Abstract
The efficiency of ceramic foam filters in removing different inclusion populations in a Fe–30Mn–9Al–1Si–0.9C–0.5Mo steel was investigated. A mold design was created utilizing fluid flow and solidification modeling software. The design utilized a common pouring cup attached to two different but balanced gating systems. One runner utilized a ceramic foam filter, while the other runner was unfiltered. Three molds were poured in sequence from a teapot-style ladle. Metallographic samples revealed extensive Al- and Mn-rich oxide bi-films in samples taken before the filter. Samples sectioned after the filter did not contain bi-films. AlN or complex AlN–MnS or AlN–MnO comprised more than 70% of all inclusions. Samples sectioned from the first two molds showed an inclusion removal efficiency of 38% and 39%, respectively. Larger inclusions greater than 3 µm were more efficiently filtered. The third mold with the greatest number of larger inclusions showed the highest inclusion removal efficiency of 55%.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lightweight high-strength steel with aluminum contents between 4 and 12wt%Al has found applications for military vehicles as well as in the automotive sector because of a combination of low density, high strength and good toughness. Fully austenitic cast steels of composition Fe–30Mn–9Al–0.9C–1.0Si–0.5Mo offer almost a 15% lower density compared to quenched and tempered SAE 4130 cast steels with equivalent strengths and dynamic fracture toughness.1 It should be noted that all compositions in the following study are in weight percent unless otherwise stated. Internal defects in FeMnAlC steels such as microporosity and the presence of faceted aluminum nitride inclusions are a major contributor for loss of toughness in these steels.2 A high aluminum content of 5–12% is also responsible for the formation of solid oxide bi-films that can be entrained during pouring and filling, and this has been linked to a significant loss in tensile strength, ductility and fatigue life in aluminum castings.3 Studies by Schulte et al.4 show that the population density of AlN inclusions directly affects the impact properties of a Fe–30Mn–9Al–1Si–0.9C–0.5Mo. In the solution-treated and aged condition, Charpy V-notch (CVN) toughness at − 40 °C decreased from 35 J to 19 J as the concentration of AlN increased from 12 to 210 inclusions/mm2.4 Clean steelmaking practices using argon cover can help reduce nitrogen pickup. However, high nitrogen in charge materials, exposure to air during metal transfer as well as during pouring and filling always result in a significant amount of AlN inclusions in these castings.
There have been constant efforts in foundries to increase cleanliness and reduce inclusions in both high and low alloy steel castings. The use of foam ceramic filters (FCF) is currently one of the best engineering solutions for increasing metal cleanliness and reducing velocity and turbulence during mold filling. The cellular structure of foam ceramic filters results in deep bed filtration leading to attachment of inclusions within the porous network. The forces of adhesion that is good wettability and the presence of a large specific area within the foam filter improve the efficiency of nonmetallic inclusion removal.5 The porous cellular structure that is present in a FCF plays a major role since it provides a high surface area and torturous flow path that increases the coefficient of mass transfer between the metal and filter surface.6 Filtration of alumina inclusions using ceramic filters has been studied by Apelian et al. for a steel composition of Fe–0.012C–0.04Ni between 12 and 20 ppm of oxygen. It was shown that inclusions greater than 2.5 µm were trapped by the filter.7 In the study by Tian et al. on steels consisting of composition Fe–0.66Mn–0.005P–0.29C–0.095Cu–0.092Cr–0.001Mo, zirconia filters were shown to have up to a 90% removal efficiency for alumina inclusions.8
The filtration efficiency expression for liquid metal filtration can be expressed as:
where η = inclusion removal efficiency, Ci = concentration of inclusions at inlet of the filter, C0 = concentration of inclusions at the outlet of the filter.7
The use of FCFs in castings has been shown to increase the yield of the castings, reduce the rejection rate and improve the machinability of the casting.6 The use of filters gives improvement in the yield and quality of steel castings, and understanding of how inclusions in FeMnAlC steels are filtered by these filters is of high priority. Although there has been some work published on the filtration of alumina inclusions utilizing foam filters, the effectiveness of these filters in inclusion removal of FeMnAl steel castings has not been investigated. The goal of this study is to determine the inclusion filtration efficiency of ceramic foam filters at removing different inclusion populations and oxide bi-films from a Fe–30Mn–9Al–0.9C–1Si–0.5Mo steel. In this regard, a mold was designed that allowed balanced filling of two identical Y-block castings in the same mold that were attached to two different but balanced rigging systems. One side of the gating systems included a 10-ppi (pores per inch) zirconia ceramic foam filter, while the other side was unfiltered. In the current study, the effectiveness of ceramic foam filters at removing different inclusion populations from the melt was evaluated directly from the filter inlet and outlet in the runner utilizing a scanning electron microscope with automated feature analysis. The effect of pouring order on the filtration efficiency was also determined in this study. Subsequent investigations will be performed to determine the effect of filtration on casting quality and mechanical properties.
