Abstract
Intravascular hemolysis occurs in many hematologic disorders which results in free hemoglobin (Hb). Haptoglobin (Hp) is an acute-phase plasma glycoprotein that binds free Hb to prevent its oxidative damages. Due to the safety issues related to the plasma-derived proteins, the production of Hp using recombinant DNA technology is considered as an alternative source. In this study, Hp gene was isolated from HepG2 cell line and cloned into pcDNA3.1(+) vector. Gene cloning was confirmed with colony PCR, restriction digestion and DNA sequencing. CHO cells were transfected with the construct, and finally a stable CHO cell line expressing human recombinant Hp (rHp) was established. The expression of rHp was confirmed with RT-PCR and Western blot analyses both at mRNA and protein levels. This is the first report of establishing a stable CHO cell line expressing human rHp as a primary step toward future rHp clinical applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Hemolytic anemia such as sickle cell anemia, thalassemia, paroxysmal nocturnal hemoglobinuria (PNH), pyruvate kinase deficiency, ABO mismatch transfusion reaction, etc. (Rother et al. 2005) is a pathologic condition mainly associated with red blood cell hemolysis. Older stored blood transfusion accelerates intravascular hemolysis that leads to the hemoglobin (Hb) release in blood circulation, reactive oxygen species (ROS) formation reactions (Alayash et al. 2013; Buehler et al. 2011) and inflammation (Rother et al. 2005). Free Hb may cause many life-threatening complications including transfusion-associated lung injury, pulmonary hypertension, post-perfusion renal and cerebral dysfunctions and aged blood-associated mortality in trauma (Buehler et al. 2011; Silverman and Weiskopf 2009).
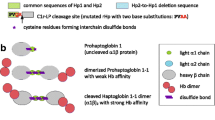
However, human physiological system has been evolved in the way that expresses chaperon proteins responsible for the free Hb detoxification. Chief among these is haptoglobin (Hp) that is the first line of defense against intravascular hemolysis (Alayash 2011). Hp is an acute phase plasma glycoprotein (Raijmakers et al. 2003, Lim et al. 2000) with an exceptionally high binding affinity to Hb (K d ~ 1×10−15 mol/L) that protects tissues from free Hb-mediated oxidative damages (Wang et al. 2014). Hp is mainly synthesized by liver, and further secreted into the plasma (Lim et al. 2000; Nantasenamat et al. 2013; Raijmakers et al. 2003). Synthesized in hepatocytes as a single polypeptide (proHp) containing αβ chains, Hp’s unusual proteolytic cleavage in endoplasmic reticulum is mediated by complement C1r-like protein (C1r-LP) prior to its entrance to the Golgi apparatus (Nielsen et al. 2007; Wicher and Fries 2004). Upon co-translational dimerization, the final tetrameric Hp protein structure will be formed consisting of two 9-kDa α-chains and two 33-kDa β-chains. In humans, one disulfide bond links the α and the β chains, and the second one crosslinks the α-chains of the αβ dimers (Hwang and Greer 1980; Kurosky et al. 1998; Wicher and Fries 2010).
In addition to the main biological function of Hp as a free Hb detoxifier, it can also play an important role in other physiological processes such as acting as an anti-inflammatory agent, antioxidant, angiogenic promoter, and immune cell regulator (Nantasenamat et al. 2013).
Recent studies demonstrated that Hp could effectively suppress the free Hb associated oxidative toxicity in both guinea pig and dog models of hemolytic anemia and also reverse the oxidative damages of old blood transfusion in guinea pigs (Alayash et al. 2013; Baek et al. 2012; Buehler et al. 2007). These findings revealed that Hp could act as a potential therapeutic agent for the detoxification and clearance of intravascular Hb in hemolytic disorders. Interestingly, plasma-derived Hp is clinically administered in Japan (Otterbein et al. 2003) for the treatment of burn injuries, blood transfusion-mediated trauma and prophylactic use prior to a number of surgeries (Hashizume et al. 1988; Miyoshi et al. 1991).
Unfortunately, Hp purification from plasma is a time-consuming process that is associated with several limitations such as the risk of virus and prion transmissions, insufficient plasma sources (Eikelboom et al. 2003; Nillson and Freiburghas 1993; Roberts et al. 2010) and heterogeneity of final purified product due to the allele variations (Yueh et al. 2007).
