Abstract
To develop a new drug delivery approach, carboxymethyl cellulose/Cu bio-nanocomposite hydrogels were successfully prepared in situ during the formation of Cu nanoparticles within swollen carboxymethyl cellulose hydrogels. The resulting hydrogels were examined by running various experimental procedures such as FT-IR, XRD, and SEM. XRD analysis confirmed the formation of Cu nanoparticles in the hydrogel matrix, while SEM micrographs showed that nanoparticles ranged from 36 to 69 nm within the same matrix. It was shown that increased Cu2+ concentration led to increased number of Cu nanoparticles. The swelling behavior of the bio-nanocomposite hydrogels was studied at pH 2.1 and 7.4, and exhibited a pH-sensitive swelling ratio, compared with the neat carboxymethyl cellulose hydrogel. The antibacterial activity of the bio-nanocomposite hydrogels was examined and mechanisms involved in their synthesis were reported; the results showed an excellent antibacterial behavior of the bio-nanocomposite hydrogel. In vitro drug release tests were carried out to assess the effectiveness of this novel type of bio-nanocomposite as a controlled drug delivery system. Sustained and controlled drug releases were observed for Cu nanoparticles containing carboxymethyl cellulose, which increased with increases in Cu nanoparticle content.
Lay Summary
The objective of this study was to prepare and characterize a group of carboxymethyl cellulose hydrogels containing copper nanoparticles. Novel carboxymethyl cellulose/Cu nanocomposite hydrogels were successfully prepared by in situ formation of Cu nanoparticles in the carboxymethyl cellulose hydrogel matrix. The effect of the concentration of the Cu nanoparticles on the swelling and drug release behavior and antibacterial activity for the Gram-negative Escherichia coli and Gram-positive Staphylococcus aureus bacteria was investigated.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hydrogels are a class of materials with the three-dimensional network of a polymer that can absorb a high amount of water or biological fluids, without being soluble under physiological conditions [1]. Also, due to their excellent properties, such as high swelling ratio, non-toxicity, biocompatibility, and biodegradability, hydrogels would have basic roles in agriculture [2, 3] and biomedical applications including wound dressing [4], tissue engineering [5, 6], and controlled drug and protein delivery [7].
Sodium carboxymethyl cellulose (Na-CMC) has much advantage due to its unique properties such as biocompatibility, hydrophilicity, and non-toxic. It has, therefore, been widely applied for many applications such as drug delivery, paper industry, nanocomposite materials, detergents, food, and oil drilling mud among others [8,9,10,11].
Recently, there has been great interest in preparing organic-inorganic nanocomposite hydrogels for biomedical applications [1]. Many nanocomposite hydrogels are found proper to be used as drug delivery carriers because they frequently exhibit remarkably improved properties compared with the pure polymer hydrogels. The introduction of nanoparticles can reduce the burst drug release effect, increase the stability of drug, and provide a slower and more continuous release mode of drugs [12, 13]. Bio-nanocomposites are a combination of biopolymers and inorganic materials, mainly metals like silver, copper, TiO2, and ZnO in nano-dimensions. Superior mechanical strength, high thermal resistance, and low permeability against gases and water vapor are a number of the properties of bio-nanocomposites. In recent years, bio-nanocomposites have been used as wound dressings and tissue engineering as well [14,15,16]. The main advantages of using inorganic nanoparticles accompanied with the organic antimicrobial mediators are their strength, stability, and extended shelf life; besides, they can be used potentially for biomedical field [17].
Recently, the nanocomposite hydrogels were prepared and investigated using biopolymers by some researchers. Yadollahi et al. prepared nanocomposite hydrogels based on CMC [18,19,20] and chitosan [21,22,23] by the addition of the nanoparticles like CuO, ZnO, and Ag by in situ generation to forma hydrogel. Gholamali et al. prepared and characterized groups of oxidized starch and carboxymethyl chitosan/poly(vinyl alcohol) hydrogels having CuO and Ag nanoparticles by oxidizing/reducing the Cu2+ and Ag+ ions in the oxidized starch and carboxymethyl chitosan/poly(vinyl alcohol) hydrogels medium in situ that improve drug-loading efficiency and drug release [1, 16].