Methodology
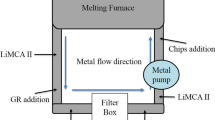
The solidification software MAGMA (5.3) was used to design the mold. The design of the mold and experimental procedure has been adapted from the paper by Chakraborty et al.9 The design consisted of two modified Y-block castings, where one of the runners is fitted with a ceramic filter (FOSECO STELEX ZR 10ppi filter) of dimensions 10 cm × 10 cm × 2.5 cm, while the other did not have any filters. The dimensions of the vertically parted molds are 70 cm × 20 cm × 35 cm. A drawing of the mold is shown in Figure 1.
The designs used similar castings, sprues, runners and gates, while the gating ratio used was different to accommodate the filter in one of them while balancing the filling into the castings. Hence, the gating ratios of the two molds were 1:2:3.8 (with filter) and 1:2.6:3.8 (without filter). Figure 2 shows the absolute velocity of filling at different stages, namely 10%, 20%, 30% and 50% full. It is shown that the presence of a filter helps in slowing down the velocity in one runner, while the filling is faster in the other runner.
Flow through the ingates of the castings takes place at a velocity of less than 0.44 m/s which is lower than the critical velocity of 0.45 m/s recommended by Campbell to minimize any air entrainment and reoxidation defects.10 Figure 3 shows the temperature at the end of filling for the steel. The steel was poured at a temperature of 1519 °C. Figure 3 shows the temperature profile just after filling with all temperatures in the casting and rigging system above 1450 °C. The liquidus temperature for this composition of steel was determined utilizing ThermoCalc thermodynamic modeling software to be 1338 °C. Figures 2 and 3 indicate that the filling of the casting happened at an absolute velocity which was less than the critical velocity and at the end of the pour, all the parts of the casting had a temperature higher than the liquidus, therefore avoiding any problems regarding premature solidification.
Thermodynamic modeling for the Fe–30Mn–9Al–1Si–0.9C–0.5Mo steel composition was performed using the Thermo-Calc 2017a software. Figure 4 shows the phases that form as a function of equilibrium cooling. The steel was modeled with 0.007% N, 0.005% O, and 0.005% S in order to determine the stability of different inclusions. Figure 4 also shows stable precipitation of Al2O3 (corundum) and AlN at temperatures well above the liquidus. MnS forms below the liquidus temperature during solidification. It should be noted that sulfur tends to highly segregate to interdendritic regions and this can increase the stability of MnS during solidification of FeMnAlC steels.11
Experimental Procedure
High-purity induction iron, ferrosilicon, ferromolybdenum, electrolytic manganese, high-purity aluminum and high-purity graphite were melted in a coreless 90.7-kg (200 lb) ferrous capacity induction furnace under argon cover with a flow rate of 25 SCFH. The target chemistry was Fe–30%Mne–9% Ale–1%Si–0.9%C–0.5%Mo. The mass of the total charge was 160 lb (72.56 kg). The molten metal was tapped at 1630 °C into a teapot-style ladle which was used to pour the metal into the three molds. The first mold consisted of metal poured from the bottom one-third of the ladle, while the second mold consisted of metal from the middle of the ladle. The last mold was poured from the metal at the top of the ladle.