Alternatively, Hp protein could be produced through recombinant DNA technology. To date, rHp was expressed in different hosts such as Escherichia coli, Saccharomyces cerevisiae and Spodoptera frugiperda Sf9 insect cells (van der Straten et al. 1986a, b; Heinderyckx et al. 1988–1989). However, the rate of expression was very low in E. coli and yeast (van der Straten et al. 1986a, b), and all these hosts lack the ability of cleaving proHp into the mature rHp protein (Heinderyckx et al. 1988; van der Straten et al. 1986a, b).
On the other hand, post-translational modifications, especially glycosylation patterns, are critical in the formation of Hp–Hb complex. Researchers have shown that the removal of 40% of Hp total carbohydrate prevents Hp–Hb interactions (Kaartinen and Mononen 1988). The glycosylation patterns of rHp expressed in the foregoing hosts were not completely human-like. Furthermore, Lack of sialic acid in protein glycosylation of baculovirus-infected insect cells, high mannose glycosylation in yeast and no glycosylation in E. coli has been reported as the major drawbacks to produce mature rHp protein (Heinderyckx et al. 1988; van der Straten et al. 1986a, b). Hence, choosing an appropriate expression system would be a promising strategy to produce functional rHp.
CHO cells are a suitable mammalian cell line capable of carrying out the necessary post-translational modifications which result in bioactive recombinant proteins compatible with human physiological system. In addition, CHO cell line is a proper host for the stable expression of human rHp due to their genome instability, adaptability to various culture conditions and less risk of contamination with human viruses (Ghaderi et al. 2012; Kim et al. 2012). Thus, it could be employed as a safe and efficient host for large-scale production of therapeutic proteins (Boeger et al. 2005; Wiberg et al. 2006).
This study aims to establish a stable CHO cell line capable of expressing human rHp that is considered as the first attempt to exploit the therapeutic potentiality of human rHp in the future to improve the health status of hemolytic patients.
2 Materials and Methods
2.1 Plasmid and Bacteria
pcDNA3.1(+) Plasmid (Invitrogen, USA) was used as a shuttle vector, and E. coli TOP10 strain (Cinnagen, Iran) was utilized as the cloning host.
2.2 Cell Culture
HepG2 (human hepatoma cell line) (Accession No: IBRC C10096) and CHO (Chinese Hamster Ovary cell line) (Accession No: IBRC C10136) were obtained from Iran’s National Center of Genetic and Biological Reservoirs. These cell lines were cultured in RPMI-1640 medium (Gibco, Germany) containing 10% fetal bovine serum (Gibco, Germany) supplemented with 100 U/ml penicillin and 100 μg/ml streptomycin (Gibco, Germany).
2.3 Isolation and PCR Amplification of Hp Gene
HepG2 cell line was used as a source for human Hp gene. Total RNA was extracted using RNX-plus (Cinnagen, Iran) reagent according to the manufacturer’s protocol. The quality and quantity of RNA was determined by Nano drop Spectrophotometer (Nanodrop, USA). Reverse transcription was performed using AccuPower® PreMix (Bioneer, Korea). Then, PCR was performed using Pfu DNA polymerase (Cinnagen, Iran) in eppendorf thermo cycler (Eppendorf, Germany). Pfu DNA polymerase is an enzyme of high fidelity which amplifies the gene of interest with high accuracy and minimum error. Hp PCR primers were designed based on the cDNA sequence of human Hp present in the GenBank [Accession No: M69197 (Erickson et al. 1992)] which amplified a 1221-bp product. The forward primer (5′-GAGCTAGCATGGTGAGTGCCCTGGGAGCTG-3′) contained a kozak sequence and NheI restriction site, and the reverse primer (5′-AGCCTCGAGTTAGTTCTCAGCTATGGTCTTCTG-3′) contained the restriction site for XhoI enzyme. The Hp sequence was PCR-amplified under the following thermal conditions: 2 min of initial denaturation at 94 °C followed by 35 cycles with a denaturation step of 30 s at 94 °C, an annealing step of 30 s at 62 °C and an extension step of 2 min and 40 s at 72 °C. The final extension step lasted for 7 min at 72 °C. Hp PCR product was analyzed on 1.5% agarose gel to confirm its size and purity. Amplification of β-actin gene (200-bp PCR product) was also performed separately.