Considering these facts, the objective of this study was to prepare and characterize a group of carboxymethyl cellulose hydrogels containing copper nanoparticles. Novel carboxymethyl cellulose/Cu nanocomposite hydrogels were successfully prepared by in situ formation of Cu nanoparticles in the carboxymethyl cellulose hydrogel matrix. Such a formation has not been reported previously. The resulting nanocomposite hydrogels were characterized using FT-IR, XRD, and SEM analyses. The effect of the concentration of the Cu nanoparticles on the swelling and drug release behavior and antibacterial activity for the Gram-negative Escherichia coli and Gram-positive Staphylococcus aureus bacteria was investigated.
Materials and Methods
Materials
Sodium carboxymethyl cellulose (CMC), degree of substitution (DS) 0.55–1.0, and viscosity 15,000 mPas/s (1% in H2O, 25 °C) were obtained from Nippon Paper Chemicals Co., Ltd. Japan. Epichlorohydrin (99.5%), CuCl2.2H2O, NaOH, and NaBH4 were purchased from Merck and used as received. Distilled water was used throughout this work.
Methods
Preparation of CMC Hydrogels
Carboxymethyl cellulose hydrogels were prepared by the following procedure: 3 g CMC was dissolved in 100 mL of 3% (w/v) NaOH solution with continuous mechanical stirring until a homogeneous viscous mixture was acquired. Then, epichlorohydrin (5 mL) was added drop-wise with continuous stirring for 2 h until a homogenous mixture was obtained. Afterward, the obtained mixture was heated at 80 °C in warm water bath for 2 h. The insoluble cross-linked CMC paste was collected and washed several times with distilled water to remove residual NaOH and epichlorohydrin. Finally, the hydrogel was dried in an oven at 50 °C for 24 h.
Preparation of CMC Nanocomposite Hydrogels Containing Cu Nanoparticles
CMC/Cu nanocomposite hydrogels were prepared according to a previously described method [24]. Typically, 0.6 g of dried CMC hydrogel was immersed in different concentrations of copper chloride solutions (0.000, 0.005, 0.010, 0.020, and 0.030 M) for 24 h. The copper ion–loaded hydrogels were washed with distilled water to remove copper ions attached to the hydrogel surface. The resulting hydrogels were placed in 100 mL of 0.2 M NaBH4 solution for 24 h. After the reduction of the bound Cu ions, the hydrogels were washed with methanol and finally dried under vacuum for 24 h. The CMC/Cu nanocomposite hydrogels with 0.000, 0.005, 0.010, 0.020, and 0.030 M of CuCl2 content will be referred to as Cu0, Cu1, Cu2, Cu3, and Cu4, respectively, in following lines.
Characterization and Analysis
Infrared spectra were recorded on a FT-IR spectrometer (Bruker Instruments, model Equinox 55, Germany) in the wave number ranging 4000–400 cm−1 at a resolution of 0.5 cm−1 as KBr pellets. The X-ray diffraction pattern of the samples was verified with Siemens-D500 diffractometer using Cu-kα radiation at 35 kV in the scan range of 2θ from 2 to 70° and scan rate of 1°/min. All of the analyzed samples were in powdery form. The morphology of the dried neat hydrogel and nanocomposite hydrogels was examined by a scanning electron microscope (SEM) (TESCAN MIRA) after coating the dried hydrogels with gold and silver films.
Antibacterial Activity
Antibacterial experiments were studied against both S. aureus (Gram-positive) and E. coli (Gram-negative) bacteria by agar diffusion test. For agar diffusion method, samples were exposed to bacteria in solid media (nutrient agar), and the inhibition zone around the samples was determined as the antibacterial effect of Cu nanoparticles and pure ibuprofen. The agar plates were inoculated with 100 μL spore suspensions of bacteria. The swelled hydrogels, each of which contained test materials at the same size, were cut and placed on the agar plate, then incubated with bacterial suspension at 37 °C for 24 h.
