Abstract
Purpose
Synthetic polymers such as poly(lactic acid) (PLA) are well suited for preparing patient-specific bone tissue scaffolds by three-dimensional (3D) printing due to their favorable mechanical properties; however, they have limited biological activity. Natural polymers have good bioactivity and provide a better cellular microenvironment for attachment, proliferation, and differentiation, but lack the mechanical strength required as a bone substitute.
Method
In this work, porous PLA scaffolds were prepared by fused filament fabrication. For uniform cell seeding and enhanced cellular function, a silk fibroin-alginate (SF/Alg) blend hydrogel loaded with human mesenchymal cells (hMSCs) was loaded into the pores of the 3D-printed hybrid scaffolds between the struts. The physicochemical properties of the scaffold and the hMSC response were characterized.
Results
The gel-loaded 3D-printed PLA scaffolds were stable over 21 days in an aqueous buffer solution. The compressive strength of the scaffolds was ≈ 10 MPa, which is similar to that of cancellous bone. The proliferation and viability of hMSCs were significantly enhanced when loaded within the SF/Alg hydrogel in the PLA scaffolds than in the neat PLA scaffold. Furthermore, the stem cells in the gel-loaded 3D-printed PLA scaffold showed markedly higher alkaline phosphatase expression and calcium phosphate deposition, which indicates higher osteogenic differentiation with the gels. These observations were corroborated by increased expressions of osteocalcin, RUNX2, and BMP-2.
Conclusion
Thus, the combination of SF/Alg hydrogel loaded with stem cells offers a promising route for enhancing the bioactivity of 3D-printed PLA scaffolds with significant clinical potential for bone tissue engineering.
Lay Summary
Owing to their good mechanical stability, 3D-printed porous scaffolds of thermoplastics such as PLA have been used for bone tissue engineering applications. However, the presence of macro-sized pores leads to low cell attachment efficiency and distribution within the scaffolds, which results in low osteogenic activity. In this work, stem cells were encapsulated within the sonicated silk fibroin and alginate blend hydrogel and embedded within the gaps of 3D-printed PLA struts. This hybrid approach maximizes the cell density and uniform distribution and leverages the mechanical integrity of the 3D-printed PLA scaffold and osteoconductive microenvironment for proliferation and differentiation offered by the silk fibroin and alginate hydrogel. The cell-laden gels loaded within 3D-printed scaffold showed improved proliferation and osteogenic activity of stem cells, which makes the system a promising bone substitute for regeneration or healing.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Bone has good intrinsic regeneration potential to self-repair in response to injuries [1]. Bone regeneration presents a complex series of biological events, including intra- and extracellular signaling pathways. Bone induction and conduction involve various cell populations to restore their structural and functional behaviors [2, 3]. However, the self-renewal and repair capacity is only limited to small bone defects of a few millimeters. Clinically, implants and devices are adapted to restore tissue function for large bone defects. In contrast, the defects arising from infections, tumors, and congenital deformations are treated with grafting, such as allografts, autografts, and xenografts [2, 4, 5]. Both implant- and grafting-based approaches are limited by their drawbacks, such as higher chances of infection and the need for secondary surgical intervention for the former, donor site morbidity, limited source, and immune rejection for the latter [2, 4]. To overcome these drawbacks, bone tissue engineering is in great demand to promote the healing and regeneration of bone defects by implanting bone-mimetic 3D scaffolds along with osteoprogenitor cells [6,7,8]. These scaffolds act as a platform for tissue regeneration and provide mechanical stability while affording cell infiltration with the rate of degradation matched to tissue regeneration for eventual osseointegration [9, 10].
The conventional fabrication approaches such as electrospinning, solvent casting, and lyophilization afford minimal control of the pore size, the shape of the pores, and pore interconnectivity in the 3D scaffolds [11]. More recently, 3D printing techniques have attracted significant interest in bone tissue regeneration to fabricate bone-mimetic scaffolds [12, 13]. These techniques utilize computer-aided designs with high precision and high reproducibility [14]. In this approach, the geometrical parameters such as pore size, pore structure, and interconnectivity as well as scaffold structure can be tuned to faithfully mimic the architecture of bone tissues for better diffusion of nutrients and oxygen than in conventional scaffolds [2, 14]. The scaffolds can be designed for the best anatomical match with the defect sites.
Among all 3D printing techniques, extrusion-based printing technique, specifically the fused filament fabrication (FFF) approach, has piqued the interest of the research community [15]. FFF is user-friendly, more cost-effective than other 3D printing techniques, and offers good printing speed with high reproducibility [15, 16]. In this approach, the material is melted through an extrusion head and deposited layer by layer with controlled geometric parameters using biodegradable polymers [13, 17]. One of the most common polymers that have been adapted for 3D printing by FFF is PLA, among others. PLA is degradable and compatible and offers good mechanical strength as a bone tissue scaffold [18]. However, hydrophobicity and lack of cell-adhesion sites impart poor bioactivity to PLA, thereby limiting its ability to induce bone formation at the defect site [18]. Uniform seeding of cells at high density in porous 3D-printed PLA scaffolds to maximize tissue regeneration is a challenge. Surface treatment of the printed scaffolds, such as alkaline treatment, grafting of polymer chains, and immobilization of bioceramics or biomolecules, have been attempted to enhance bioactivity [19,20,21]. However, the difficulties with efficient and uniform cellular colonization of the macroporous scaffolds persist.
Hydrogels have been employed for bone tissue regeneration with enhanced cellular adhesion, proliferation, and osteogenesis, such as injectable formulations, disks, and 3D printed constructs [22, 23]. 3D bioprinted cell-laden hydrogels do not suffer from non-uniform cell distribution. The 3D bioprinted gel or hydrogel disks lack sufficient mechanical and structural integrity when implanted in sites requiring support [11]. Silk fibroin (SF) derived from silkworms has been widely used as a biomaterial. SF is reported to activate molecular signaling to promote osteogenesis underscoring its promise in bone tissue engineering [24, 25]. Blends of SF and alginate (Alg) are reported to be stable and offer a favorable microenvironment to support cell function [26].
