Abstract
Portugal is a country rich in hydromineral resources mostly encompassed by alkaline sulphurous groundwater. These waters are mostly confined in the so-called Ancient Massif and result from the infiltration of meteoric waters that may reach great depths giving groundwater special physicochemical characteristics. The main aim of the research was to evaluate the hydrogeochemical evolution of the sulphurous mineral waters of Entre-os-Rios, as well as to achieve a better knowledge of the hydromineral system and to improve its hydrogeological conceptual model. Entre-os-Rios thermal baths are sited in NW Portugal and are recognised for their thermal spa tradition that dates back at least to the middle of sixteenth century. The hydromineral resources of Entre-os-Rios emerge from a confined granitic aquifer, deep seated, and are geotectonically controlled. Hydrochemical data was collected, both from the main mineral spring waters (Torre, Curveira, Arcos Esquerda, and Arcos Direita) and Barbeitos well. Hydrochemical analyses were gathered, including organoleptic characteristics, physicochemical properties, major anions, and cations and minor elements from the 1938 to 2017 periods. In addition, some historical data, from late 19th to early 20th centuries, were included. Moreover, some isotopic hydrology data were discussed (oxygen-18, deuterium, and tritium). Entre-os-Rios groundwater is colourless, with no turbidity, and has a foul odour of rotten eggs, has a low temperature, 17°–21 °C, is alkaline, pH values 8.4–8.8, a total mineralisation lower than 500 mg/L and a high total sulphuration, about 24 mg/L. Major anions follow the tendency HCO3 > Cl > F > SO4 > CO3, with high concentrations of fluoride, 18–21 mg/L and major cations follow the tendency Na > Ca > K > Li > NH4 > Mg. Among minor elements, boron, caesium, and tungsten have the highest concentrations, 986 µg/L, 247 µg/L, and 186 µg/L, respectively. The dominant hydrogeochemical facies are Na–HCO3. Isotopic data indicate a meteoric origin for Entre-os-Rios mineral waters, with a long residence time in the aquifer system, and that these waters are, most probably, sub-modern, recharged prior to 1952. The mineral waters of Entre-os-Rios are one of the most important sulphurous groundwater in Portugal and their hydrogeochemical status exhibited a good equilibrium through the last 100 years.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
According to Mays (2007), the sustainability of water resources is “the ability to use water in sufficient quantities and quality from the local to the global scale to meet the needs of humans and ecosystems for the present and the future to sustain life, and to protect humans from the damages brought about by natural and human-caused disasters that affect sustaining life”.
From the earliest times, humankind has associated certain springs with divine powers of healing, so for centuries springs have been visited for their curative properties. The basics of balneology appeared as early as the fifth century BC when Herodotus called attention to the methods of prescription and application of mineral waters (e.g., Albu et al. 1997; van Tubergen and van der Linden 2002; Gómez et al. 2017).
Mineral waters provide a source of valuable groundwater resources at local and regional levels, and are a resource of high economic importance considering their utilisation in the thermal spa (e.g., Carvalho 1993, 1996, 2006; LaMoreaux and Tanner 2001; TERMARED 2011; Corral et al. 2015; Juncosa et al. 2017). Nowadays, the historic thermal towns, or spa towns, constitute an interesting network for promoting hydromineral resources centres focused on thermal tourism, wellness, and healing (EHTTA 2016).
Mineral waters can be defined as (e.g., Moret 1946; Albu et al. 1997; LaMoreaux and Tanner 2001; Marques et al. 2018): natural groundwater originated from meteoric waters which infiltrate into the unsaturated zone and circulate underground via deep-crustal discontinuities (e.g., major faults, shear zones), acquiring distinct physical and chemical composition, that results from a water–rock interaction, and temperature, which are constant over time; these waters are normally located in a singular geologic and morphotectonical framework and have usually a deep source.
Since the last century, mineral and thermal waters have become progressively more endangered by human intervention, both in quantity and quality, by accelerated modification of their natural conditions (e.g., Albu et al. 1997).
Mineral and thermal springs have been admired across Europe for over 4000 years. Over the centuries, the Greeks, Romans, Ottomans, and others established bathing traditions and built interesting bath complexes (EHTTA 2016). The Romans were the first to capture and develop thermal and mineral springs on a large scale (e.g., Wittfogel 1956; Mays 2011; EuroGeoSurveys 2016; Gómez et al. 2017). The first Portugal overview of groundwater with therapeutic characteristics dates to the early eighteenth century (Henriques 1726) with an exhaustive inventory made by the personal physician of King John V of Portugal. As stated by Schöeller (1982) that impressive sentence: “Few countries have been as interested in thermomineral waters as Portugal, as evidenced by the beautiful publications I have in my library”. Most of the natural low-temperature geothermal springs that occur in Portugal belong to the Na–HCO3–sulphurous type and occur at temperatures in the range 20°–69 °C. Their geographical distribution is not uniform being most sites located in the northern and Central parts of the Portuguese mainland and are associated with granitic and schistose rocks of Variscan age, frequently related to major regional active faults (e.g., Acciaiuoli 1952/1953; Calado 2001; EuroGeoSurveys 2016).