2.4 Preparation of Recombinant pcDNA3.1-Hp Plasmid
To construct the recombinant plasmid, the amplified full-length human Hp was cleaned up using high pure PCR product purification kit (Roche, Germany). Both the PCR product and pcDNA3.1(+) plasmid were digested with NheI and XhoI restriction enzymes (Fermentase, USA). Then, Hp gene was ligated into pcDNA3.1(+) plasmid using T4 DNA ligase (Cinnagen, Iran) to construct recombinant vector (pcDNA3.1-Hp). Finally, competent E. coli TOP10 strain was transformed with pcDNA3.1-Hp. The accuracy and orientation of the Hp gene in pcDNA3.1-Hp was confirmed by colony PCR, restriction digestion and DNA sequencing.
2.5 Transfection of CHO Cell Line with pcDNA3.1-Hp Recombinant Vector
pcDNA3.1-Hp was linearized using BglII restriction enzyme (Fermentase, USA). Then, CHO cells were seeded and upon reaching 80% confluency, were transfected with 1.5 µg of linearized pcDNA3.1-Hp (CHO-Hp) using X-tremeGENE HP DNA transfection reagent (Roche, Germany) according to the manufacturer’s instruction. Additionally, CHO cells transfected with empty vector (CHO-V) were used as control group. For stable expression, the transfected cells were selected following cultivation in a selective medium containing 200 μg/ml geneticin (Bio Basic, Canada). Single clones of stable CHO cells transfected with pcDNA3.1-Hp (CHO-Hp) were selected after their subsequent cultivation in 96-well culture plates. Geneticin-resistant clones were chosen at the 10th and 20th passages in order to verify the stable expression of human recombinant Hp by RT-PCR and western blot analyses.
2.6 RT-PCR Analysis of rHp Expression
RT-PCR was performed using Taq DNA polymerase (Bioneer, Korea) to investigate the expression of rHp at mRNA level in transfected CHO cells. Hp internal primers included the forward (5′-GTATGTCATGCTGCCTGTGG-3′) and the reverse (5′-AGATCCCAGTCGCATACCAG-3′) which amplified a 233-bp product. PCR amplification using internal primer was performed according to the following program: initial denaturation at 95 °C for 5 min followed by 35 cycles of denaturation at 95 °C for 30 s, annealing at 55 °C for 30 s and extension at 72 °C for 30 s. The final extension step at 72 °C lasted for 5 min.
PCR program with total primer (1221-bp PCR product) included 30 s at 95 °C for denaturation, 30 s at 60 °C for annealing and 1 min at 72 °C for extension repeated for 32 cycles. For normalization, expression of β-actin was examined.
2.7 Western Blot Analysis of rHp Expression
Cell culture supernatants of CHO-Hp and CHO-V (negative control) were collected and concentrated using Vivaspin®6 Centifugal Concentrator equipped with a 3 kDa-cut-off filter (Sartorius, Germany). The electrophoretic separation of protein samples was carried out on a 12.5% sodium dodecyl sulfate (SDS) polyacrylamide gel. Then, the protein bands were subsequently trans-blotted onto a PVDF membrane (Hi-bond Amersham Biosciences, USA) using the Semi-dry western blot system (PeQLab, Germany). The membrane was blocked with 5% skim milk solution for an overnight. The blocked membrane was washed with phosphate buffer saline (PBS). In order to detect the Hp protein band, the membrane was incubated with rabbit monoclonal anti-human Hp β-chain antibody (Abcam, England) at 25 °C. Next, the membrane was washed with PBS containing 0.1% Tween-20 (PBST), and incubated with polyclonal goat anti-rabbit horseradish peroxidase (HRP)-coupled secondary antibody (Cell Signalling, USA). At the following stage, the membrane was washed with PBST and further developed with ECL western blotting substrate kit (Abcam, England). Finally, ChemiDoc XRS + system (Biorad, USA) and Image Lab software (Biorad, USA) were used for imaging.
3 Results
3.1 pcDNA3.1-Hp was Successfully Cloned
Full-length human Hp cDNA (1221 bp) was amplified with Pfu DNA polymerase (Fig. 1). Then, the isolated gene was cloned into pcDNA3.1(+) vector and transformed into the competent E. coli TOP10 strain. pcDNA3.1(+) shuttle vector was used for both cloning and expression.
Colony PCR (Fig. 2a), restriction digestion (Fig. 2b) and DNA sequencing (data not shown) of recombinant pcDNA3.1-Hp plasmid confirmed the correct identity and orientation of the Hp insert inside the vector.