Specimens were sectioned for microstructural and inclusion analysis at a distance of 10 mm from the inlet and outlet side of the filter as shown in Figure 5b. The chemistry analysis was performed by optical emission arc spectroscopy and combustion analysis using the LECO CS 500 for carbon and sulfur and a LECO TC 600 for determining the total oxygen and nitrogen contents. Specimens were sectioned before and after the filter in the same location for each of the three castings, and three sets of samples were obtained from each of them, as shown in Figure 5c, and observed under an optical microscope. A representative optical micrograph of a sample taken from the inlet side of the filter is shown in Figure 5d to contain a large bi-film defect. Polishing was performed utilizing 240–800 grit SiC papers followed by polishing with diamond compound to a 0.05-micron finish. Inclusion analysis was performed using automated inclusion analysis and was conducted utilizing an ASPEX PICA 1020 scanning electron microscope (SEM). The chemistries of the inclusions and the matrix were also observed using energy-dispersive X-ray spectroscopy (EDS). The bi-films were quantified using ImageJ software.
(a) Design showing the position of the filter. (b) Representative image showing how the filter was sectioned. (c) Representative image showing where the metallographic and inclusion analysis samples sectioned from each side of the filter. (d) Optical micrograph of one of the samples taken before the filter.
Results
The computer filling simulation exhibited a filling time of 9 s. This was in close accordance with the actual filling time of the first mold which was recorded to be 9 s. The filling time for molds two and three was 12 s and 17 s. This could be because of factors like less metallostatic pressure as the ladle was drained and had slight differences in tilt speed during pouring. The simulation produced the results in close accordance with the first mold poured. The total oxygen, nitrogen and sulfur contents results are shown in Table 1 and were measured using inert gas fusion and combustion infrared detection techniques. The samples were taken in the runner area directly after the filter in all three castings as shown in Figure 5b, c as well as from the unfiltered runner area in the same relative position (Figure 5a). Table 1 compares the results. Nitrogen was largely constant at 45 ppm regardless of pouring order in the samples taken in the unfiltered runner area. However, the filtered runners show a decrease in total nitrogen content when compared to the samples taken from the unfiltered runners. It is important to remind the reader that these molds were poured from a teapot ladle in which the first metal from the ladle may contain the cleanest metal, while the last metal poured from the ladle will likely have the highest amount of possible slag as well as oxide and nitride inclusions. However, throughout the three molds all the samples taken in the unfiltered runner and after filtration showed oxygen levels less than 10 ppm and this did not appear to be influenced by filtration. Total nitrogen decreased with pouring order from 34 to 22 ppm in filtered specimens. Sulfur was also fairly invariant of pouring order and filtration with a value around 32 ppm as shown in Table 1.
Table 2 gives the target and measured chemistry of the steel in weight percent as measured by optical emission spectroscopy, OES, for all the elements except carbon and sulfur, which were measured by combustion infrared detection techniques in a LECO C/S analyzer. The certified standards used for calibration of the OES had chemistries similar to the composition of the steel. The measured chemistry is reasonably close to the target chemistry. It should be noted that the molybdenum level was slightly higher than the anticipated, 0.7% Mo when 0.5% Mo was expected and the carbon content was slightly lower, 0.8% C when 0.9% C was expected.
Optical micrographs of samples sectioned directly before the filter for all the three molds are presented in Figure 6a–c. The matrix consists of mainly austenite with less than 10–15% ferrite. The most notable feature in Figure 6a–c is the presence of extensive oxide bi-films that are increasing in prevalence in the order of filling. These networks of oxide bi-films were found in of all the samples taken before the filters in all of the molds. In some cases, they are associated with areas of porosity caused through thickness failure of inlet gating system resulting from bi-film separation. The microstructures of samples taken after the filter are shown in Figure 7. Bi-films were quantified by determining the average area coverage utilizing image analysis on optical micrographs. Bi-films were not observed in filtered specimens as shown in Figure 7. It should be noted that the areas presented in Figures 6 and 7 were sectioned in exactly the same positions before and after the filter in each of the three gating systems as shown in Figure 5b, c. The composition of the bi-film defects was characterized utilizing an SEM with EDS and compared with the matrix austenite chemistries. Figure 8 shows the backscattered electron images of a network of bi-film defects from the area before the filter in mold 1. EDS analysis confirmed that the bi-films are mainly composed of aluminum and manganese oxides as shown in Table 3. Previous unpublished work by the authors shows that nitrogen and possibly hydrogen gas may nucleate on the bi-films during solidification, causing porosity and growth of coarse AlN plates during subsequent heat treatment, making these defects even more detrimental to casting quality.