Natural Mineral Waters are bacteriologically suitable groundwater with physical and chemical specificities that are stable at the origin, within the range of natural fluctuations, which may result in possible therapeutic properties or favourable health effects. These waters in Portugal are protected by the 2015 legal framework (Law 54/2015, from June 22nd), defining them as a geological resource. Several theories and hydrogeological conceptual models were proposed to assess the origin of Natural Mineral Waters occurring on the Portuguese mainland and the mechanisms of their upward flow from the reservoir towards the surface (e.g., Marques et al. 2003, 2018).
The mineral waters of Entre-os-Rios are one of the most important sulphurous waters in Portugal, because of the uniqueness of its water composition, namely the highest sulphur content. These mineral waters are recognised since the middle of the XVI century and are utilised for balneotherapy since the end of the XIX century. The main goal of this study was to evaluate the hydrogeochemical evolution throughout the last century of the mineral waters of Entre-os-Rios, to a better understanding and improvement of the hydrogeological conceptual model of this hydromineral system.
Entre-os-Rios mineral waters: regional background
Entre-os-Rios site is an area which has had a thermal tradition that dates back at least to the middle of sixteenth century (e.g., Tavares 1810; Ramalho Ortigão 1875; Baptista 1884, 1912; Lopes 1892; Amorim 1900; Ferreira da Silva 1896, 1909; Acciaiuoli 1952/1953).
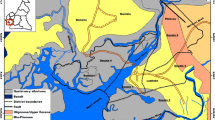
Entre-os-Rios thermal baths (formerly Torre thermal baths) are situated in Lugar da Torre (Eja), municipality of Penafiel, district of Porto (NW Portugal), (Fig. 1). This region has an Atlantic mild temperate climate, with an annual precipitation of 1300–1350 mm, an annual air temperature of 14 °C, an annual surface runoff of 600–650 mm, an annual real evapotranspiration of 500–550 mm and an annual recharge of 150–200 mm (Labcarga 2009; Teixeira 2011).
The hydromineral resources of Entre-os-Rios are controlled by: (i) lithology, namely the geological contact between the porphyritic coarse-grained granite and granodiorites, and by the presence of two-mica microgranite; (ii) tectonic constraints, mainly the deep-crustal fracture systems N–S to NNE–SSW and ENE–WSW, and the regional fracture systems NW–SE and NE–SW, (Fig. 1), (Medeiros et al. 1964, 1980; Labcarga 2009; Afonso et al. 2016).
Materials and methods
Hydrochemical data was collected from the overall period of 1938–2017, from the old thermal springs, Torre (Ts), Curveira (Cs), Arcos Esquerda (AEs) and Arcos Direita (ADs) and from the Barbeitos well (Bw) (Canto Machado 1985, 1987, 1988, 1991; ACavaco 1987, 1990; Teixeira 2011; Ferreira 2013).
Concerning the springs, the global period for Ts was 1938–1990 (7 samples), since after 1990 this spring had a decline in yield and virtually dried. For the rest of the springs the global studied period was 1938–2012 (25 samples). Regarding Barbeitos well (depth of 115 m), the period was 1990–2017 (63 samples), since this well was built in 1990. Since 1990 this well is the only exploited for thermal baths purposes. In addition, some historical data from the end of XIX century to the early XX century (Ferreira da Silva 1896, 1909) were analysed and integrated in the discussion. Nearly all the water samples were not collected by the authors. Instead hydrochemical data were gathered on the bibliographic archive of Entre-os-Rios thermal baths.
Hydrochemical parameters included: (i) organoleptic characteristics, smell, colour, and turbidity for springs and Barbeitos well; (ii) several physicochemical properties, namely, temperature, pH, electrical conductivity, dry residue at 180 °C, silica and total sulphuration, for springs and Barbeitos well; (iii) major anions, specifically, bicarbonate, carbonate, sulphate, chloride and fluoride for springs and Barbeitos well and silicate, hydrosulphide and nitrate for Barbeitos well; (iv) major cations, namely, sodium, calcium, magnesium, potassium, lithium and ammonia for springs and Barbeitos well. As well, 33 minor elements were considered for Barbeitos well for the period 2005–2014. Moreover, since the use of environmental isotope geochemistry is an important tool in the study of mineral waters (e.g., IAEA 1981; Mook 2000), we gathered and discussed some isotopic data, oxygen-18, deuterium and tritium for the years 1995 (Calado 2001) and 2011. The δ2H, δ18O and 3H isotopes content for the year 2011 was determined at the Stable Isotopes and Instrumental Analysis Facility (SIIAF) Laboratory, University of Lisbon (Portugal), by mass spectrometry continuous flow (CF-IRMS). The results are expressed as δ % V-SMOW and the analytical accuracy is < 0.1. Microsoft Excel 2016 and AquaChem v.5.1 software was used for the hydrochemical interpretation.