Swelling Behavior
The swelling ratio (SR%) of CMC/Cu nanocomposite hydrogels was measured in distilled water [10] and buffer solutions (pH values of 2.1 and 7.4) at room temperature. About 0.1 g of CMC/Cu nanocomposite hydrogels was immersed in 50 mL of prepared buffer solutions with a desired pH at room temperature for 450 min to reach maximum swelling capacity. The equilibrium swelling of nanocomposite hydrogels was determined according to Eq. 1.
where W0 is the weight of the initial dried samples, and Wt is the weight of the sample after swelling for 450 min.
In vitro Drug Release Studies
As a model drug, ibuprofen was employed in drug release experiments. UV-Vis spectroscopy was employed to study the drug release behavior of the hydrogel. About 0.2 g of prepared dry hydrogel was added to 20 mL of ibuprofen solution (100 ppm in distilled water) at 25 °C for 2 days to load the drugs into the hydrogel. Then, the resulting solution was filtered, and the content of ibuprofen was calculated using UV-Vis spectroscopy at 275 nm. The results of drug loading were calculated using Eq. 2.
The drug release profiles of hydrogels were investigated in pH 7.4 for 24 h. In a typical experiment, 0.2 g of the drug-bearing nanocomposite hydrogel was set in 10 mL of the chosen release medium maintained at 37 °C under a constant rotation speed of 50 rpm. In order to measure the amount of released drug at a certain time, sufficient amounts of sample solutions were picked up and its recorded absorption was determined using a UV spectrophotometer. In order to keep the volume of buffer constant, the removed volume was replaced with fresh buffer solution. The drug samples released from CMC/Cu nanocomposite hydrogel were quantified by the UV spectrophotometer, and the quantity of ibuprofen was determined using a standard calibration curve obtained under the same conditions.
Results and Discussion
Preparations of Carboxymethyl Cellulose/Cu Bio-Nanocomposite Hydrogels
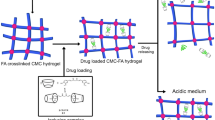
Scheme 1 schematically represents the formation of Cu nanoparticles inside the network of CMC hydrogel. CMC can interact with many metal cations, including Mo6+, Al3+, Zn2+, Co2+, and Cu2+ [25]. Due to the negative charge on the carboxylate groups of CMC (–CO2−), the CMC hydrogels easily bind to the positively charged copper ions in aqueous solutions of copper chloride via electrostatic force. With the suitable reducing agent such as NaBH4, copper ions are reduced to Cu nanoparticles. This procedure is a facile and economical method for in situ preparation of Cu nanoparticles, not requiring heat or any other tools for nanoparticle synthesis.
FT-IR Analysis
Figure 1 represents FT-IR spectra of the pure CMC hydrogel, CMC/Cu nanocomposite hydrogel, and CMC/Cu-ibuprofen nanocomposite hydrogel. The FT-IR spectra of CMC hydrogel displayed the broad band centered at 3500 cm−1, assigned to the stretching of –OH groups and intermolecular and intramolecular hydrogen bonds [10, 26]. The peak about C-H stretching associated with the methane hydrogen atoms appeared at 2913 cm−1. The peaks at 1422 and 1607 cm−1 belong to the symmetrical and asymmetrical stretching vibrations of the carboxylate groups. Also, it can be seen from the spectrum of CMC hydrogel that the absorption bands between 1000 and 1200 cm−1 were related to the –C-O- stretching on the polysaccharide skeleton [19].
The FT-IR spectra CMC/Cu nanocomposite hydrogel (Fig. 1(b)) represent a characteristic band at 1647 cm−1 associated with Cu nanocomposite hydrogel. Compared with the FT-IR spectra of pure CMC hydrogel, CMC/Cu nanocomposite hydrogel indicates the new peaks in the 400–800 cm−1 regions. These peaks were attributed to the incorporation of M-O and O-M-O bonds into the hydrogel. The FT-IR spectrum of CMC/Cu-ibuprofen is a combination of ibuprofen, Cu nanoparticles, and CMC with the shift of the O-H vibrational band. The obtained results could reveal the formation of hydrogen bonds between the carboxylic group of ibuprofen and the hydroxyl group of CMC [27].