The goal of this work was to develop mechanically robust, degradable polymer scaffolds with uniform cell seeding and good bioactivity. In this work, a hybrid 3D-printed scaffold was prepared. 3D-porous scaffolds of PLA were prepared by FFF. Subsequently, a cell-laden hydrogel was loaded into the 3D-printed PLA scaffold. The hydrogel was prepared from a blend of SF and Alg (SF/Alg), which encapsulated bone marrow-derived human mesenchymal stem cells (hMSCs). The ability of the SF/Alg gel to support stem cell proliferation and osteogenesis in the 3D-printed PLA scaffolds was studied.
Materials and Methods
Fabrication of PLA Scaffolds by 3D Printing
The porous scaffold was fabricated by an FFF-based 3D printer (FabX3.0, Reddx Technologies, India). The 3D structure of the scaffold with dimensions of 9 mm diameter and 2.0 mm height was designed using Sketchup make software. The structure consisted of four layers; each layer was made up of 5 layers and 0.1 mm in height. The 3D scaffolds were printed in a layer-by-layer fashion by the melt extrusion of the PLA filament from the extruder nozzle at the speed of 15 mm·s−1. The temperature of the extrusion nozzle was maintained at 210 °C. The scaffolds were immersed in 5.0 M sodium hydroxide (NaOH) (Sigma-Aldrich, USA) for 1 h. The scaffolds were washed repeatedly with distilled water until the pH was 7.0. The scaffolds were air dried at room temperature, stored for further experiments, and are hereafter denoted as PLA-OH, whereas the as-printed constructs are referred to as PLA scaffolds.
Preparation of Silk Fibroin and Alginate Solution to Form Hydrogels
The SF protein was isolated from silk cocoons following a standard protocol. Briefly, B. Mori cocoons were obtained from a sericulture farm (Chennai). B. Mori cocoons were cut into small pieces and washed thoroughly. A total of 10 g of cocoons were boiled in an aqueous solution of 0.02 M Na2CO3 for 30 min at 100 °C and washed three times with distilled water. The degummed SF was dried overnight in an oven at 45 °C and dissolved in 9.3 M lithium bromide (LiBr) (SRL, India) solution at 60 °C for 4 h. The resulting solution was centrifuged at 5000 rpm to remove undissolved debris and dialyzed using a 12-kDa cutoff dialysis membrane for 4 days. The resultant SF solution obtained was 4% (w/v) by measuring the drying weight of the casted membrane at 60 °C for 1 h. The SF solution was concentrated to 8% (w/v) by dialyzing against 20% PEG-700 for 15 h. The obtained SF solution was autoclaved and stored at 4 °C for 1 week. To induce gelation, a 4 ml aliquot of 8% (w/v) was sonicated for 10, 20, or 40 s at 20 W. The resultant 8% (w/v) SF solution was mixed homogeneously with 4 wt% sodium alginate (Sigma-Aldrich, USA) on a magnetic stirrer for 4 h at 37 °C and used for the hydrogel preparation.
Fabrication of SF/Alginate Hydrogel Embedded PLA Scaffold
A total of 5 ml of a homogenous solution of 8% (w/v) SF and 4 wt% Alg (SF/Alg) was sonicated for 20 s at 20 W. The solution was drop cast in a well plate containing 100 mM calcium chloride (CaCl2) (Sigma-Aldrich, USA) for 10 min and washed with distilled water. To obtain SF/Alg-embedded PLA scaffolds, the sonicated solution of SF/Alg was drop cast over the PLA-OH scaffolds and placed under a vacuum for 15 min. These scaffolds, hereafter referred to as PLA-OH/SF/Alg, were immersed in 100 mM calcium chloride for 10 min and washed three times with distilled water. The fabricated SF/Alg hydrogel and SF/Alg embedded PLA scaffolds were used for further characterization.
Chemical Characterization of Silk Fibroin/Alginate Blend
Fourier Transform Infrared (FTIR) Spectroscopy
Attenuated total reflectance Fourier transform infrared spectroscopy (ATR-FTIR, Perkin-Elmer Frontier IR/NIR systems, USA) 100 FT-IR spectrometer) was used to analyze the chemical modification of the SF and alginate solutions. All the IR spectra were recorded in the range of 650 to 4000 cm−1 with a resolution of 4 cm−1. A total of 16 scans were performed for each sample. Each sample was examined in triplicate.
X-Ray Diffraction (XRD)
XRD was performed to characterize the conformational changes in SF, Alg, SF/Alg blend with or without sonicated pre-polymer solution using XPERT Pro of PAN employed with Cu Kα radiation of wavelength λ = 1.506 Å operated at 40 kV and 30 mA.
Physical Characterization of PLA-OH and SF/Alg Embedded PLA-OH Scaffolds
Scanning Electron Microscopy (SEM)
The microstructures of PLA-OH, SF/Alg gel post-sonication (SF/Alg20), and PLA-OH/SF/Alg20 were characterized using a scanning electron microscope (SEM, Ultra 55 FESEM, Karl Zeiss Mono, Germany) operated at an accelerating voltage of 5 kV. The SF/Alg20 gel and PLA-OH/SF/Alg20 were frozen at − 80 °C and freeze dried for 16 h. The dried samples were sputter coated with gold prior to imaging. Pore size quantification was performed using ImageJ 1.43 software from the SEM images for at least 20 random pores of each condition.
Porosity Measurement
The liquid displacement method was used to measure the porosity of PLA-OH, SF/Alg20, and PLA-OH/SF/Alg20 scaffolds. The scaffold of weight ‘W’ was immersed into the known volume (V1) of ethanol in a graduated cylinder for 5 min for saturation. The total volume of ethanol and ethanol-saturated scaffold was recorded as (V2). Ethanol-impregnated scaffolds were removed from the cylinder, and the residual ethanol left was recorded as (V3). The experiments were carried out in triplicates for each condition of the scaffolds.
The porosity (ϵ) of the scaffold was evaluated as follows:
Mechanical Properties
The compressive strength of PLA-OH, PLA-OH/SF20, and PLA-OH/SF/Alg20 scaffolds (dimension of 20 mm height and 9 mm diameter) was measured using a universal testing machine (Instron 5967, USA) with 1-kN load cell with a crosshead speed of 5 mm/min. The compressive strength of the scaffold was determined by calculating the slope of the strain–stress curve between 25 and 40% compressive strain.