Results and discussion
Table 1 summarises the most representative data from the mineral and the normal groundwater of the study region.
Organoleptic features
The mineral groundwater of Entre-os-Rios are colourless, with no turbidity and have a characteristic foul odour of rotten eggs, given by hydrogen sulphide, as it will be shown later on major anions description. The same characteristics were described by Ferreira da Silva (1896, 1909).
Physicochemical properties
Concerning temperature, these groundwater are cold, with median temperatures in the range 17°–21 °C. Among the old springs, Torre (Ts) had a higher temperature, 19 °C, while for Barbeitos well the median temperature was 21 °C; this means that the temperature of Barbeitos well is 2–4 °C higher than the springs. Since the mean annual air temperature is 14 °C, this groundwater is considered thermal according to Schöeller’s criterion (Schöeller 1962). In 1895 and 1908, the recorded temperatures were similar to these (Ferreira da Silva 1896, 1909): 17.7 °C and 18.6 °C for Ts and 17.6 °C for AEs and ADs.
Concerning pH, these waters are clearly alkaline, being the values very similar, both for the old thermal springs and Barbeitos well. Median values of 8.68, 8.59, 8.41, 8.84 and 8.80, were registered for Torre (Ts), Curveira (Cs), Arcos Esquerda (AEs), Arcos Direita (Ads) and Barbeitos well (Bw), respectively, (Fig. 2). The observed pH values are consistent with the circulation of these waters through granitic rocks, where the dissolution of minerals, such as silicates and aluminosilicates, tend to raise the pH to alkaline values (Langmuir 1997). According to Canto Machado (1988) and Calado (2001); in addition, are included in the subgroup of Portuguese sulphurous waters with pH values of 8.35–9.00, along with others sulphurous waters in Northern and Central Portugal located in similar geologic settings, such as Carlão, S. Lourenço and Moledo (Marques et al. 2000, 2018) and Caldas da Cavaca (Teixeira et al. 2015).
Electrical conductivity (EC) measurements can give a good approximation of dry residue and total mineralization. The springs presented similar values of EC, with median EC in the range 552–596 µS cm−1, while Barbeitos well had a slightly higher median value of 609 µS cm−1. The dry residue at 180 °C varied in springs from 399 mg/L to 428 mg/L and the median concentration for Barbeitos well was, also, somewhat higher, 437 mg/L. Finally, total mineralization was only calculated for Barbeitos well, being in the range 444–542 mg/L, with a median concentration of 493 mg/L. The results for dry residue at 180 °C are very comparable with the historical values presented by Ferreira da Silva (1896, 1909) for the old springs, ranging from 417 mg/L to 439 mg/L. In addition, the mineralization results are consistent with those proposed by Canto Machado (1988) for the subgroup of sulphurous waters with pH values in the range 8.35–9.00, 432 ± 123 mg/L.
About silica, Torre spring registered the highest value, 57 mg/L, while the rest of the springs and Barbeitos well had very similar values, with a median value of 44 mg/L. These silica concentrations are lower than those indicated by Ferreira da Silva (1896, 1909), between 43.9 mg/L and 74.1 mg/L. Although, the values are close to 10% of total mineralization, that is pointed out by Canto Machado (1988) as typical for sulphurous waters. High silica values (range 41–57 mg/L), usually representing more than 15% of total mineralization, were found by Marques et al. (2000) in Carlão, S. Lourenço and Moledo waters. Teixeira et al. (2015) recorded for Caldas da Cavaca waters high silica contents around 55 mg/L, that represent nearly 21% of total mineralization.
Concerning total sulphuration, the available data for springs were from the 1979–1990 period, while for Barbeitos well were from 1990 to 2017. Among springs, only Torre is represented in the chart, since the other springs only have three values for the analysed period. Except for Arcos Direita spring, that showed a median value of 18 mg/L, the rest of the springs and Barbeitos well have similar concentrations, with median values in the range 23.8–24.9 mg/L (Fig. 3-left). According to Canto Machado (1988), these concentrations are the highest in the subgroup of sulphurous waters with pH values in the range 8.35–9.00, with mean values of 1.1 ± 0.7 mg/L. This way, Entre-os-Rios groundwater are an anomaly in this subgroup. In addition, Ferreira da Silva (1896, 1909) presented a graphic including several Portuguese mineral waters where Entre-os-Rios groundwater were highlighted for having the highest sulphuration (Fig. 3-right).