XRD Analysis
The XRD patterns of the CMC/Cu nanocomposite hydrogel and pure CMC hydrogel in the 2θ range of 2–70° are shown in Fig. 2. The diffractogram of CMC/Cu nanocomposite hydrogel is assigned to diffractions at 2θ values of about 43° and 50°, assigned to the (111) and (200) diffractions of Cu crystals, respectively. All the peaks match well with those of monoclinic phase Cu crystals and confirm the formation of Cu particles in the CMC hydrogel matrix. None of the other peaks can be observed in the XRD pattern that indicates the high purity of obtained Cu particles [28, 29]. A wide peak at 20° is due to the polymer networks [9].
Scanning Electron Microscopy
Scanning electron microscopy (SEM) was employed for investigation of the surface morphology of the samples, shape, size, and porosity of the hydrogel matrices. SEM of the neat hydrogel and CMC/Cu nanocomposite hydrogels is given in Fig. 3. A clear and flat surface morphology can be seen from Fig. 3(a) for the neat CMC hydrogel. On the other hand, copper nanoparticles are visible as spherical particles that were observed for CMC/Cu nanocomposite hydrogels (Fig. 3(b, c)). SEM results revealed that the Cu nanoparticles were uniformly dispersed and some were embedded in the CMC matrix in the sample with copper chloride concentration of 0.01 M (sample C2), and the particle size range was between 36 and 55 nm (Fig. 3(b)). However, some aggregation and bigger particle sizes (40 and 69 nm) can be observed in the CMC/Cu nanocomposite hydrogels containing the highest copper chloride concentration of 0.03 M (Fig. 3(c)).
Antibacterial Activity
The in vitro antibacterial activities of pure CMC hydrogel, CMC/Cu nanocomposite hydrogels, and pure ibuprofen were studied comparatively against Gram-negative E. coli and Gram-positive S. aureus bacteria by disk diffusion test. Inhibition zone around the tested samples for bacterial growth was detected visually and listed in Table 1. The inhibition zones are given in Fig. 4. The results showed that the Cu nanoparticle–embedded nanocomposite hydrogel had a more toxic effect on bacteria than pure CMC hydrogel under similar conditions. The results in Table 1 revealed that the antibacterial efficiency of the nanocomposite hydrogels was influenced by increasing the concentration of the Cu nanoparticles. In the case of E. coli bacteria, after 24 h of incubation, the zone of inhibition for CMC/Cu hydrogels reached 0 mm during a day, but they showed better activity toward S. aureus. The antibacterial effect of CMC/Cu nanocomposite hydrogels could be associated with the attachment of Cu nanoparticles to the bacterial cell wall which damages the cell wall, causing damage of the cell as well. Qualitative in vitro antibacterial results show that pure ibuprofen samples in concentrations of 5 and 2.5 (mg/mL) did not inhibit the growth of S. aureus nor E. coli bacteria [30, 31]. Therefore, the proteins and other intracellular constituents could be leaked out and ultimately causes cell death [19, 32].
Effect of pH on Swelling Behavior
In order to evaluate the pH sensitivity, swelling behavior of the prepared hydrogels was analyzed in the pH of 2.1 and 7.4 buffer solutions. Figure 5 shows swelling of all of the hydrogels that enhanced with time, first quickly and then slowly, reaching a maximum constant swelling. It was observed that the swelling ratio of test at pH 2.1 was lower than that of pH 7.4. This was due to the dissociation carboxyl groups on the CMC chains in aqueous media and conversion to negatively charged carboxylate ions, leading in higher electrostatic repulsion; thus, water would be taken up and swelling ratio increased [26, 33]. In addition, the results in Fig. 5 show that as compared with the pure CMC hydrogel, CMC/Cu nanocomposite hydrogels revealed a higher swelling ratio. The enhancement of the swelling degree of the CMC/Cu nanocomposite hydrogels may be originated from the presence of Cu nanoparticles with different sizes, morphologies, and surface charges. The charged copper nanoparticles lead to the penetration of more water molecules in order to balance the build-up ion osmotic pressure, which causes the swelling of hydrogels [27, 34]. Moreover, formation of CuNPs in the hydrogel network could cause the expansion of hydrogel network and thus enhance the pores and free spaces within the hydrogel matrix, and as a consequence, CMC/Cu samples could absorb more water [28]. Therefore, CMC/Cu swells quickly and achieves higher swelling ratio. However, as compared with the CMC/Cu1 hydrogel, CMC/Cu2, CMC/Cu3, and CMC/Cu4 hydrogels revealed less swelling degree. In other words, swelling degree decreased by increasing the Cu nanoparticles content [22, 35]. This behavior may be related to the role of Cu nanoparticles as the knot-tying functions, which restricts the expanding of polymer chains. The knot-tying function of Cu nanoparticles could be because of the chelation of some hydroxyl and carboxyl groups of the CMC with copper nanoparticles [20, 23].