Swelling Ratio
The swelling behavior was evaluated by determining the hydration kinetics of the SF and SF/Alg hydrogel-embedded PLA scaffolds. PLA-OH, PLA-OH/SF20, PLA-OH/SF/Alg, and PLA-OH/SF/Alg20 scaffolds were weighed (Wd) and then transferred to 2 ml PBS at 37 °C under constant shaking (60 rpm). The scaffolds (n = 3) were allowed to swell to equilibrium and weighed at different time intervals (Ws). The swelling ratio was determined as follows:
Degradation Behavior
The integral stability of SF and SF/Alg hydrogel was evaluated by determining the mass loss behavior of the hydrogel-embedded PLA-OH scaffold. The PLA-OH, PLA-OH/SF20, PLA-OH/SF/Alg, and PLA-OH/SF/Alg20 scaffolds were freeze-dried and weighed initially (Wi). The scaffolds were immersed in 2 ml PBS at physiological temperature (37 °C) under constant shaking (60 rpm). To measure the weight loss, PLA-OH and PLA-OH/SF/Alg scaffolds (n = 3) were removed from PBS at different time points (1, 3, 5, 7, 14, or 21 days) and freeze-dried prior to weighing (Wf). The degradation rate was determined as follows:
In Vitro Cytocompatibility of Scaffolds
Primary hMSCs (Lonza, USA) were cultured in Dulbecco’s minimum essential medium (DMEM) containing 10% (v/v) MSC-qualified FBS (Gibco, Life Technologies, USA). Antibiotic penicillin–streptomycin (Gibco, Life Technologies, USA) was added at 1% (v/v) concentration. Cells were passaged with trypsin–EDTA (Gibco, Life Technologies, USA) and subsequently sub-cultured. Cells of passage 4 were used for all the reported studies.
A total of 1 × 105/ml hMSCs per scaffold were seeded on PLA-OH scaffold, whereas for SF/Alg20 and PLA-OH/SF/Alg20 scaffolds, hMSC density was fixed at 2 × 106/ml. To encapsulate the cells within the hydrogel, sterilized 4 wt% sodium alginate powder and autoclaved 8% (w/v) SF solution were mixed homogenously and kept for 2 h at 37 °C and sonicated for 20 s. Then 2 × 106 hMSCs were added to the SF/Alg20 solution and mixed gently by pipetting for cell dispersion. The cell-laden hydrogel was drop-casted over the PLA-OH scaffolds and placed under a vacuum for 3 min to facilitate the ingress of the gel into the pores of the PLA-OH scaffold. The SF/Alg20 hydrogel and PLA-OH/SF/Alg-20 scaffolds were then immersed in 100 mM CaCl2 solution for 10 min and washed three times with PBS. The scaffolds were then transferred into 48-well plates containing the complete culture medium and placed in an incubator at 37 °C with 5% CO2.
The cell attachment and proliferation rate of hMSCs on PLA-OH, SF/Alg20, and PLA-OH/SF/Alg20 were quantitatively and qualitatively assessed by the metabolic assay (WST-1, Thermo Scientific, USA) and live-dead assay (LDA, Thermo Scientific, USA), respectively. WST-1 was used to evaluate the viability and proliferation of the encapsulated cells on days 1, 4, and 7. The cell-seeded scaffolds (PLA-OH, SF/Alg 20, and PLA-OH/SF/Alg 20) were washed with PBS and incubated in WST-1 reagent for 3 h. The absorbance was measured with a spectrophotometer (Biotek, USA) at 440 nm. All measurements were done in triplicates. At 1, 4, and 7 days, the cell-laden scaffolds (PLA-OH, SF/Alg, and PLA-OH/SF/Alg) were incubated in the LDA solution containing Calcein-AM and ethidium homodimer-1 following the instructions from the manufacturer and imaged using a laser scanning confocal microscope (LSCM, Olympus, USA).
Osteogenic Differentiation Studies
For osteogenic studies, hMSCs on all the scaffolds were cultured initially in the growth medium for 3 days and subsequently replaced with osteogenic medium (GM containing 100 nM dexamethasone, 50 µM ascorbic acid, and 20 mM glycerophosphate). The medium was replenished every alternate day. The osteogenic activity of hMSCs on the scaffolds was evaluated by assessing the alkaline phosphatase (ALP) activity, calcium deposition, and gene expression of specific osteogenic markers at days 7 and 14.
ALP activity was measured on days 7 and 14 with the ALP colorimetric assay, which uses p-nitrophenylphosphate (pNPP, Sigma Aldrich, USA) as a phosphate substrate. Briefly, the scaffolds (PLA-OH, SF/Alg, and PLA-OH/SF/Alg) with hMSCs were washed with PBS and lysed in 1% triton-X for 1 h followed by three freeze–thaw cycles of 15 min each. Thereafter, a total of 8 0 µl of total cell lysate was added to the 50 µl pNPP, and the absorbance was measured at 405 nm using a spectrophotometer (BioTek, USA). The ALP activity normalized to the DNA content as measured by the PicoGreen assay (Thermo Scientific, USA).
Calcium deposition by the hMSCs in the scaffolds (PLA-OH, SF/Alg, and PLA-OH/SF/Alg) was evaluated at 7 and 14 days by staining with alizarin red S (ARS) dye that binds to calcium salts. hMSCs were fixed with 4% paraformaldehyde for 30 min, followed by washing, and subsequently incubated for 45 min in 40 mM ARS solution. The scaffolds were incubated in a solution of 5% sodium-dodecyl sulfate and 0.5 N hydrochloric acid for 20 min to elute the stain. The absorbance was measured at 405 nm using a spectrophotometer (BioTek). The absorbance was normalized to DNA content determined using the PicoGreen dsDNA assay (Thermo Scientific, USA) to quantify the calcium content.