During long time exploitation, considering the data from Barbeitos well, only temperature and total sulphuration show a slight tendency to increase (ca. 1 °C) and decrease (ca. 1.4 mg/L), respectively.
Major anions
The available data for the old thermal springs were from the 1938–2012 period, while for Barbeitos well were from 1990 to 2017 (Fig. 4). The springs not represented in the charts are those that have less than 5 values for the analysed period.
The major anions analysed both in springs and Barbeitos well, bicarbonate (HCO3), carbonate (CO3), sulphate (SO4), chloride (Cl) and fluoride (F), show the same rating in terms of median concentrations: HCO3 > Cl > F > SO4 > CO3. Barbeitos well usually presented the highest concentrations. In addition, SO4 and CO3 usually display the highest variation.
Median values for HCO3 are in the range 150–175 mg/L and vary between 155.6 mg/L (AEs) and 174.0 mg/L (Bw). For Cl, median values are in the range 44–61 mg/L and vary between 44.4 mg/L (Cs) and 60.4 mg/L (Ts). The HCO3 and Cl concentrations presented by Ferreira da Silva (1896, 1909), 169–221 mg/L and 53–64 mg/L, respectively, are similar to those.
Median concentrations for F are in the range 18–21 mg/L and vary between 18.1 mg/L (Cs) and 21.0 mg/L (Bw). These values of F are consistent with the assumptions of Canto Machado (1988) and Calado (2001), which consider concentrations of F higher than 5 mg/L are typical of sulphurous groundwater. Moreover, Teixeira et al. (2015) registered also for Caldas da Cavaca waters high-fluoride concentrations of up to 14 mg/L. For SO4, median values are in the range 2–7 mg/L and vary between 2.1 mg/L (Cs) and 6.5 mg/L (ADs). For CO3, the median values are 3.8 mg/L (Ts) and 5.8 mg/L (Bw).
Considering the other anions only analysed in Barbeitos well, silicate (H3SiO4), hydrosulphide (HS) and nitrate (NO3), the median values are 7.4 mg/L, 25.1 mg/L and 0.19 mg/L, respectively.
Marques et al. (2000) have also registered for Carlão, S. Lourenço and Moledo waters the presence of HCO3 and CO3, HCO3 as the dominant anion, the presence of reduced species of sulphur (HS, 0.3–3.5 mg/L), and high F concentrations (2.5–14 mg/L). According to Marques et al. (2000, 2018) the presence of F can be faced as a sign of water–rock interaction mainly associated with granitic rocks. Moreover, Michard (1990) states that high F values indicate that the reservoir rock should be mainly granite. Teixeira et al. (2015) have also recorded for Caldas da Cavaca mineral waters the presence of HS, around 0.9 mg/L.
During long time exploitation, considering the data from Barbeitos well, only CO3 and SO4 show a tendency to decrease (ca. 7 mg/L) and increase (ca. 8 mg/L), respectively.
Major cations
The available data for the old thermal springs were from the 1938–2012 period, while for Barbeitos well were from 1990 to 2017 (Fig. 5). The springs not represented in the charts are those that have less than five values for the analysed period.
The major cations analysed both in springs and Barbeitos well, sodium (Na), calcium (Ca), potassium (K), magnesium (Mg), lithium (Li) and ammonia (NH4), show the same rating in terms of median concentrations: Na > Ca > K > Li > NH4 > Mg. The dominance of Na among cations corroborates the conclusions by Canto Machado (1988) and Calado (2001), that refer Na as the mostly major cation among sulphurous groundwater. All cations have similar concentrations in the springs and Barbeitos well. Furthermore, NH4 and Mg usually display the highest variation. Marques et al. (2000) have also identified Na as the dominant cation for Carlão, S. Lourenço and Moledo waters. Median values for Na are in the range 130–145 mg/L and vary between 130.4 mg/L (ADs) and 142.0 mg/L (Bw). The Na concentrations presented by Ferreira da Silva (1896, 1909), 124–141 mg/L are comparable to those.
Ca and K have close median values, being Ca slightly higher (range 3.0–5.5 mg/L) than K (range 2.8–3.0 mg/L). The lowest and the highest values for Ca were registered, respectively, in Bw and Cs. The Ca and K concentrations registered by Ferreira da Silva (1896, 1909), 2.7–5.1 mg/L and 2.3–3.2 mg/L, respectively, are comparable to those.
For Li and NH4, their median values are close, 1.3 mg/L and 1.0 mg/L, respectively. The Li concentrations registered by Ferreira da Silva (1896, 1909) were lower, 0.5–0.9 mg/L, while the NH4 values are similar, 0.7–1.0 mg/L. Concerning NH4, Canto Machado (1988) and Calado (2001) refer that this cation is found in all the sulphurous groundwater, and therefore is characteristic of these waters.
Finally, Mg shows most of the values below 0.2 mg/L. Ferreira da Silva (1896, 1909) recorded higher concentrations, below 3.2 mg/L.