Drug Release Behavior
The relation between encapsulation and release of ibuprofen in CMC/Cu nanocomposite hydrogels was examined in detail in this section and results are presented in Table 2 and show the amounts of ibuprofen incorporated as pure drug and fractional release of the model drug in these nanocomposite. It is clear that the amount of ibuprofen incorporated as pure drug in the hydrogel decreases with increasing the amount of Cu nanoparticles. This behavior could be originated from the smoother and tighter surface of CMC/Cu hydrogels which prevents the penetration of drug molecules into the hydrogels. However, porous structure of the pure CMC hydrogel could make capillary forces, which facilitated the penetration of fluids into the hydrogels. The in vitro drug release profiles were performed in pH 7.4 phosphate buffer solution (PBS) for 24 h. Figure 6 shows the relation of time versus cumulative release of the drug from the hydrogels with different Cu nanoparticle contents. As it is obvious in Fig. 6, there is an obvious decrease in the cumulative ibuprofen release from the hydrogels by the increase of Cu nanoparticle content. The role that CuNPs play in the CMC/Cu bio-nanocomposite hydrogels is the main reason for the prolonged drug release behavior. The release time of ibuprofen from CMC/Cu hydrogel is extended because of a longer path needed for ibuprofen to migrate from Cu nanoparticles containing hydrogels to the buffer solution, as compared with the neat CMC hydrogels [23, 35]. The drug release mechanism of CMC/Cu bio-nanocomposite hydrogels is shown in Scheme 2.
Conclusion
In this project, antibacterial CMC/Cu bio-nanocomposite hydrogels were prepared based on the combination of Cu nanoparticles and biopolymer carboxymethyl cellulose. Pure CMC hydrogels were successfully synthesized via cross-linking CMC with epichlorohydrin in an alkaline medium. Cu nanoparticles were loaded by reduction of copper chloride in CMC hydrogels with NaBH4 at room temperature. Structural details of CMC/Cu nanocomposite hydrogels were provided by FT-IR, XRD, and SEM analyses. Their swelling behavior was studied in the pH of 2.1 and 7.4 and salt solutions. XRD analysis studies confirmed the formation of Cu nanoparticles in the hydrogel matrix. SEM micrographs clearly showed that Cu nanoparticles with size ranging from 65 to 80 nm were formed within the hydrogel matrix, and the number of Cu nanoparticles increased with the increase of Cu+2 concentration. CMC/Cu nanocomposite hydrogels revealed a higher swelling capacity in comparison with neat CMC hydrogel which was dependent upon the Cu nanoparticle content. Antibacterial activity of the prepared nanocomposite hydrogels was studied against E. coli (Gram-negative) and S. aureus (Gram-positive) using the agar diffusion test. The results showed an excellent antibacterial activity of CMC/Cu nanocomposite hydrogels for S. aureus bacteria. Based on these findings, the prepared CMC/Cu bio-nanocomposite hydrogels could be helpful candidates for the controlled delivery of drugs. Drug release studies revealed that CuNPs prolonged the release of drug from CMC hydrogels. The release time of drug molecules from CMC/Cu hydrogels is extended due to a longer path for drugs to migrate from nanocomposite hydrogel to the media.