The total RNA of hMSCs from PLA-OH, SF/Alg20, and PLA-OH/SF/Alg20 was extracted using the RNeasy RNA kit (Qaigen, USA) by following the manufacturer’s instructions. Complementary DNA (cDNA) was synthesized by reverse transcription of mRNA using an iScript cDNA synthesis kit (Bio-Rad, USA) in a thermal cycler. The quantitative real-time polymerase chain reaction (qRT-PCR) was performed using SYBR green reagent for the quantification of osteogenic markers such as osteocalcin (OCN), bone morphogenic protein-2 (BMP-2), and Runt-related transcription factor 2 (RUNX2), and ALP. GAPDH was used as an endogenous control. The fold change was based on the relative quantification method. The cycling condition for the PCR reaction for all the samples: 95 °C for 7 min, 40 cycles at 95 °C for 15 s, and 60 °C for 30 s. Primer sequences are mentioned in Table 1.
Statistical Analysis
All quantitative experiments were run in triplicate for each sample. Each experiment was conducted at least thrice. Statistical analysis was performed by 2-way analysis of variance (ANOVA) with Tukey’s posttest to determine the statistical significance of the results.
Results and Discussion
Fabrication of Functionalized 3D PLA Scaffold
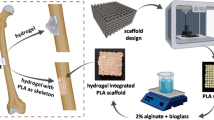
Figure 1 schematically presents the strategy used in this work, wherein an SF/Alg hydrogel was used to fill the pores of a 3D-printed PLA scaffold. The 3D-PLA porous scaffolds were successfully printed using FFF, a well-established additive manufacturing technique. The dimensions of the as-printed PLA scaffolds closely matched the dimensions of the 3D computer model. It has been reported that PLA is hydrophobic, which results in poor cell attachment and proliferation. To improve the water-wettability of the PLA scaffolds, the surface was modified by NaOH treatment. In alkaline conditions, PLA degrades to yield carboxyl and hydroxyl groups at the surface, which improves the water-wettability for better cell attachment and growth.
Scaffold architecture is an important consideration as open and interconnected pores facilitate cell ingrowth as well as nutrient exchange and oxygen diffusion throughout the scaffold. Figure 2 shows the surface morphology of the PLA and PLA-OH scaffolds. The diameter obtained for both PLA and PLA-OH ranges from 420 to 490 µm with no observable differences. However, the surface of PLA scaffolds was smoother as compared to the PLA-OH scaffolds, which arises due to the degradation of the polymer under alkaline conditions. The observation is consistent with our earlier observations [19].
A SEM micrographs showing surface structure of a PLA (magnification × 30), b PLA-OH (× 30), c SF/Alg20 (× 200), and d PLA-OH/SF/Alg20 (× 30) (scale bar = 200 µm). B and C FTIR and XRD spectra, respectively, showing chemical characterization of the SF, Alg, SF/Alg blend, sonicated SF, and SF/Alg20 blend confirming the bonding of SF chains with Alg via hydrogen bonding and hydrophobic interactions in SF/Alg blend and the formation of β sheet in sonicated SF and SF/Alg blend
SF and SF/Alg blends were sonicated for different intervals to assess their ability to form stable gels to encapsulate the cells. Firstly, 8% SF with sonication time (5, 10, 20, 30, 40, and 50 s) were investigated. The sonication time of 20 s was found to induce gelation within a time window of 30 min for 8% SF. Shorter sonication times (5 or 10 s) resulted in 120 min gelation window, whereas 30 and 40 s of sonication yielded 15 min window. When sonicated for 50 s, SF gelled during sonication. A similar trend was found with a blend of 8% SF and 4% Alg sonicated for 20 s. Thus, for the subsequent studies, 20 s sonication time was used for gelation as it provides the optimal time window for cell encapsulation within blend and casting. Figure 2 (c, d) shows the surface morphology of SF/Alg20 and PLA-OH/SF/Alg20 scaffolds. The pores showed good interconnectivity with pore sizes ranging from 50 to 100 µm for SF/Alg20 scaffold and 200 to 300 µm in PLA-OH/SF/Alg20. The porosity of PLA-OH, SF/Alg20, and PLA-OH/SF/Alg20 scaffolds was calculated to be 66.4%, 86.4%, and 86.9%, respectively.
Chemical and Physical Characterization of SF and SF/Alg Blend Hydrogel
Figure 2B presents the FTIR spectra of SF, Alg, and its blend before and after sonication for 20 s. The IR spectra of SF and Alg are reported in the literature [27, 28]. The IR spectral region of SF from 1700 to 1600 cm−1 is assigned to the amide I (C = O stretching vibrations), 1600 to 1500 cm−1 to amide II (NH bending and C–H stretching), and the region from 1350 to 1200 cm−1 to amide III (C–N stretching and C = O bending vibrations). Amide I vibration directly depends on the secondary structure of the protein backbone of SF. The IR spectrum of SF shows peaks at 1644 cm−1 for amide I, 1533 cm−1 for amide II, and 1240 cm−1 for amide III. The IR spectrum observed here is characteristic of the amorphous structure of SF with silk type I conformation (random coil) [29]. The IR spectrum of SF after 20 s of sonication showed shifting of the peaks to lower wavenumber at 1625 cm−1 in the amide I region and 1514 cm−1 in the amide II region, which is indicative of silk type II structure rich in the β-sheet arrangement. It plays an important role in facilitating hydrogel network formation. Sonication leads to gelation, which depends on the self-assembly of protein chains into physically crosslinked β-sheet crystals [30]. Sonication induces hydration of the hydrophobic groups on SF chains, which accelerates the intra- and inter-chain hydrophobic interactions related to β-sheet formation [30,31,32]. However, the sonication time is insufficient for gelation as we fixed the sonication time for 20 s, as mentioned above. Thus, SF was blended with alginate and cross-linked with CaCl2. Ca+2 can neutralize the electrostatic repulsion and facilitate the formation of salt bridges between SF protein molecules and Alg molecules [29]. The IR spectra of Alg showed characteristic peaks at 1655, 1422, and 1027 cm−1 due to –COO, –COO–, and C–O–C stretching, respectively. The IR spectra of the SF/Alg blend without sonication showed the characteristic peaks of both SF and Alg, whereas SF/Alg blend with 20-s sonication and calcium crosslinking showed peaks at 1625 and 1516 cm−1 along with the SF and Alg characteristic peaks, which confirms the physical crosslinking between the molecules to form a stable hydrogel network.