During long time exploitation, considering the data from Barbeitos well, only Mg and NH4 show a slight tendency to decrease (ca. 1 mg/L) and increase (ca. 1 mg/L), respectively.
Minor elements
The median concentration values available for the 33 minor elements are represented in Fig. 6. Three groups can be emphasized: aluminium (Al), manganese (Mn) selenium (Se) and nickel (Ni) in the range 1–10 µg/L, strontium (Sr), rubidium (Rb) and iron (Fe) in the range 10–100 µg/L, and boron (B) caesium (Cs) and tungsten (W) in the range 100–1000 µg/L, with the uppermost concentrations, 986 µg/L, 247 µg/L and 186 µg/L, respectively. The highest values of boron validate the results presented by Machado (1988) that concludes Entre-os-Rios mineral waters are an exception in the Portuguese sulphurous waters with pH inferior to 9.00.
The dominance of the major ions HCO3, Na, F and Li, and the Cs–Sr–Rb-rich nature and the high pH values are common in granite-sourced thermal springs (Michard 1990; Lottermoser and Cleverley 2007).
Considering the major anions and cations, the analysis allows to conclude that the hydrogeochemical signature of Entre-os-Rios mineral waters is dominantly Na-HCO3, being this facies different from the normal groundwater of the region, Na–Ca–Cl–SO4 (Fig. 7). These normal waters have a low pH (4–6) and very low mineralisations (EC < 150 µS cm− 1) and their facies should be related to contamination, namely from agriculture. They correspond to shallow groundwater with a short residence time in the aquifers.
Isotopic data
Concerning the isotopic approach, representative analyses of groundwater samples are presented in Table 2. The relationship between δ18O and δ2H is shown in Fig. 8 with the Global Meteoric Water Line (GMWL, Craig 1961) and the Local Meteoric Water Line (LMWL) for Porto meteorological station (Carreira et al. 2005). As shown in Fig. 8, all the water samples fall near the GMWL and the LMWL, implying that, both normal and mineral groundwater are of meteoric origin. Moreover, Entre-os-Rios mineral waters do not show evidences of water–rock interaction at high temperatures, since there is no oxygen-18 shift. Marques et al. (2003, 2018) have reached the same conclusion for Caldas de Moledo thermal waters. Considering the 3H content in the mineral groundwater, values are less than 1 TU, that is compatible with a recharge prior to 1952, which means these waters are, most probably, sub-modern. According to Calado (2001), mineral groundwater has a long residence time in the aquifer system, that is located at circa 3.2 km depth, estimated by chemical geothermometry. Marques et al. (2000) also measured low 3H values for Carlão, S. Lourenço and Moledo thermal waters, considering this indicates a considerable residence time at depth. However, according to Clark and Fritz (1997) recurring to 3H to date groundwater assumes a qualitative character, and it is not possible to make quantitative interpretations concerning the average residence times of groundwater’s.
δ18O vs δ2H signatures of the groundwater samples from Entre-os-Rios groundwater. The Porto precipitation data (Carreira et al. 2005), the Global Meteoric Water Line (GMWL: δ2H = 8δ18O + 10; Craig 1961) and the Local Meteoric Water Line (LMWL: δ2H = 6.20δ18O + 1.12; Carreira et al. 2005) were plotted as reference
Hydrogeological conceptual site model
Environmental hydrogeological conceptualisation and geo-visualisation techniques have become essential tools in understanding groundwater and hydromineral systems (e.g., Chaminé et al. 2013, 2015; Kresik and Mikszewski 2013; Chaminé 2015). The previous hydrogeochemical and isotopic approach helped to understand and to improve the hydrogeological conceptual model for these groundwater systems (Fig. 9). The area of Entre-os-Rios is characterised by three aquifer types:
-
A shallow and unconfined aquifer associated with the highly weathered granite and the granitic residual soil. This aquifer has a key role to the recharge of the underlying aquifers;
-
An unconfined to semi-confined aquifer, located in the fractured and weathered granitic rock mass. Normal groundwater is characterised by a Na–Ca–Cl–SO4 facies, with pH around 4–6, electrical conductivities lower than 150 µS cm−1 and very low mineralisations;
-
A deep confined hydromineral aquifer, placed in the slightly weathered granite dominated by a deep fault zone. Barbeitos well is in the coarse-grained granite, close to the geological contact with the granodiorites. Groundwater is characterised by a pH around 8.4–8.9, electrical conductivities around 552–610 µS cm−1 and an intermediate mineralization of circa 493 mg/L. Moreover, these mineral waters are sulphurous, fluoridated and have a Na–HCO3 facies. In addition, groundwaters have a meteoric origin and a long residence time in the aquifer system, that is located at circa 3.2 km depth.