As seen from Table 3, the bi-films were composed of mainly alumina and possibly in combination with manganese oxides. However, the high amount of Mn in the matrix contributes to a background effect, and thus, the presence of Mn in these bi-films is difficult to resolve. Inclusion analysis of samples sectioned before and after the filter was accomplished utilizing an ASPEX PICA 1020 SEM with automated feature analysis. A backscattered electron detector (BSED) and a magnification of 500 × were used for the analysis.
An emission current of 32–34 µA with a dwell time of 12 µs was considered for the analysis. Areas of bi-films and pores were excluded from the inclusion analysis based on size (greater than 10 µm for bi-films) and chemistry. For example, pores and bi-films were found to have either high carbon levels, since the diamond paste used for polishing can accumulate in the cracks in the bi-films and pores, or high concentrations of iron and manganese. AlN and MnS inclusions were differentiated from the bi-films as the nitrogen and/or sulfur levels in these inclusions were always greater than 4%. From Table 1, it is observed that the amount of total oxygen in chemistry samples was low (< 10 ppm). Additionally, EDS is not very accurate at determining oxygen. Thus, inclusions were classified depending on the amount of Al, Mn, S and N. The representative chemistries of different inclusions by type are shown in Table 4. Most of the inclusions observed were aluminum nitride AlN, manganese sulfide MnS and complex inclusions consisting of an AlN core with a capping layer of MnS. Some representative backscattered electron, BSE, images of AlN and complex AlN–MnS are shown in Figure 9. Figure 9a shows singular AlN inclusion that has nucleated and grown in the liquid. As the steel solidifies, sulfur will be enriched in the liquid and MnS inclusions will precipitate below the liquidus, utilizing AlN as a nucleation site as shown in Figure 9b, c.
Automated inclusion analysis was performed on samples sectioned from identical locations from each of the mold gating systems before and after the filter as shown in the drawings in Figure 5b, c. The following nomenclature will be adopted to identify the respective samples: mold 1, before filter (M1BF), mold 1, after filter (M1AF), mold 2, before filter (M2BF), mold 2, after filter (M2AF), mold 3, before filter (M3BF), mold 3, after filter (M3AF). Figure 10 shows the inclusion density by type for specimens sectioned before the filter. It was found that the AlN inclusions formed the majority of the inclusions followed by AlN–MnO and AlN–MnS. MnO and MnS were observed to precipitate on AlN. MnS inclusions had an inclusion density of less than 5/mm2. It should be noted that the MnS inclusions will form below the liquidus temperature as observed from Figure 4 and are thus unaffected by filtration.
Complex oxysulfides of Al and Mn and Ti–Mo carbides were found in trace amounts in all the three molds. However, these inclusions accounted for only 1–1.5% of the total amount of inclusions and were therefore excluded from the analysis. Figure 11 shows the inclusion density by type for samples sectioned after the filter in all three molds. The density of AlN decreased in the filtered samples by 27–28% in the first two molds and by 38% in the last mold poured. The percentage of MnS increases slightly; however, MnS forms after filling of the gating system and during solidification, and thus, for analysis of the filtration effectiveness, MnS is excluded from the analysis. The inclusions forming in the liquid, and thus subject to filtration, were considered to be AlN and complex AlN–MnS and AlN–MnO inclusions. The total area fraction of inclusion coverage in the filtered and unfiltered samples as a function of mold pouring order is given in Figure 12. Mold 1 showed the highest area fraction of inclusions before filtration at 456 ppm followed by mold 2 and mold 3 at 405 and 345 ppm, respectively. The samples taken after the filter were much cleaner and showed an average decrease in inclusion area of 174 ppm. The efficiency of inclusion removal by the filter was greatest in the last mold poured with a 54% overall reduction in the area fraction of inclusions. Figure 13a–c shows size distribution plot between the average inclusion size and the inclusion density for inclusions that are stable during filling. All the three molds show a reduction in the inclusion density between the unfiltered and filtered samples. From Figure 13a, b, it can be noted that the majority of inclusions were between 2 and 3 µm. The inclusion removal efficiency for inclusions greater than 3 µm was found to be 30.3% and 28.6% for the molds 1 and 2, while for the third mold it was 58.2%.