Cloning of human Hp into pcDNA3.1 plasmid. a Colony PCR of 8 randomly-selected plasmids. Bacterial colonies No. 1, 3 and 4 contained human Hp gene. b Digestion of the extracted plasmids with restriction enzymes. M: 1 kbp DNA marker, Lane 1: digested recombinant plasmid, Lane 2: un-digested recombinant plasmid and Lane 3: empty undigested vector. The 1221 bp band confirmed the insertion of Hp into pcDNA3.1 plasmid
3.2 CHO Cells Stably Expressed Human rHp at Both mRNA and Protein Levels
pcDNA3.1-Hp was transfected to CHO cells using X-tremeGENE HP DNA transfection reagent(CHO-Hp). CHO-Hp single cell clones were identified under the selective pressure of 200 μg/μl geneticin. RT-PCR analysis showed that CHO-Hp could express human rHp at mRNA level at the 10th and 20th passages, but there was no rHp expression in CHO-V (negative control). The expected size of the rHp PCR products were 233 bp using internal primer and 1221 bp using total primer sets (Fig. 3a, b).
RT-PCR analysis of human rHp expression in CHO-Hp cells at the 10th and 20th passages. a Lane 1: RT-PCR of rHp expression in CHO-Hp using internal primers at the 10th passage. Lane 2: rHp RT-PCR product of CHO-V using internal primers. M: 100 bp DNA marker. Lane 3: rHp RT-PCR product of CHO-V using total primers. Lane 4: RT-PCR of rHp expression in CHO-Hp using total primers at the 10th passage. β-Actin was used as internal control. b Lane 1: RT-PCR of rHp expression in CHO-Hp using internal primers at the 20th passage, Lane 2: rHp RT-PCR product of CHO-V using internal primers. M: 100 bp DNA marker, Lane 3: rHp RT-PCR product of CHO-V using total primers. Lane 4: RT-PCR of rHp expression in CHO-Hp using total primers at 20th passage. β-actin was used as internal control. Transfection of CHO cells with recombinant pcDNA3.1-Hp resulted in stable expression of human rHp at mRNA level while no expression was detected in transfected CHO cells with empty pcDNA3.1 vector
To detect the expression of rHp at protein level, the concentrated supernatant of CHO-Hp cells at the 10th and 20th passages was collected and prepared for western blot analysis. The results showed that CHO-Hp cells expressed rHp protein, while CHO-V control group did not (Fig. 4a, b).
Western blot analysis of rHp expression in CHO-Hp cells at 10th and 20th passages. a Lane 1: Concentrated supernatant of CHO-V cells. Lane 2: Concentrated supernatant of CHO-Hp cells at the 10th passage. Lane 3: Concentrated supernatant of CHO-Hp cells at the 20th passage. b β-Actin was used as control for both CHO-V and CHO-Hp at the 10th and 20th passages. Transfection of CHO cells with recombinant pcDNA3.1-Hp resulted in expression and secretion of human rHp protein while no protein expression was detected in transfected CHO cells with empty pcDNA3.1 vector. Western blot was performed using Hp β chain specific monoclonal antibody
4 Discussion
Potential therapeutic application of Hp for the inactivation and clearance of free Hb has been of great clinical importance in detoxification of extracellular Hb associated with blood transfusion, malaria, sickle cell disease and other hemolytic anemia (Alayash et al. 2013; Wang et al. 2014). Since Hp purification from plasma is a challenging task, the production of rHp through advanced biotechnological techniques might be a viable alternative and reasonable strategy with regard to the future employment in clinical settings.
In the present study, human Hp gene was isolated from HepG2 cell line. Then, pcDNA3.1 shuttle vector was used for cloning Hp gene in E. coli, and finally rHp was expressed in CHO cells, a mammalian cell line capable of expressing mature human-compatible glycoproteins in their secretory forms. The expression of rHp was consequently confirmed both at transcriptional and translational levels.