Furthermore, conformational changes in SF and Sf/Alg blend after 20-s sonication were also determined by XRD. Figure 2C shows the XRD pattern of SF and SF/Alg, with or without sonication. SF, SF20, SF/Alg, and SF/Alg20 presented a peak ≈ 19.7° corresponding to 4.4 Å and representative of silk type I structure. Alginate typical peak and halo were observed at 13.9 and 29.6°, corresponding to 6.32 and 3.12 Å, respectively. In SF/Alg, with and without sonication, showed the presence of peak and halo around 13.9°, 29° indicated the alginate component existed in SF/SA. Moreover, during gelation, both the silk fibroin component and sodium alginate component interact with each other via hydrophobic interactions and hydrogen bonding.
Degradation and Swelling Characteristics
Figure 3a shows the degradation rate of SF and SF/Alg hydrogels embedded in PLA-OH scaffolds, with and without sonication. No degradation was observed for the PLA-OH scaffold over the period of 21 days. Thus, in this work, the only measurable degradation arises due to that of SF and SF/Alg hydrogels embedded within PLA-OH scaffolds. The PLA-OH scaffold embedded with SF20 hydrogel exhibited ≈ 99% degradation within three days of incubation in dPBS (pH = 7.3). This suggested that gelation induced by sonication alone is insufficient to form a stable gel network. In contrast, blending SF with Alg improved the stability of hydrogel, where Ca+2 induced stable crosslinking within alginate and also hydrogen bonding and hydrophobic interaction between Alg and SF molecules. Both PLA-OH/SF/Alg20 and PLA-OH/SF/Alg, with or without sonication, showed 67% and 71% degradation, respectively, over the 21 days. No significant difference in the degradation rate was observed between gels prepared with or without sonication of the blend. It has been reported that sonication can degrade the polysaccharides, yielding lower molecular weight fragments of Alg, which leads to the decreased stability of hydrogel [33]. In addition, hydrogel also contains low molecular weight SF molecules, which remain unblended with Alg molecules and cannot participate in gel network formation. However, sonicated SF/Alg showed improved stability compared to that of PLA-OH and PLA-OH/SF/Alg20 scaffolds and could be further assessed for tissue repair and regeneration.
Figure 3b presents the swelling of PLA-OH alone and PLA-OH scaffolds embedded with SF and SF/Alg, with or without sonication, over 24 h. Swelling is an essential attribute of implantable tissue scaffolds as it determines the diffusion and affords the exchange of nutrients and removal of metabolic wastes through the scaffold. No swelling was observed for the PLA-OH scaffold. PLA-OH/SF20 attained equilibrium within 8 h of incubation in water, whereas PLA-OH/SF/Alg and PLA-OH/SF/Alg20 attained equilibrium within 12 h of incubation. It was observed that the swelling ratio was higher for PLA scaffolds embedded with SF/Alg and SF/Alg20 hydrogel. It is widely reported that SF protein has both hydrophilic and hydrophobic domains. During gelation, the inter- and intra-chain interactions lead to gel formation via hydrophobic interactions, which leads to reduced water uptake. On the other hand, Alg is superabsorbent owing to its hydrophilic nature and augments water uptake in the blend compared to neat SF hydrogel. Similar to the degradation results, there was no significant observable difference between the swelling ratio of SF/Alg hydrogels with and without sonication.
Mechanical Strength
For bone tissue engineering applications, the mechanical strength of scaffolds plays an important role in providing mechanical stability during bone tissue regeneration and remodeling. Figure 3C shows the compressive strength obtained for PLA-OH, PLA-OH/SF20, and PLA-OH/SF/Alg20. No statistically significant difference was observed between the compressive strength of PLA-OH (7.25 ± 1.34 MPa) and PLA-OH/SF20 (8.92 ± 1.5 MPa). On the other hand, PLA-OH/SF/Alg 20 s showed a significant difference (p < 0.5) with PLA-OH. However, there was no large change in mechanical strength. The increase in compressive strength is attributed to the cross-linking of SF/Alg hydrogel. The range of compressive strength obtain for all the scaffolds lies within the range of the cancellous bone compressive strength (1.5 to 45.0 MPa). This observation suggests that the embedding of hydrogel in the PLA scaffold yields constructs that match the biomechanical properties of the bone tissues in vivo.
Cell-material interactions
In vitro studies of the cellular response to a biomaterial provide essential evidence to predict the performance of the scaffold in vivo. The scaffolds in this study were designed to facilitate diffusion and promote cellular function. However, the large pore diameter of ≈ 420–490 µm2 on PLA-OH scaffolds results in poor cell attachment during cell seeding as a large fraction of the cells pass through the scaffolds and adhere to the tissue culture plate surface. Thus, there is a considerable loss of cell density, which can compromise tissue formation in the PLA-OH scaffolds. To improve the cell density within PLA-OH scaffolds for faster tissue formation, the pores of the scaffolds were embedded with SF/Alg hydrogel. The blend was sonicated for 20 s and crosslinked with Ca+2 ions to serve as the carrier for the encapsulated stem cells. Further, all the scaffolds (PLA-OH, SF/Alg20, and PLA-OH/SF/Alg20) were tested qualitatively and quantitatively for cell viability and proliferation by LDA and WST-1.
Cell Viability and Proliferation
Figure 4a shows the cell viability and proliferation of hMSCs grown on PLA-OH scaffolds and when encapsulated within SF/Alg20 and PLA-OH/SF/Alg20 scaffolds. All the scaffolds showed good viability with minimal toxicity, which indicates all the scaffolds are cytocompatible. The cell number increased steadily from day 1 to day 7 in all the scaffolds; however, SF/Alg20 and PLA-OH/SF/Alg20 showed higher cell density and growth over the time points as compared to that on PLA-OH.