Concluding remarks
The use of hydrogeochemical and environmental isotopic data provided a clear characterisation of Entre-os-Rios hydromineral system. Comparison between actual and historic hydrochemistry data gave important insights about water evolution in this hydromineral region.
Organoleptic features, physical properties and major ion chemistry are stable with only relatively slight variations. Minor elements are dominated by boron, caesium and tungsten. According to isotopic analysis, the groundwaters are of meteoric origin and are most probably sub-modern. Therefore, Entre-os-Rios mineral waters revealed a good constancy throughout the last century.
The attained results showed that tectonics strongly control the occurrence of natural mineral waters ascribed to a special geological environment, responsible for the development of a Na–HCO3 hydrogeochemical signature with a pH in the range 8–9. All the data and the hydrogeological conceptual model will permit to define priority areas to develop complementary studies (e.g., isotopic analysis). In addition, they will contribute to a better evaluation of wellhead protection areas, to accomplish a sustainable management of the groundwater resources of the region.
Future work should focus on developing a multivariate statistical analysis, to establish the common relationships between measured hydrogeochemical variables, more isotopic studies to define the recharge area of these waters and to estimate their age and analyse the role of hydrogeochemical characteristics on the beneficial effects on human health.
References
ACavaco (1987) Estudo hidrogeológico das Caldas de Entre-os-Rios. Sondagens e Fundações ACavaco Lda, Lisboa (unpublished report)
ACavaco (1990) Furos de pesquisa e eventual captação de água mineral no Centro do INATEL em Entre-os-Rios. Sondagens e Fundações ACavaco Lda, Lisboa (unpublished report)
Acciaiuoli LMC (1952/1953) Le Portugal hydrominéral, vol 2. Direction Générale des Mines et des Services Géologiques, Lisbonne
Afonso MJ, Ferreira MR, Teixeira J, Chaminé HI (2016) The sulphurous mineral waters of Entre-os-Rios (NW Portugal): a hydrogeochemical assessment. In: Faílde JM, Formella A, Fraiz JÁ, Gómez-Gesteira M, Pérez F, Vázquez VR (eds.), Proceedings Ist international congress on water healing spa and quality of life/I Congreso Internacional del Auga, Termalismo y Calidad de Vida (Ourense, Spain, 23–24 September 2015), Campus da Auga, Vicerrectoría del Campus de Ourense, Universidade de Vigo, p. 169–174
Albu X, Banks X, Nash X (1997) Mineral and thermal groundwater resources. Chapman & Hall, London
Amorim HS (1900) Therapeutica thermal (aguas de Entre-os-Rios). Escola Medico-Cirurgica do Porto, Typographia de A.F. Vasconcellos Succursal, Porto. http://hdl.handle.net/10216/17232 (Graduation Dissertation). Accessed 1 June 2017
Baptista A (1884) Aguas minero-medicinaes do Concelho de Penafiel. Escola Medico-Cirurgica do Porto. Typographia de Viúva Gandra, Porto. http://hdl.handle.net/10216/16811 (Graduation Dissertation)
Baptista A (1912) As aguas d’Entre-os-Rios e a sua estância (Torre). Typographia a vapor da Empreza Guedes, Porto
Calado CMA (2001) A ocorrência de água sulfúrea alcalina no Maciço Hespérico: quadro hidrogeológico e quimiogénese. Universidade de Lisboa. (PhD Thesis)
Canto Machado MJ (1985) Análise química de elementos maiores das diferentes emergências das Termas de Entre-os-Rios. Ministério da Indústria Energia e Exportação, Direcção-Geral de Geologia e Minas, Laboratório da DGGM, São Mamede Infesta (Unpublished Report)
Canto Machado MJ (1987) Estudo químico completo das águas das várias nascentes das Termas de Entre-os-Rios. Direcção-Geral de Geologia e Minas, Laboratório, Secção de Hidroquímica, Relatório n.º11, São Mamede Infesta (Unpublished Report)
Canto Machado MJ (1988) O quimismo das águas sulfúreas portuguesas. Est Not Trab Serv Fom Min Porto 30:37–49
Canto Machado MJ (1991) Estudo físico-químico completo da água do furo de Barbeitos das Termas de Entre-os-Rios. Direcção-Geral de Geologia e Minas, Laboratório, Secção de Hidroquímica, Relatório n.º11, S. Mamede de Infesta (Unpublished Report)
Carreira PM, Araújo MF, Nunes D (2005) Isotopic composition of rain and water vapour samples from Lisbon region: characterization of monthly and daily events. IAEA-TECDOC-1453, Isotopic composition of precipitation in the Mediterranean Basin in relation to air circulation patterns and climate—final report of a coordinated research project 2000–2004. IAEA, Viena, pp 141–155