References
Gholamali I, Hosseini SN, Alipour E, Yadollahi M. Preparation and characterization of oxidized starch/CuO nanocomposite hydrogels applicable in a drug delivery system. Starch/Stärke. 2019;71(3–4).
Ullah F, Othman MBH, Javed F, Ahmad Z, Akil HM. Classification, processing and application of hydrogels: a review. Mater Sci Eng C. 2015;57:414–33.
Wu T, Li Y, Lee DS. Chitosan-based composite hydrogels for biomedical applications. Macromol Res. 2017;25(6):480–8.
Namazi H, Rakhshaei R, Hamishehkar H, Samadi Kafil H. Antibiotic loaded carboxymethyl cellulose/MCM-41 nanocomposite hydrogel films as potential wound dressing. Int J Biol Macromol. 2016;85:327–34.
Park SA, Lee SH, Kim WD. Fabrication of hydrogel scaffolds using rapid prototyping for soft tissue engineering. Macromol Res. 2011;19(7):694–8.
Van Vlierberghe S, Dubruel P, Schacht E. Biopolymer-based hydrogels asscaffolds for tissue engineering applications: a review. Biomacromolecules. 2011;12:1387–408.
Hoare TR, Kohane DS. Hydrogels in drug delivery: progress and challenges. Polym. 2008;49:1993–2007.
Yadollahi M, Namazi H. Synthesis and characterization of carboxymethyl cellulose/ layered double hydroxide nanocomposites. J Nanopart Res. 2013;15:1563–72.
Yadollahi M, Namazi H, Barkhordari S. Preparation and properties of carboxymethyl cellulose/layered double hydroxide bionanocomposite films. Carbohydr Polym. 2014;108:83–9.
Barkhordari S, Yadollahi M, Namazi H. pH sensitive nanocomposite hydrogel beads based on carboxymethyl cellulose/layered double hydroxide as drug delivery systems. J Polym Res. 2014;21(6):454–62.
Ashraf S, Park HK, Park H, Lee SH. Snapshot of phase transition in thermoresponsive hydrogel PNIPAM: role in drug delivery and tissue engineering. Macromol Res. 2016;24(4):297–304.
Jayaramudu T, Raghavendra GM, Varaprasad K, Sadiku R, Raju KM. Development of novel biodegradable Au nanocomposite hydrogels based on wheat: for inactivation of bacteria. Carbohydr Polym. 2013;92:2193–200.
Jayaramudu T, Raghavendra GM, Varaprasad K, Sadiku R, Ramam K, Raju KM. Iota-Carrageenan-based biodegradable Ag0 nanocomposite hydrogels for the inactivation of bacteria. Carbohydr Polym. 2013;95:188–94.
Khorasani MT, Joorabloo A, Moghaddam A, Shamsi H, Mansoori Moghadam Z. Incorporation of ZnO nanoparticles into heparinised polyvinyl alcohol/chitosan hydrogels for wound dressing application. Int J Biol Macromol. 2018;114:1203–15.
Song F, Li X, Wang Q, Liao L, Zhang C. Nanocomposite hydrogels and their applications in drug delivery and tissue engineering. J Biomed Nanotechnol. 2015;11:40–52.
Gholamali I, Asnaashariisfahani M, Alipour E. Silver nanoparticles incorporated in pH-sensitive nanocomposite hydrogels based on carboxymethyl chitosan-poly (vinyl alcohol) for use in a drug delivery system. Regen Eng Transl Med. 2019:1–16.
Rasoulzadeh M, Namazi H. Carboxymethyl cellulose/graphene oxide bio-nanocomposite hydrogel beads as anticancer drug carrier agent. Carbohydr Polym. 2017;168:320–6.
Yadollahi M, Gholamali I, Namazi H, Aghazadeh M. Synthesis and characterization of antibacterial carboxymethylcellulose/CuO bio-nanocomposite hydrogels. Int J Biol Macromol. 2015;73:109–14.
Yadollahi M, Gholamali I, Namazi H, Aghazadeh M. Synthesis and characterization of antibacterial carboxymethyl cellulose/ZnO nanocomposite hydrogels. Int J Biol Macromol. 2015;74:136–41.