The area fraction of the aluminum nitride inclusions as a function of the average size of inclusions for the three molds considering filtered and unfiltered sections is represented in Figure 14a–c. The area fraction of AlN was shown to decrease in filtered samples taken from each of the molds. The results are similar to those presented for the total number of stable inclusions in the melt presented in Figure 12. Larger AlN inclusions, > 2–3 μm, were removed from the melt more efficiently by the filter than smaller AlN inclusions. The filtration is most significant in the third mold, as shown in Figure 14c as there is a higher percentage of larger-sized aluminum nitride inclusions in the unfiltered sections. It is also observed that the filtered areas from mold 3 have a maximum inclusion size of 6–8 µm, indicating that the larger-sized AlN inclusions have been mostly removed by the filter.
Discussion
Figure 15 shows the measured N, O and S contents from samples taken from unfiltered runners and from samples taken just behind the filter as described in Figure 5. The amount of sulfur and oxygen was not much different in the unfiltered and filtered runners and that did not appear to vary with pouring order. The sulfur concentration corresponds directly to the amount of sulfide inclusions (MnS and complex AlN–MnS) in the castings. Thermodynamic modeling in Figure 4 shows that MnS is stable below the liquidus near the end of solidification and this is consistent with the work of Vaz Penna et al. who show MnS precipitation after the liquidus in a Fe–30Mn–9Al–1C–1Si–0.5Mo steel with 40–400 ppm S.12 Thus, MnS did not form until after filling and during solidification, and therefore, the consistency of the sulfur concentrations in Figure 15 between the filtered and unfiltered runner areas is understandable. This is consistent with the work of others who have shown that inclusions in these steels mainly consist of AlN and AlN–MnS complex inclusions in which MnS is often found to precipitate heterogeneously on AlN during solidification.1,2,4,11 The amount of measured total oxygen in Figure 15 is low in all the samples and was less than 10 ppm.
This is consistent with the inclusion analysis presented in Figures 10 and 11 which shows less than 10 inclusions/mm2 were complex oxide inclusions. In general, endogenous oxide inclusions such as alumina and manganese spinel are not as prevalent as AlN in castings poured from FeMnAlC steels that are induction melted and this has been reported by several studies.1,2,4,11 Samples taken from the unfiltered runner show no real difference in the amount of nitrogen as a function of pouring order as shown in Figure 15. However, filtered specimens show a substantial decrease in total nitrogen, decreasing from around 45 ppm N to 35 ppm N in mold 1 to less than 22 ppm N in mold 3. The amount of nitrogen in these steels appears to be directly correlated to the amount aluminum nitride inclusions. This is consistent with the inclusion analysis presented in Figures 11 and 14 which shows a reduction in the area fraction and number of AlN and complex AlN inclusions with filtration. The total nitrogen contribution from the inclusions was determined from samples sectioned after the filter and compared to the measured nitrogen in the unfiltered and filtered runners. The method used for calculating the nitrogen content based on inclusion was obtained from the work by M Harris et al.13 The areal average elemental composition of inclusions is calculated for each element as follows:
%m is the areal average mass percent of a given element, %x is the amount of respective element in an individual inclusion, and Ainclusion and Atotal are the area of the individual inclusion and total area of all measured inclusions. The mass balance calculation was performed using the compositional data obtained from the EDS inclusion analysis and the following equation.13
In the above equation, Mppm is the mass fraction in ppm of a given element in the steel sample contained within the inclusions, Af is the total inclusion area fraction, and ρi and ρm are the densities of the inclusions associated with the given element and the density of the matrix, respectively. wi is the mass fraction of the given element in the associated inclusion compound.13
As shown in Figure 16, nitrogen decreases after filtration and the filtered nitrogen contents are in very good agreement with the nitrogen contribution from the inclusions. The efficiency of solid inclusion filtration increases with pouring order as shown from Figure 13, and this is supported by the measured nitrogen contents in Figures 15 and 16.
The measured amount of total nitrogen observed in the filtrated steels is also somewhat lower than previous induction-melted Fe–30Mn–(3–9)Al–(0.9–1.8) C steels in which the total nitrogen content that varied between 50 and 150 ppm.2 It should be noted that results indicate that the steel in the current study was very clean even in the unfiltered condition with total inclusion densities before filtration ranging from 50 to 70 inclusions/mm2 as shown in Figure 11. The total inclusion density of the current steel can be compared with the results of Bartlett et al.2, who showed total inclusion densities ranging from 70 to 146 inclusions/mm2 in induction-melted Fe–30Mn–(3–9)Al–(0.9–1.8)C steels.
