cathode and application to degradation of synthetic dye basic blue 3 in aqueous medium. J Electroanal Chem 616(1–2):71–78
Palma MSA, Paiva JLD, Zilli M, Converti A (2007) Batch phenol removal from methyl isobutyl ketone by liquid–liquid extraction with chemical reaction. Chem Eng Process 46(8):764–768
Patra AK, Dutta A, Bhaumik A (2013) Mesoporous core–shell Fenton nanocatalyst: a mild, operationally simple approach to the synthesis of adipic acid. Chem A Eur J 19(37):12388–12395
Pereira NS, Zaiat M (2009) Degradation of formaldehyde in anaerobic sequencing batch biofilm reactor (ASBBR). J Hazard Mater 163(2–3):777–782
Pignatello JJ, Oliveros E, MacKay A (2006) Advanced oxidation processes for organic contaminant destruction based on the Fenton reaction and related chemistry. Crit Rev Environ Sci Technol 36(1):1–84
Rezakazemi M, Khajeh A, Mesbah M (2018) Membrane filtration of wastewater from gas and oil production. Environ Chem Lett 16(2):367–388
Sheng Y, Song S, Wang X, Song L, Wang C, Sun H, Niu X (2011) Electrogeneration of hydrogen peroxide on a novel highly effective acetylene black-PTFE cathode with PTFE film. Electrochim Acta 56(24):8651–8656
Tengrui L, Al-Harbawi A, Jun Z, Bo LM (2007) The effect and its influence factors of the Fenton process on the old landfill leachate. J Appl Sci 7(5):724–727
Tsantaki E, Velegraki T, Katsaounis A, Mantzavinos D (2012) Anodic oxidation of textile dyehouse effluents on boron-doped diamond electrode. J Hazard Mater 207:91–96
Zhang G, Yang F, Gao M, Fang X, Liu L (2008) Electro-Fenton degradation of azo dye using polypyrrole/anthraquinonedisulphonate composite film modified graphite cathode in acidic aqueous solutions. Electrochim Acta 53(16):5155–5161
Zhang DQ, Eng CY, Stuckey DC, Zhou Y (2017) Effects of ZnO nanoparticle exposure on wastewater treatment and soluble microbial products (SMPs) in an anoxic-aerobic membrane bioreactor. Chemosphere 171:446–459
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Adimi, M., Mohammad Mohebizadeh, S., Poor, M.M. et al. Treatment of Shazand Petrochemical Co. Effluent using Electro-Fenton Method Modified with Iron Nanoparticles and Anodic Aluminum Oxide Electrode: A Comparison. Iran J Sci Technol Trans Sci 43, 2799–2806 (2019). https://doi.org/10.1007/s40995-019-00766-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40995-019-00766-6