In this study, CHO cell line was employed as a new expression host. CHO cells are able to carry out post-translational modifications which would result in compatible, bioactive and stable proteins in humans (Kim et al. 2012). Specifically, the protein glycosylation patterns of CHO cell line is very similar to that of humans (Ghaderi et al. 2012). Less immunogenicity and less risk of human virus contamination makes it the most preferred host cell line for the production of therapeutic proteins. In addition, due to their genome instability and capability of adapting to suspension culture, CHO cells are considered as a suitable machinery choice for large scale production of recombinant biologics (Boeger et al. 2005; Wiberg et al. 2006).
rHp was produced in different expression systems. van der Straten et al. expressed human rHp in S. cerevisiae. They transformed yeast with two recombinant plasmids, i.e., pRIT12597 and pRIT12598. Their study showed that not only the yeast cells poorly expressed the rHp protein, but they also were not capable of secreting this protein into the culture medium (van der Straten et al. 1986a). Other drawbacks of yeast expression system include lack of human-like glycosylation, high immunogenicity of the recombinant product due to hyper-mannosylation, lack of terminal sialic acid and the decreased or absent amount of fucose (Ghaderi et al. 2012).
In another study, van der Straten et al. expressed human Hp in E. coli. Various human Hp cDNA fragments were cloned in pCQV2 expression vector. Then, E. coli bacteria were transformed with the recombinant vector. The rHp expressed in the E. coli system was a truncated non-glycosylated protein aggregated in the form of inclusion bodies; therefore, additional steps of resolubilization and refolding will be definitely required for obtaining a functional high-quality protein (van der Straten et al. 1986b). Generally, bacterial expression systems lack enzymatic machinery and compartmentalization required for mammalian-type glycosylation (Ghaderi et al. 2012). Unfortunately, immature non-glycosylated rHp expressed in prokaryotic expression systems is not a proper choice for therapeutic use.
Lai et al. expressed human Hp subunits in E. coli to delineate major antioxidant domain of Hp. They cloned cDNA of Hp subunits in pQE30 expression vector, and expressed the construct in E. coli. Their results showed that all subunits were primarily expressed in the form of inclusion bodies (Lai et al. 2007). This study supported the previous findings that declared recombinant protein aggregation and inclusion body formation occurs in E. coli expression system (Khow and Suntrarachun 2012; van der Straten et al. 1986b).
Expression of human rHp was also reported by Heinderyckx et al. They utilized baculovirus Autographa californica nuclear polyhedrosis virus (AcNPV) as an expression vector. Insect cells were transfected with AcNPV vectors carrying the pre-proHp cDNA using calcium phosphate. Their expression system resulted in high levels of un-cleaved glycosylated pro-Hp in the culture medium. Lacking subunit maturation, the secreted recombinant pro-Hp was able to bind Hb in vitro albeit less efficiently than plasma-derived Hp (Heinderyckx et al. 1988–1989).
One serious issue regarded to the insect expression system is the lack of complex-type glycans with terminal sialic acids that makes the insect cells inappropriate hosts to produce human proteins for therapeutic purposes (Ghaderi et al. 2012). Heinderyckx’s group transfected S. frugiperda Sf9 insect cells using calcium phosphate. Herein, CHO cells were transfected with a cationic non-liposomal transfection reagent called x-tremeGENE HP DNA transfection reagent which is highly recommended for the generation of stable cell lines. It shows low toxicity and high rate of transfection compared with calcium phosphate conventional method.
In conclusion, we took the first step toward the production of therapeutic rHp through the establishment of a stable CHO cell line capable of expressing human rHp. We confirmed that the CHO cell line expressed rHp both at the transcriptional and translational levels. Interestingly, we showed that rHp was successfully secreted into the CHO culture medium that indicated the functional expression of human rHp in eukaryotic expression system.
To the best of our knowledge, this is the first report of establishing a stable CHO cell line expressing human Hp in its secretory form. However further studies are required with regard to rHp purification, its biological characterization, large scale protein production, in vivo studies and hopefully future clinical trials.