The optical microstructural analysis of the samples in Figures 6 and 8 revealed extensive bi-film networks in samples taken before the filter with high amounts of porosity. The percentage of area covered by bi-films in samples taken before and after the filters in molds one, two and three were 1.78, 2.04 and 2.46%, respectively. Bi-films were not observed in samples taken after the filter. The velocity of the metal was the highest at the base of the downsprue and before the filter expansion area as shown in Figure 2. In some regions, the velocity reaches as high as 2 m/s, which is above the critical value 0.45 m/s. This leads to mixing of the metal with the air and the formation of a surface skin of the metal oxide that folds onto itself with along with a volume of entrapped air, leading to formation of aluminum oxide bi-films and associated porosity. These phenomena of bi-film formation and air entrainment have been widely reported in aluminum castings.14,15,16 The high amount of aluminum in these steels makes these alloys particularly susceptible to bi-film formation, and EDS elemental analysis of the bi-film defects in the current study shows that they are made of primarily aluminum oxide and perhaps in some cases Al–Mn–O spinel as shown in Figure 8 and Table 3. The filter was effective at removing the bi-film defects from the steel as shown in Figure 8. This is an encouraging result that has not been previously documented for high Mn and Al steels. It should be noted that the bi-films may have contributed to some inclusion removal because of their large area to volume ratio and a possible “inclusion netting” mechanism. However, inclusions were not observed to be attached or associated with bi-films when observed both optically and with an SEM. Additionally, the high initial velocity in front of the filter of greater than 1 m/s, as shown in Figure 2, may tend to push inclusions through these networks suggesting that most of the inclusions were captured by the filter. Further studies are planned to determine the mechanism of inclusion attachment within the filter.
Inclusions in the FeMnAlC steel consisted mainly of AlN and complex AlN–MnS and AlN–MnO inclusions. Very few oxide inclusions were observed, other than bi-film defects, and this is consistent with previous studies that report that inclusions in these steels are mainly AlN and complex AlN–MnS- and AlN–MnO-type inclusions as previously noted for industrially induction-melted and cast Fe–30Mn–9Al–(0.6–1.6)Si–0.9C–0.5Mo steels.1,2 The presence of mainly AlN and complex AlN–MnS and AlN–MnO inclusions in this study is consistent with previous studies of similar compositions of Fe–30Mn–XAl–0.9C steels with Al contents between 3 and 9% Al.1,2,4,17 A comparison of the inclusion removal of samples taken from before and after the filter for all the three molds is shown in Figure 12. The density of AlN decreased in the filtered samples by 27–28% in the first two molds and by 38% in the last mold poured. The filtration efficiency has been calculated using Eqn. 1 and the area fraction of inclusions before and after filtration as per the method used by Apelian et al.7 Filtration efficiency of all the nitride-based inclusions that form in the liquid (AlN, AlN–MnO and AlN–MnS, namely) was between 38 and 39% for the first two molds poured and increased to 55% in the last mold poured. It is shown in Figure 13, that although the incoming inclusion densities are largely similar, the last molds poured tended to have a higher percentage of larger inclusions, > 3 μm, and these larger inclusions tend to be filtered out more efficiently. The inclusion removal efficiency for inclusions greater than 3 µm was found to be 30.3% and 28.6% for the first two molds, while for the third molds it was 58.2%. A study for a similar mold design and inclusion filtration study was recently performed by Chakraborty et al. on a SAE 316 steel that was deoxidized by aluminum, generating a large amount of solid alumina inclusions.9 The efficiency of solid nitride inclusion filtration in the current study in mold 1 and mold 3 was higher by 20% and 10%, respectively, than in the study by Chakraborty et al.9.
From Figures 10 and 11, it was observed that aluminum nitride inclusions had the highest inclusion density among all inclusions. The analysis of aluminum nitride inclusions across the three sets of molds becomes of paramount importance since it plays a major role in determining the impact toughness of the steel.4 It was also observed that the filter displayed an efficiency of 37% removal of AlN inclusions in the first mold followed by 45% and 61% removal of AlN inclusions in the second and third molds, respectively. Figure 13 shows that the samples taken after the filter had a higher amount of smaller-sized inclusions than large-sized inclusions The difference in the bar graphs in Figure 13 shows that since the larger inclusions float to the top and since mold three was poured at the end, it consisted of a high number of larger (> 3 µm)-sized inclusions. A similar trend is observed in the filtration of alumina inclusions by Chakraborty et al.9. This is understandable since the smaller-sized inclusions would have passed through the pores of the ceramic filter while the larger-sized inclusions are more easily filtered out.