Carvalho JM (1993) Mineral and thermal water resources development in the Portuguese Hercynian Massif. In: Banks D, Banks S (eds) Hydrogeology of Hard Rocks, International Association of Hydrogeologists, IAH, Oslo, 24(1):548-561
Carvalho JM (1996) Mineral water exploration and exploitation at the Portuguese Hercynian Massif. Environ Geol 27:252–258
Carvalho JM (2006) Prospecção e pesquisa de recursos hídricos subterrâneos no Maciço Antigo Português: linhas metodológicas. http://hdl.handle.net/10773/5016 Universidade de Aveiro. (PhD Thesis)
Chaminé HI (2015) Water resources meet sustainability: new trends in environmental hydrogeology and groundwater engineering. Environ Earth Sci 73(6):2513–2520
Chaminé HI, Carvalho JM, Afonso MJ, Teixeira J, Freitas L (2013) On a dialogue between hard-rock aquifer mapping and hydrogeological conceptual models: insights into groundwater exploration. Eur Geol J 35:26–31
Chaminé HI, Carvalho JM, Teixeira J, Freitas L (2015) Role of hydrogeological mapping in groundwater practice: back to basics. Eur Geol J 40:34–42
Clark ID, Fritz P (1997) Environmental isotopes in hydrogeology. CRC Press, Lewis Publishers, Boca Raton
Corral MM, Galindo E, Ontiveros C, Díaz-Muñoz J (2015) Hydrogeochemical areas as background for specific mineral and thermal waters of Spain. Environ Earth Sci 73(6):2683–2697
Craig H (1961) Isotopic variations in meteoric waters. Science 133:1702–1703
EHTTA—European Historic Thermal Towns Association (2016) Discover the European route of thermal heritage. European Historic Thermal Towns Association, Italy. http://www.ehtta.eu/. Accessed May 2017
EuroGeoSurveys (2016) Wonder water the value of water. The Geological Surveys of Europe, Brussels
Ferreira MRN (2013) Evolução hidrogeoquímica das águas sulfúreas de Entre-os-Rios: avaliação preliminar. Instituto Superior de Engenharia do Porto, ISEP, Porto http://hdl.handle.net/10400.22/6157 (MSc Dissertation)
Ferreira da Silva AJ (1896) Memoria e estudo chimico sobre as aguas minero-medicinais de Entre-os-Rios (Quinta da Torre). Com um appendice contendo as noticias e observações clinicas sobre estas afamadas aguas, publicadas em 1815–1817 pelo medico de Penafiel dr. Antonio de Almeida. Porto. Typographia do Commercio do Porto, Porto
Ferreira da Silva AJ (1909) As aguas mineraes de Entre-os-Rios (Estância da Torre): memória e estudo chimico e bacteriológico. Typographia a vapor da Empreza Guedes, Porto
Gómez CP, González-Soutelo S, Mourelle ML, Legido JL (2017) Spa techniques and technologies: from the past to the present. Sustain Water Resour Manag. https://doi.org/10.1007/s40899-017-0136-1
Henriques F (1726) Aquilégio medicinal. Lisboa Ocidental na Oficina da Música, Lisboa
IAEA—International Atomic Energy Agency (1981) Stable isotope hydrology. Deuterium and Oxygen-18 in the Water Cycle. IAEA, Vienna. Technical Reports Series, 210
Juncosa R, Meijide-Failde R, Delgado J (2017) Therapeutic characteristics of Galician mineral and thermal waters (NW-Spain) ascribed to their local/regional geological setting. Sustain Water Resour Manag. https://doi.org/10.1007/s40899-017-0112-9
Kresik N, Mikszewski A (2013) Hydrogeological conceptual site models: data analysis and visualization. CRC Press, Taylor and Francis Group, Florida
Labcarga—Laboratório de Cartografia e Geologia Aplicada (2009) Estudo geomorfológico e geológico-estruturalda concessão hidromineral HM-23 de Entre-os-Rios (Quinta da Torre) e área envolvente: implicações no desenvolvimento de recursos hídricos subterrâneos. Laboratório de Cartografia e Geologia Aplicada, ISEP, Porto (unpublished report)
LaMoreaux PE, Tanner JT (2001) Springs and bottled waters of the world: ancient history, source, occurrence, quality and use. Springer, Berlin
Langmuir D (1997) Aqueous environmental geochemistry. Prentice Hall, Upper Saddle River
Lima A (2010) Composição e origem das águas minerais naturais: exemplo das Caldas da Saúde. Edições Almedina, Coimbra
Lopes AL (1892) Águas minero-medicinais de Portugal. Edição M. Gomes, Livreiro-Editor, Lisboa
Lottermoser BG, Cleverley JS (2007) Controls on the genesis of a high-fluoride thermal spring: innot hot springs, north Queensland. Austral J Earth Sci 54:597–607