References
Alayash AI (2011) Haptoglobin: old protein with new functions. Clin Chim Acta 412(7–8):493–498. https://doi.org/10.1016/j.cca.2010.12.011
Alayash AI, Andersen CB, Moestrup SK, Bülow L (2013) Haptoglobin: the hemoglobin detoxifier in plasma. Trends Biotechnol 31(1):2–3. https://doi.org/10.1016/j.tibtech.2012.10.003
Baek JH, D’Agnillo F, Vallelian F, Pereira CP, Williams MC, Jia Y et al (2012) Hemoglobin-driven pathophysiology is an in vivo consequence of the red blood cell storage lesion that can be attenuated in guinea pigs by haptoglobin therapy. J Clin Invest 122(4):1444–1458. https://doi.org/10.1172/JCI59770
Boeger H, Bushnell DA, Davis R, Griesenbeck J, Lorch Y, Strattan JS et al (2005) Structural basis of eukaryotic gene transcription. FEBS Lett 579:899–903. https://doi.org/10.1016/j.febslet.2004.11.027
Buehler PW, D’Agnillo F, Hoffman V, Alayash AI (2007) Effects of endogenous ascorbate on oxidation, oxygenation, and toxicokinetics of cell-free modified hemoglobin after exchange transfusion in rat and guinea pig. J Pharmacol Exp Ther 323(1):49–60. https://doi.org/10.1124/jpet.107.126409
Buehler PW, Karnaukhova E, Gelderman MP, Alayash AI (2011) Blood aging, safety, and transfusion: capturing the “radical” menace. Antioxid Redox Signal 14(9):1713–1728. https://doi.org/10.1089/ars.2010.3447
Eikelboom MR, Bird R, Blythe D, Coyle L, Gan E, Harvey M et al (2003) Recombinant activated factor VII for the treatment of life-threatening haemorrhage. Blood Coagul Fibrinolysis 14(8):713–717. https://doi.org/10.1097/01.mbc.0000061358.73802.9c
Erickson LM, Kim HS, Maeda N (1992) Junctions between genes in the haptoglobin gene cluster of primates. Genomics 14(4):948–958
Ghaderi D, Zhang M, Hurtado-Ziola N, Varki A (2012) Production platforms for biotherapeutic glycoproteins. Occurrence, impact, and challenges of non-human sialylation. Biotechnol Genet Eng Rev 28:147–175. https://doi.org/10.5661/bger-28-147
Hashizume M, Kitano S, Yamaga H, Sugimachi K (1988) Haptoglobin to protect against renal damage from ethanolamine oleate sclerosant. Lancet 2(8606):340–341. https://doi.org/10.1016/S0140-6736(88)92400-2
Heinderyckx M, Jacobs P, Bollen A (1988) Secretion of glycosylated human recombinant haptoglobin in baculovirus-infected insect cells. Mol Biol Rep 13(4):225–232
Hwang PK, Greer J (1980) Interaction between hemoglobin subunits in the hemoglobin–haptoglobin complex. J Biol Chem 255:3038–3041
Kaartinen V, Mononen I (1988) Hemoglobin binding to deglycosylated haptoglobin. Biochim Biophys Acta 953(3):345–352
Khow O, Suntrarachun S (2012) Strategies for production of active eukaryotic proteins in bacterial expression system. Asian Pac J Trop Biomed 2(2):159–162. https://doi.org/10.1016/S2221-1691(11)60213-X
Kim JY, Kim YG, Lee GM (2012) CHO cells in biotechnology for production of recombinant proteins: current state and further potential. Appl Microbiol Biotechnol 93:917–930. https://doi.org/10.1007/s00253-011-3758-5
Kurosky A, Barnett DR, Lee TH, Touchstone B, Hay RE, Arnott MS et al (1998) Covalent structure of human haptoglobin: a serine protease homolog. Proc Natl Acad Sci USA 77:3388–3392
Lai IH, Tsai TI, Lin HH, Lai WY, Mao SJ (2007) Cloning and expression of human haptoglobin subunits in Escherichia coli: delineation of a major antioxidant domain. Protein Expr Purif 52(2):356–362. https://doi.org/10.1016/j.pep.2006.09.012
Lim YK, Jenner A, Ali AB, Wang Y, Hsu SI, Chong SM et al (2000) Haptoglobin reduces renal oxidative DNA and tissue damage during phenylhydrazine-induced hemolysis. Kidney Int 58(3):1033–1044. https://doi.org/10.1046/j.1523-1755.2000.00261.x
Miyoshi H, Ohshiba S, Matsumoto A, Takada K, Umegaki E, Hirata I (1991) Haptoglobin prevents renal dysfunction associated with intravariceal infusion of ethanolamine oleate. Am J Gastroenterol 86(11):1638–1641
Nantasenamat C, Prachayasittikul V, Bulow L (2013) Molecular modeling of the human hemoglobin-haptoglobin complex sheds light on the protective mechanisms of haptoglobin. PLoS ONE 8(4):e62996. https://doi.org/10.1371/journal.pone.0062996