Conclusions
The filtration of inclusions from the melt of a Fe–30Mn–9Al–1Si–0.9C–0.7Mo steel was studied as a function of pouring order and utilizing a novel mold design consisting of two castings connected to two separate but balanced rigging designs. One of the gating systems utilized a 10-ppi zirconia foam filter in the runner system, while the other one was unfiltered. The three molds were prepared using no-bake sand, and the metal was poured using a teapot ladle. The results showed a decrease in the number and area fraction of inclusions with filtration as well as elimination of bi-film defects in samples sectioned directly after the filter in the runner system. Samples taken before the filter were covered with bi-films on their surface, which were mainly composed of aluminum and manganese oxides. Samples taken after the filter showed an elimination of bi-films. These results show that filters are extremely effective at removal of bi-film defects from high manganese and aluminum steels. AlN inclusions contributed to approximately 50% of the total inclusion population, while AlN–MnS and AlN–MnO contributed about 20% of the total inclusions. The filtration efficiency of inclusions from the melt increased from 38 to 39% in mold 1 and mold 2 to 55% for mold 3. The filter displayed an efficiency of AlN removal from 37 to 61% from mold 1 to mold 3. Mold 3 exhibited the highest amount of large-size inclusions in the size distribution analysis and showed the highest inclusion filtration rate. These results show that ceramic foam filters are very effective at decreasing bi-film defects and at filtration of large inclusions from the steel and should be used when a high degree of cleanliness and toughness is desired. Future work will quantify the effect of filtration on casting quality and toughness and verify the inclusion capture mechanism within the filter.
References
L. Bartlett, D. Van Aken, High manganese and aluminum steels for the military and transportation industry. JOM 66(1), 1770–1784 (2014)
L.N. Bartlett, D.C. Van Aken, Effect of aluminum and carbon on dynamic fracture toughness of FeMnAlC steels. American Foundry Society, AFS Transactions, Paper 13-1344 (2013)
R. Gopalan, N.K. Prabhu, Oxide bifilms in aluminum alloy castings: a review. Mater. Sci. Technol. 27(12), 1757–1769 (2011)
A.M. Schulte, S.N. Lekakh, D.C. Van Aken, V.L. Richards, Phosphorus mitigation in cast lightweight Fe–Mn–Al–C steel. 114th MetalCasting Congress, Orlando, Florida (March 2013)
A.N. Leonov, M.M. Dechko, Theory of design of foam ceramic filters for cleaning molten metals. Refract. Ind. Ceram. 40(11–12), 537–542 (1999)
V.N. Antsiferov, S.E. Porozova, Foam ceramic filters for molten metals: reality and prospects. Powder Metall. Met. Ceram. 42(9–10), 474–479 (2003)
D. Apelian, R. Mutharasan, S. Ali, Removal of inclusions from steel melts by filtration. J. Mater. Sci. 20, 3501–3514 (1985)
C. Tian, On the Removal of Nonmetallic Inclusions from Molten Steel Through Filtration. Thesis: Mining and Metallurgical Engineering, McGill University (May 1990)
S. Chakraborty, L. Bartlett, R. O’Malley, M. Xu, Efficiency of solid inclusion removal from the steel melt by foam ceramic filter: design and Experimental Validation. 122nd AFS Metal Casting Congress, American Foundry Society (2018)
J. Campbell, Complete Casting Handbook (Elsevier Ltd., Oxford, 2011)
R. Vaz Penna, L.N. Bartlett, T. Constance, Understanding the role of inclusions on the dynamic fracture toughness of high strength lightweight FeMnAl Steels. Int. J. Met. Cast. 13, 286–299 (2018)
G. Gigacher, W. Krieger, P.R. Scheller, C. Thomser, Non metallic inclusions in high manganese alloy steels. Steel Res. Int. 76(9), 644–649 (2005)
M. Harris et al., Evolution of non- metallic inclusions in foundry steel casting processes processes, in Proceedings of the 69th Annual Technical and Operating Conference, Steel Founders Society of America (SFSA) (2015, Chicago Illinois), Steel Founders society of America (SFSA) (Dec 2015)
D. Dispinar, J. Campbell, Porosity, hydrogen, and bi-film content in Al alloy castings. Mater. Sci. Eng. 528, 3860–3865 (2011)
G. Bozchaloei, N. Varahram, P. Davami, S. Kim, Effect of oxide bi-films on the mechanical properties of cast Al–7Si–0.3Mg alloy and the roll of runner height after filter on their formation. Mater. Sci. Eng. A 548, 99–105 (2012)