Marques JM, Aires-Barros L, Graça R (2000) Genesis of low-temperature sulphurous mineral waters (Northern Portugal): a geochemical and isotopic approach. In: Proceedings World Geothermal Congress 2000, Kyushu-Tohoku, Japan, p. 1407–1412
Marques JM, Espinha Marques J, Carreira PM, Graça RC, Aires-Barros L, Carvalho JM, Chaminé HI, Borges FS (2003) Geothermal fluids circulation at Caldas do Moledo area, Northern Portugal: geochemical and isotopic signatures. Geofluids 3:189–201
Marques JM, Carreira PM, Neves O, Espinha Marques J, Teixeira J (2018) Revision of the hydrogeological conceptual models of two Portuguese thermomineral water systems: similarities and differences. Sustain Water Resour Manag. https://doi.org/10.1007/s40899-018-0218-8
Mays LW (2007) Water resources sustainability. McGraw-Hill, New York
Mays LW (2011) Ancient water technologies. Springer, Dordrecht
Medeiros AC, Pilar L, Fernandes AP (1964) Carta Geológica de Portugal, na escala 1/50000. Notícia Explicativa, Folha 13-B (Castelo de Paiva). Serviços Geológicos de Portugal, Lisboa
Medeiros AC, Pereira E, Moreira A (1980) Carta Geológica de Portugal, na escala 1/50000. Notícia Explicativa, Folha 9-D (Penafiel). Serviços Geológicos de Portugal, Lisboa
Michard G (1990) Behaviour of major elements and some trace elements (Li, Rb, Cs, Sr, Fe, Mn, W, F) in deep hot waters from granitic areas. Chem Geol 89:117–134
Mook WG (2000) Environmental isotopes in hydrological cycle. Technical documents in hydrology, 39 (I). UNESCO, IAEA, Paris
Moret L (1946) Les sources thermominérales. Masson & cie, Paris
Ramalho Ortigão JD (1875) Banhos de caldas e águas mineraes. Livraria Universal. Magalhães & Moniz Editores, Porto
Schöeller H (1962) Les eaux souterraines. Masson & cie, Paris
Schöeller H (1982) Sur les eaux thermominérales et leur origine. In: Romariz C (ed), Actas da III Semana de Hidrogeologia (Lisboa 10-14 Maio 1982), Faculdade de Ciências Universidade de Lisboa, Lisboa. p. 37–43
Tavares F (1810) Instrucções e cautelas practicas sobre a natureza, diferentes especies, virtudes em geral, e uso legitimo das águas mineraes, principalmente de Caldas; com a noticia daquellas, que são conhecidas em cada huma das Provincias do Reino de Portugal, e o methodo de preparar as aguas artificiais, vol 2. Real Imprensa da Universidade, Coimbra
Teixeira J (2011) Hidrogeomorfologia e sustentabilidade de recursos hídricos subterrâneos. Universidade de Aveiro http://hdl.handle.net/10773/8308 (PhD Thesis). Accessed 1 June 2017
Teixeira J, Chaminé HI, Espinha Marques J, Carvalho JM, Pereira AJ, Carvalho MR, Fonseca PE, Pérez-Alberti A, Rocha F (2015) A comprehensive analysis of groundwater resources using GIS and multicriteria tools (Caldas da Cavaca, central Portugal): environmental issues. Environ Earth Sci 73(6):2699–2715
TERMARED (2011) Catálogo de manantiales termales del espacio SUDOE / Catálogo de nascentes termais do espaço SUDOE / Catalogue des sources thermales de l’espace SUDOE. Xunta de Galicia, Santiago de Compostela
van Tubergen A, van der Linden S (2002) A brief history of spa therapy. Ann Rheum Dis 61:273–275
Wittfogel KA (1956) The hydraulic civilizations. In: Thomas WL (ed) Man’s role in changing the face of the Earth. The University of Chicago Press, Illinois, pp 152–164
Acknowledgements
The authors are grateful to INATEL Foundation, namely A. Vilela and M.C. Soares, for consenting the access to the archive of Entre-os-Rios thermal baths. Special thanks are due to J.M. Carvalho, J. Teixeira, M.R. Ferreira, M.R. Carvalho and J.M. Marques for all inputs, discussions and some shared data. The present work is dedicated to the outstanding chemist and Professor A. J. Ferreira da Silva (1853–1923) who developed numerous valuable hydrochemical assessments in Portuguese thermal spas. We acknowledge the anonymous reviewers for the constructive comments that helped to improve the focus of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the special issue on Sustainable Resource Management: Water Practice Issues.
Rights and permissions
About this article
Cite this article
Afonso, M.J., Chaminé, H.I. Environmental hydrogeochemistry assessment as a tool for sustainable hydromineral resources management (Entre-os-Rios thermal baths, NW Portugal). Sustain. Water Resour. Manag. 5, 147–159 (2019). https://doi.org/10.1007/s40899-019-00300-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40899-019-00300-x