Nielsen MJ, Petersen SV, Jacobsen C, Thirup S, Enghild JJ, Graversen JH et al (2007) A unique loop extension in the serine protease domain of haptoglobin is essential for CD163 recognition of the haptoglobin-hemoglobin complex. J Biol Chem 282(2):1072–1079. https://doi.org/10.1074/jbc.M605684200
Nillson IM, Freiburghas C (1993) Treatment of patients with factor VII and IX inhibitors. Thromb Haemost 70:56–59
Otterbein LE, Soares MP, Yamashita K, Bach FH (2003) Heme oxygenase-1: unleashing the protective properties of heme. Trends Immunol 24(8):449–455. https://doi.org/10.1016/S1471-4906(03)00181-9
Raijmakers MTM, Roes EM, te Morsche RHM, Steeger EAP (2003) Haptoglobin and its association with the HELLP syndrome. J Med Genet 40:214. https://doi.org/10.1136/jmg.40.3.214
Roberts HR, Monroe DM, Hoffman M (2010) Molecular biology and biochemistry of the coagulation factors and pathways of hemostasis. In: Beutler E, Lichtman MA, Coller BS, Kipps TJ, Seligsohn U (eds) William’s hematology, 8th edn. Churchill-Livingstone, New York, pp 1409–1434
Rother RP, Bell L, Hillmen P, Gladwin MT (2005) The clinical sequelae of intravascular hemolysis and extracellular plasma hemoglobin: a novel mechanism of human disease. JAMA 293(13):1653–1662. https://doi.org/10.1001/jama.293.13.1653
Silverman TA, Weiskopf RB (2009) Hemoglobin-based oxygen carriers: current status and future directions. Anesthesiology 111:946–963. https://doi.org/10.1097/ALN.0b013e3181ba3c2c
van der Straten A, Falque JC, Loriau R, Bollen A, Cabezón T (1986a) Expression of cloned human haptoglobin and alpha 1-antitrypsin complementary DNAs in Saccharomyces cerevisiae. DNA 5(2):129–136. https://doi.org/10.1089/dna.1986.5.129
van der Straten A, Loriau R, Herzog A, Bollen A (1986b) The alpha 2 cDNA sequence of human haptoglobin carries a bacterial promoter functional in vivo. Biosci Rep 6(4):363–373. https://doi.org/10.1007/BF01116423
Wang Y, Zhang Y, Zhao L, Yin Y, Wang Q, Zhou H (2014) Addition of haptoglobin to RBCs storage, a new strategy to improve quality of stored RBCs and transfusion. Med Hypotheses 82(2):125–128. https://doi.org/10.1016/j.mehy.2013.09.020
Wiberg FC, Rasmussen SK, Frandsen TP, Rasmussen LK, Tengbjerg K, Coljee VW et al (2006) Production of target-specific recombinant human polyclonal antibodies in mammalian cells. Biotechnol Bioeng 94:396–405. https://doi.org/10.1002/bit.20865
Wicher KB, Fries E (2004) Prohaptoglobin is proteolytically cleaved in the endoplasmic reticulum by the complement C1r-like protein. Proc Natl Acad Sci USA 101(40):14390–14395. https://doi.org/10.1073/pnas.0405692101
Wicher KB, Fries E (2010) Evolutionary aspects of hemoglobin scavenger. Antioxid Redox Signal 12:249–259. https://doi.org/10.1089/ars.2009.2760
Yueh SC, Lai YA, Chen WL, Hsu HH, Mao SJ (2007) An improved method for haptoglobin 1-1, 2-1, and 2-2 purification using monoclonal antibody affinity chromatography in the presence of sodium dodecyl sulfate. J Chromatogr B Anal Technol Biomed Life Sci 845(2):210–217. https://doi.org/10.1016/j.jchromb.2006.08.012
Acknowledgements
This study was supported by the Iran National Science Foundation (INSF) and resulted from an M.Sc. thesis in medical biotechnology authorized by High institute for Education and Research in Transfusion Medicine.
Funding
This study was funded by High institute for Education and Research in Transfusion Medicine (Grant No. 22) and also supported by the Iran National Science Foundation (INSF).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Movahed, M., Roudkenar, M.H., Bahadori, M. et al. Establishment of Stable CHO Cell Line Expressing Recombinant Human Haptoglobin: Toward New Haptoglobin-Based Therapeutics. Iran J Sci Technol Trans Sci 42, 1097–1103 (2018). https://doi.org/10.1007/s40995-017-0381-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40995-017-0381-z