Yadollahi M, Namazi H, Aghazadeh M. Antibacterial carboxymethyl cellulose/Ag nanocomposite hydrogels cross-linked with layered double hydroxides. Int J Biol Macromol. 2015;79:269–77.
Farhoudian S, Yadollahi M, Namazi H. Facile synthesis of antibacterial chitosan/CuO bio-nanocomposite hydrogel beads. Int Biol Macromol. 2016;82:837–43.
Yadollahi M, Farhoudian S, Barkhordari S, Gholamali I, Farhadnejad H, Motasadizadeh H. Facile synthesis of chitosan/ZnO bio-nanocomposite hydrogel beads as drug delivery systems. Int J Biol Macromol. 2016;82:273–8.
Yadollahi M, Farhoudian S, Namazi H. One-pot synthesis of antibacterial chitosan/silver bio-nanocomposite hydrogel beads as drug delivery systems. Int J Biol Macromol. 2015;79:37–43.
Hashem M, Sharaf S, Abd El-Hady MM, Hebeish A. Synthesis and characterization of novel carboxymethylcellulose hydrogels and carboxymethylcellulose-hydrogel-ZnO-nanocomposites. Carbohydr Polym. 2013;95:421–7.
Franco AP, Recio MAL, Szpoganicz B, Delgado AL, Felcman J, Mercê ALR. Complexes of carboxymethylcellulose in water. Part 2. Co2+ and Al3+ remediation studies of wastewaters with Co2+, Al3+, Cu2+, VO2+ and Mo6+. Hydrometallurgy. 2007;87:178–89.
Barkhordari S, Yadollahi M. Carboxymethyl cellulose capsulated layered double hydroxides/drug nanohybrids for Cephalexin oral delivery. Appl Clay Sci. 2016;121–122(121):77–85.
Javanbakht S, Pooresmaeil M, Hashemi H, Namazi H. Carboxymethylcellulose capsulated Cu-based metal-organic framework-drug nanohybrid as a pH-sensitive nanocomposite for ibuprofen oral delivery. Int J Biol Macromol. 2018;119:588–96.
Tokarek K, Hueso JL, Kuśtrowski P, Stochel G, Kyzioł A. Green synthesis of chitosan-stabilized copper nanoparticles. Eur J Inorg Chem. 2013;2013(28):4940–7.
Salavati-Niasari M, Davar F, Mir N. Synthesis and characterization of metallic copper nanoparticles via thermal decomposition. Polyhedron. 2008;27(17):3514–8.
Mohd Sebri NJ, Mat Amin KA. Gellan gum/ibuprofen hydrogel for dressing application: mechanical properties, release activity and biocompatibility studies. Int J Appl Chem. 2016;12:483–98.
Abdul Hussein A, AL-Janabi S. In Vitro antibacterial activity of ibuprofen and acetaminophen. J Global Infect Dis. 2010;2(2):105–8.
Ingle AP, Duran N, Rai M. Bioactivity, mechanism of action, and cytotoxicity of copper based nanoparticles: a review. Appl Microbial Biotechnol. 2014;98:1–9.
Zakhireh S, Mahkam M, Yadollahi M, Jafarirad S. Investigation of pH-sensitive galactopyranoside glycol hydrogels as effective vehicles for oral drug delivery. J Polym Res. 2014;21:398–404.
Gils PS, Ray D, Sahoo PK. Designing of silver nanoparticles in gum arabic based semi-IPN hydrogel. Int J Biol Macromol. 2010;46:237–44.
Zare-Akbari Z, Farhadnejad H, Furughi-Nia B, Abedin S, Yadollahi M, Khorsand GM. PH-sensitive bionanocomposite hydrogel beads based on carboxymethyl cellulose/ZnO nanoparticle as drug carrier. Int J Biol Macromol. 2016;93:1317–27.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gholamali, I. Facile Preparation of Carboxymethyl Cellulose/Cu Bio-Nanocomposite Hydrogels for Controlled Release of Ibuprofen. Regen. Eng. Transl. Med. 6, 115–124 (2020). https://doi.org/10.1007/s40883-019-00133-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40883-019-00133-2