A Fluorescence images of the hMSC-seeded PLA-OH, hMSC encapsulated SF/Alg hydrogel, and PLA-OH/SF/Alg20 scaffolds stained with the live-dead dyes (magnification × 10, scale bar = 100 µm). B Cytocompatibility of the fabricated scaffolds assessed by WST-1 proliferation assay. Significant differences in cell growth were observed between PLA-OH and PLA-OH/SF/Alg20 on day 4 (* p < 0.05) and day 7 (**p < 0.01), C SEM microphotograph showing the proliferation of hMSC on PLA-OH and lyophilized SF/Alg embedded PLA-OH scaffolds (magnification × 500; scale bar = 20 µm)
To further validate the high cell density and proliferation rate of encapsulated hMSCs within SF/Alg20 and PLA-OH/SF/Alg20 scaffolds were measured by proliferation assay (WST-1). Figure 4b shows the proliferation or mitochondrial activity of encapsulated hMSCs within all scaffolds (PLA-OH, SF/Alg20, and PLA-OH/SF/Alg20). The proliferation rate increased from day 1 to day 7 on all the scaffolds. However, it was observed that the PLA-OH/SF/Alg20 showed a significantly higher proliferation of encapsulated hMSCs as compared to that of PLA-OH on day 4 (p < 0.5) and day 7 (p < 0.01). No significant difference was observed between SF/Alg20 and PLA-OH/SF/Alg20. However, PLA-OH/SF/Alg20 showed enhanced proliferation of encapsulated hMSCs. Figure 4c shows the SEM micrographs of hMSCs seeded on PLA-OH and PLA-OH/SF/Alg 20 on day 7. It was observed that the hMSCs formed monolayer sheets over the porous PLA-OH/SF/Alg20 scaffolds in contrast to PLA-OH. This experiment suggested that incorporating cell-encapsulated hydrogel in PLA-OH 3D-printed scaffold improved the retention of cells initially post-seeding. PLA scaffolding provides mechanical stability to the hydrogel, which acts as the extracellular matrix. This scaffold, thus, mimics the native microstructure of bone and thus provides a conducive microenvironment for proper attachment and proliferation of hMSCs for bone repair and regeneration-based application.
Osteogenic Differentiation on Scaffolds
ALP Activity
ALP is an early marker of osteogenic differentiation generally expressed by differentiated osteoblasts, which facilitates bone mineralization [34]. Figure 5a shows the ALP activity of differentiating hMSCs on PLA-OH, Alg/SF20, and PLA-OH/SF/Alg20 scaffolds on days 7 and 14. It was observed that the hMSCs encapsulated within PLA-OH/SF/Alg20 showed significantly increased ALP activity compared to PLA-OH at day 14 (p < 0.01). The ALP activity in PLA-OH/SF/Alg20 was higher than in SF/Alg20, but the difference was not statistically significant. This trend suggested that SF/Alg20 gel effectively facilitates osteogenic differentiation of hMSCs.
Osteogenic potential of hMSCs on the different scaffolds at 7 and 14 days: a quantitative alkaline phosphatase activity, b quantitative measurement of calcium deposition (the data for both ALP and mineralization normalized to the amount of DNA (mmol/ng)), c digital photograph showing mineralization in the scaffolds by alizarin red staining. Statistical significance was observed on day 14 between PLA-OH and PLA-OH/Alg/SF20 for both ALP and mineralization activity (* represents p < 0.5 and ** represents p < 0.01)
Mineralization Activity
The calcium nodules, which are the later markers observed during osteogenic differentiation [35], were characterized on the different scaffolds by alizarin red staining. The calcium deposition was quantitatively analyzed, as shown in Fig. 5b. It was observed that hMSCs encapsulated in PLA-OH/SF/Alg20 scaffolds showed significantly higher calcium deposition at day 14 in comparison to the PLA-OH scaffold (p < 0.5). Figure 5c shows the photograph of alizarin red staining of the PLA-OH and PLA-OH/Sf/Alg20 scaffold on day 14. PLA-OH/SF/Alg20 scaffold appeared deep red in comparison to the PLA-OH scaffold. The results underscored that the PLA-OH/SF/Alg20 facilitates osteogenic induction of hMSCs and bone mineralization. Similar to ALP activity, there was no statistically significant difference between the mineralization in SF/Alg and PLA-OH/SF/Alg, although PLA-OH/SF/Alg20 showed higher mineralization than SF/Alg.
Osteogenic Markers Expression
To further confirm the enhanced osteogenic differentiation of hMSCs in PLA-OH/SF/Alg20, the expression levels of RUNX2, OCN, BMP2, and ALP were examined by qRT-PCR, as shown in Fig. 6 (a, b, c, and d). It was observed that SF/Alg20 and PLA-OH/SF/Alg20 showed upregulation of osteogenic markers on both days 7 and 14 compared to PLA-OH. The relative fold change is normalized data to the expression of osteogenic markers on PLA-OH.
Graph showing quantitative RT-PCR of osteogenic markers expression on PLA-OH, SF/Alg20, and PLA-OH/SF/Alg20 scaffolds: a RUNX2, b osteocalcin, c BMP2, and d ALP. The data (mean ± S.D. for n = 3) are normalized to the gene expression on PLA-OH scaffolds for the given day. Statistical significance was observed on day 14 between PLA-OH and PLA-OH/Alg/SF20 for all markers (** represents p < 0.01 and **** p < 0.0001)
On day 7, no significant difference was observed between the PLA-OH and PLA-OH/SF/Alg20. However, on day 14, cells on PLA-OH/SF/Alg20 showed significantly higher expression of osteogenic markers (RUNX2 (p < 0.01), OCN (p < 0.01), BMP2 (p < 0.001)). RUNX2 is an early marker of osteoblast differentiation and is essential for hMSC differentiation into the osteogenic lineage [36]. hMSCs on PLA-OH/SF/Alg20 showed 0.2 and 2.1-fold higher expression of RUNX2 than on SF/Alg and PLA-OH, respectively. RUNX2 upregulation induces the activation of osteogenesis-related genes such as OCN, Osetrix, and OPN [36, 37]. OCN is expressed by mature osteoblasts and plays an important role in bone mineralization [38]. The OCN expression by differentiating hMSCs was 0.4 and 1.9-fold higher on PLA-OH/SF/Alg than on SF/Alg and PLA-OH, respectively. Similarly, BMP2 plays an important role during bone development and can influence osteogenesis [39]. It induces the RUNX2 signaling, which activates the osteogenesis of differentiated hMSCs. hMSCs showed 0.8- and 2.5-fold higher expression of BMP2 and 0.2- and 1.5-fold higher expression of ALP on PLA-OH/SF/Alg20 compared to SF/Alg20 and PLA-OH. These data further corroborate the results above, underscoring that PLA-OH scaffold embedded with cell-laden SF/Alg hydrogels offers a route to prepare scaffolds that are mechanically robust with good bioactivity to augment bone tissue regeneration.