B. Farhoodi, R. Raiszadeh, M. Ghanaatian, Role of double oxide film defects in the formation of gas porosity in commercial purity and Sr-containing Al alloys. J. Mater. Sci. Technol. 30, 154–162 (2014)
L. Bartlett, A. Dash, D. Van Aken, V. Richards, K. Peaslee, Dynamic fracture toughness of high strength cast steels. Int. J. Metalcast. 7, 17–33 (2013)
Acknowledgements
The authors greatly acknowledge the contributions of the many undergraduate research assistants that contributed to this research: Kyle Dunsford, Trevor Constance, Ryan Van Dyke and Steen Anthony. This project was supported in part by the Wolf Endowed Funds for metalcasting education and research at Missouri University of Science and Technology. The authors also greatly acknowledge MAGMA LLC for their support of the metalcasting program at Missouri S&T and the contribution to the modeling work within this manuscript.
Funding
Funding was provided by AIST Foundation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Technical Discussion Appendix
Technical Discussion Appendix
Comments and discussion between reviewers and the authors.
Reviewer #2: The nitrogen decreased while pouring in the filtered molds. Do the authors have any theory on why? It seems counter intuitive.
Authors’ reply: “The authors appreciate this question and we understand why this may be somewhat counter intuitive. As shown in Figure 16in the original version of the manuscript, the measured nitrogen after the filter decreased from mold 1 to 3. This is most likely the effect of more efficient filtration of aluminum nitride inclusions with increasing inclusion loading within the filter. The molds were poured with a teapot ladle. Assuming some amount of floatation time, the metal in mold 1 should be the cleanest with increasing levels of inclusions in each successive mold that was poured. The higher amount of initial inclusions into the filter, the greater the filtration efficiency. This is because as a greater number of inclusions attach themselves to the walls of the filter, this actually increases the likelihood that subsequent inclusions will collide with the walls of the filter and become attached. The inclusions may also increase the surface roughness of the filter as they attach and this could help to decrease metal velocity and increase the amount of time and surface area for attachment. Therefore, it is reasonable that the filtered runner section from Mold 3 showed the lowest nitrogen content.”
Reviewer #2: Figure 16 lists that N was calculated, but the details of the calculation are not present in the text. Explain calculation.
Authors’ reply: The authors would like to convey that the values for calculated nitrogen before and after the filter have calculated utilizing the method by Harris et al. (Reference 13 in the modified paper). The software assumes the area fraction of each inclusion is equal to the volume fraction. Then, for each inclusion the software multiplies the area of the inclusion by the percentage of nitrogen. To obtain the amount of nitrogen on that inclusion, the sum of the total area of the inclusions is divided by the scan area and this gives the mass fraction of the nitrogen in ppm. A method of calculating a mass balance from SEM-EDS data was used that allows the study of elemental content contained within inclusions. The areal average elemental composition of inclusions is calculated for each element. The mass balance calculation was performed using the compositional data obtained from the SEM- EDS inclusion analysis. A passage explaining this method has been added to the discussion on page 11.
Reviewer #3: Were the simulation results (e.g. same filling time) validated?
Authors’ reply: “The simulation in MagmaSoft exhibited a filling time of 9 seconds. This was in close accordance with the actual filling time of the first mold which was recorded to be 9 s. The filling time for molds two and three were 12 s and 17 s. This could be because of factors like less metallostatic pressure as the ladle was drained and slight differences in tilt speed during pouring. The simulation produced results in close accordance with the first mold poured.”
Rights and permissions
About this article
Cite this article
Balasubramanian, K., Bartlett, L.N., O’Malley, R. et al. Filtration Efficiency of Inclusions in Lightweight FeMnAl Steels. Inter Metalcast 14, 328–341 (2020). https://doi.org/10.1007/s40962-019-00372-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40962-019-00372-7