The mechanical strength of PLA scaffolds can match that of the native bone tissues. However, they are limited by the poor attachment and proliferation of cells. The results of this work demonstrate that an SF/Alg blend hydrogel system can be embedded with stem cells in PLA-OH scaffolds to effectively improve their growth and differentiation. The improved osteogenic activity in the presence of the SF-based gel is attributed to the inherent ability of mineralization by the β sheet crystalline region present in SF. These sheets act as nucleating sites to facilitate calcium deposition in a manner that is similar to collagen mineralization in healing bone tissue [24]. In addition, it also has been reported that amorphous linkages within the β sheet of SF mimic the anionic, non-collagenous proteins, which induce the deposition of hydroxyapatite on these nucleation sites [40]. Thus, the SF/Alg hydrogel system is an effective matrix wherein SF induces osteogenic activity, and Alg provides integrity and stability for potential applications in bone tissue engineering.
Conclusion
In this study, hMSC-seeded SF/Alg gel-embedded PLA-OH scaffolds were prepared by 3D printing. The macroporous architecture of the PLA scaffolds with large, open facilitated nutrient and oxygen diffusion. The scaffold system showed good water uptake capacity with a swelling ratio of 160% and remained stable over the period of 21 days with 67% degradation. The compressive strength of the gel-embedded scaffold matches the compressive strength of cancellous bone. Both the hydrogel system alone and hydrogel embedded PLA-OH scaffold showed high cell viability and proliferation rate. Furthermore, the high mineralization and alkaline phosphatase activity demonstrated that SF/Alg provided a conducive microenvironment to support osteogenic induction, which was corroborated by the high expressions of osteogenic markers (RUNX2, OCN, BMP-2, and ALP). Thus, stem cell encapsulation in SF/Alg remarkably improved the osteogenic processes in vitro, including cell proliferation and osteogenesis, while PLA provides mechanical stability to the scaffolds for bone tissue regeneration. Taken together, this approach of combining two different types of materials offers an effective strategy for engineering bone tissue scaffolds with a good combination of mechanical and biological activity.
References
Dimitriou R, Jones E, McGonagle D, Giannoudis PV. Bone regeneration: current concepts and future directions. BMC Med. 2011;9(1):1–10.
Amini AR, Laurencin CT, Nukavarapu SP. Bone tissue engineering: recent advances and challenges. Crit Rev™ Biomed Eng. 2012;40(5):363–408.
Orciani M, Fini M, Di Primio R, Mattioli-Belmonte M. Biofabrication and bone tissue regeneration: cell source, approaches, and challenges. Front Bioeng Biotechnol. 2017;5:17.
Kinoshita Y, Maeda H. Recent developments of functional scaffolds for craniomaxillofacial bone tissue engineering applications. Sci World J. 2013;2013:863157.
Perez JR, Kouroupis D, Li DJ, Best TM, Kaplan L, Correa D. Tissue engineering and cell-based therapies for fractures and bone defects. Front Bioeng Biotechnol. 2018;6:105.
Melke J, Midha S, Ghosh S, Ito K, Hofmann S. Silk fibroin as biomaterial for bone tissue engineering. Acta Biomater. 2016;31:1–16.
Tang G, Liu Z, Liu Y, Yu J, Wang X, Tan Z, Ye X. Recent trends in the development of bone regenerative biomaterials. Front Cell Dev Biol. 2021;9:665813.
Mravic M, Péault B, James AW. Current trends in bone tissue engineering. BioMed Res Int. 2014;2014:865270.
Bose S, Roy M, Bandyopadhyay A. Recent advances in bone tissue engineering scaffolds. Trends Biotechnol. 2012;30(10):546–54.
Nikolova MP, Chavali MS. Recent advances in biomaterials for 3D scaffolds: a review. Bioactive materials. 2019;4:271–92.
Buyuksungur S, Hasirci V, Hasirci N. 3D printed hybrid bone constructs of PCL and dental pulp stem cells loaded GelMA. J Biomed Mater Res, Part A. 2021;109(12):2425–37.
Haleem A, Javaid M, Khan RH, Suman R. 3D printing applications in bone tissue engineering. J Clin Orthop Trauma. 2020;11:S118–24.
Su X, Wang T, Guo S. Applications of 3D printed bone tissue engineering scaffolds in the stem cell field. Regen Ther. 2021;16:63–72.
Buj-Corral I, Bagheri A, Petit-Rojo O. 3D printing of porous scaffolds with controlled porosity and pore size values. Materials. 2018;11(9):1532.
Rezania N, Asadi-Eydivand M, Abolfathi N, Bonakdar S, Mehrjoo M, Solati-Hashjin M. Three-dimensional printing of polycaprolactone/hydroxyapatite bone tissue engineering scaffolds mechanical properties and biological behavior. J Mater Sci - Mater Med. 2022;33(3):1–14.
Kumar A, Kargozar S, Baino F, Han SS. Additive manufacturing methods for producing hydroxyapatite and hydroxyapatite-based composite scaffolds: a review. Front Mater. 2019;6:313.
Chung JJ, Im H, Kim SH, Park JW, Jung Y. Toward biomimetic scaffolds for tissue engineering: 3D printing techniques in regenerative medicine. Front Bioeng Biotechnol. 2020;8:586406.
Gregor A, Filová E, Novák M, Kronek J, Chlup H, Buzgo M, Blahnová V, Lukášová V, Bartoš M, Nečas A. Designing of PLA scaffolds for bone tissue replacement fabricated by ordinary commercial 3D printer. J Biol Eng. 2017;11(1):1–21.
Jaidev L, Chatterjee K. Surface functionalization of 3D printed polymer scaffolds to augment stem cell response. Mater Des. 2019;161:44–54.
Nilawar S, Chatterjee K. Surface decoration of redox-modulating nanoceria on 3D-printed tissue scaffolds promotes stem cell osteogenesis and attenuates bacterial colonization. Biomacromolecules. 2021;23(1):226–39.
Zhang B, Wang L, Song P, Pei X, Sun H, Wu L, Zhou C, Wang K, Fan Y, Zhang X. 3D printed bone tissue regenerative PLA/HA scaffolds with comprehensive performance optimizations. Mater Des. 2021;201:109490.
Liu Q, Li Q, Xu S, Zheng Q, Cao X. Preparation and properties of 3D printed alginate–chitosan polyion complex hydrogels for tissue engineering. Polymers. 2018;10(6):664.
Bakhtiary N, Liu C, Ghorbani F. Bioactive inks development for osteochondral tissue engineering: a mini-review. Gels. 2021;7(4):274.
Midha S, Murab S, Ghosh S. Osteogenic signaling on silk-based matrices. Biomaterials. 2016;97:133–53.
Midha S, Chameettachal S, Dey E, Ghosh S. Nonmulberry silk braids direct terminal osteocytic differentiation through activation of wnt-signaling. ACS Biomater Sci Eng. 2017;3(6):1062–74.
Wang Y, Wang X, Shi J, Zhu R, Zhang J, Zhang Z, Ma D, Hou Y, Lin F, Yang J. A biomimetic silk fibroin/sodium alginate composite scaffold for soft tissue engineering. Sci Rep. 2016;6(1):1–13.
Ming J, Zuo B. A novel silk fibroin/sodium alginate hybrid scaffolds. Polym Eng Sci. 2014;54(1):129–36.
Rajput M, Bhandaru N, Barui A, Chaudhary A, Paul RR, Mukherjee R, Chatterjee J. Nano-patterned honey incorporated silk fibroin membranes for improving cellular compatibility. RSC Adv. 2014;4(84):44674–88.
Silva R, Singh R, Sarker B, Papageorgiou DG, Juhasz JA, Roether JA, Cicha I, Kaschta J, Schubert DW, Chrissafis K. Soft-matrices based on silk fibroin and alginate for tissue engineering. Int J Biol Macromol. 2016;93:1420–31.
Wang X, Kluge JA, Leisk GG, Kaplan DL. Sonication-induced gelation of silk fibroin for cell encapsulation. Biomaterials. 2008;29(8):1054–64.
Kadakia P, Jain E, Hixon K, Eberlin C, Sell S. Sonication induced silk fibroin cryogels for tissue engineering applications. Mater Res Exp. 2016;3(5):055401.
Yucel T, Cebe P, Kaplan DL. Vortex-induced injectable silk fibroin hydrogels. Biophys J. 2009;97(7):2044–50.
Zhou C, Ma H. Ultrasonic degradation of polysaccharide from a red algae (Porphyra yezoensis). J Agric Food Chem. 2006;54(6):2223–8.
Prins H-J, Braat AK, Gawlitta D, Dhert WJ, Egan DA, Tijssen-Slump E, Yuan H, Coffer PJ, Rozemuller H, Martens AC. In vitro induction of alkaline phosphatase levels predicts in vivo bone forming capacity of human bone marrow stromal cells. Stem Cell Res. 2014;12(2):428–40.
BouAssaf R, Fayyad-Kazan M, Al-Nemer F, Makki R, Fayyad-Kazan H, Badran B, Berbéri A. Evaluation of the osteogenic potential of different scaffolds embedded with human stem cells originated from Schneiderian membrane: an in vitro study. BioMed Res Int. 2019;2019:1–10.
Zou L, Kidwai FK, Kopher RA, Motl J, Kellum CA, Westendorf JJ, Kaufman DS. Use of RUNX2 expression to identify osteogenic progenitor cells derived from human embryonic stem cells. Stem Cell Reports. 2015;4(2):190–8.
Wang L-T, Lee Y-W, Bai C-H, Chiang H-C, Wang H-H, Yen BL, Yen M-L. A rapid and highly predictive in vitro screening platform for osteogenic natural compounds using human runx2 transcriptional activity in mesenchymal stem cells. Front Cell Dev Biol. 2020;8:607383.
De Giglio E, Bonifacio MA, Ferreira AM, Cometa S, Ti ZY, Stanzione A, Dalgarno K, Gentile P. Multi-compartment scaffold fabricated via 3D-printing as in vitro co-culture osteogenic model. Sci Rep. 2018;8(1):1–13.
Cai H, Zou J, Wang W, Yang A. BMP2 induces hMSC osteogenesis and matrix remodeling. Mol Med Rep. 2021;23(2):1–1.
Mandal BB, Kundu S. Non-mulberry silk gland fibroin protein 3-D scaffold for enhanced differentiation of human mesenchymal stem cells into osteocytes. Acta Biomater. 2009;5(7):2579–90.
Acknowledgements
The authors acknowledge Anindo Roy for SEM imaging.
Funding
This work was supported by the Department of Science and Technology (DST), Government of India (DST/NM/NB/2018/119(G)).
Author information
Authors and Affiliations
Contributions
M.R. and K.C. designed the study. M.R. performed the experiments, analyzed the data, and prepared the first draft of the manuscript. S.N. performed XRD and FTIR experiments and assisted in 3D printing. K.C. edited the manuscript and is the senior author overseeing the work.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rajput, M., Nilawar, S. & Chatterjee, K. Embedding Silk Fibroin-Alginate Hydrogel in a 3D-Printed Porous Poly(Lactic Acid) Bone Tissue Scaffold Augments Stem Cell Function. Regen. Eng. Transl. Med. 9, 384–396 (2023). https://doi.org/10.1007/s40883-022-00286-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40883-022-00286-7