Abstract
Decline and death of young vines is a worldwide problem for viticulture that may lead to economic loss. Fungal pathogens have been associated with trunk and root diseases, including the black foot disease that affects the performance of the vineyards. Fungicides have not worked efficiently to control the disease and alternative methods should be investigated. We evaluated the potential of Bacillus subtilis strain F62 for controlling the disease caused by different strains in grapevine rootstocks 1103P (Vitis berlandieri × V. rupestris) and SO4 (V. berlandieri × V. riparia). The in vitro antagonism of B. subtilis F62 was evaluated on mycelial growth, by diffusible and volatile compounds, and conidia germination, by bacterial suspension and cell-free filtrate. In the in vivo assay, cuttings and micropropagated rootstocks were submitted to four different treatments: control, Bac (B. subtilis inoculation), Pat (pathogen inoculation) and Bac + Pat. According to our results, the bioagent was able to inhibit the mycelial growth of all the three fungal isolates by diffusible compounds and conidial germination by bacterial suspension and cell-free filtrate. In the in vivo assay, cuttings of SO4 treated with B. subtilis F62 showed higher shoot nodes and length of primary shoot, while cuttings of 1103P had a longer primary shoot. In micropropagated plants, B. subtilis F62 promoted plant growth in both rootstocks and reduced the frequency of D. macrodidyma re-isolation to 24.6% in SO4 and 29.5% in 1103P. The results demonstrated the potential of B. subtilis F62 on plant growth promotion and in the biocontrol of black foot disease on micropropagated plants and cuttings of grapevine rootstocks 1103P and SO4.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The young grapevine decline and death have increased in recent years affecting production of grape and wine worldwide due to the reduction in the vine productivity and the elimination of young vineyards (Berlanas et al. 2017; Gramaje et al. 2018). Although several factors have been associated with the syndrome, fungal strains associated with black foot disease have been considered the main cause for the vineyard decline (Aroca et al. 2010; Gramaje and Armengol 2011).
Black foot is a fungal disease that causes a significant impact on grapevine production and may be caused by different genera such as Cylindrocarpon, Campylocarpon, Dactylonectria, Ilyonectria, Neonectria, Cylindrocladiella and Thelonectria (Gramaje and Armengol 2011; Agustí-Brisach and Armengol 2013; Halleen and Fourie 2016). These fungi attack the grapevine trunk and roots through wounds causing necrosis of woody tissue, gum exudation, dark xylem discoloration, necrotic lesions and root biomass reduction (Gramaje and Armengol 2011; Agustí-Brisach and Armengol 2013). The external symptoms appear at the beginning of the vegetative cycle, and are characterized by reduced vigor, leaves with interveinal chlorosis and necrosis, shortening of internodes and wilting (Garrido et al. 2004; Abreo et al. 2010). Use of healthy mother vines is key for preventing the disease because asymptomatic plants may be already infected by these fungi (Waite et al. 2015). Other measures are important to prevent plant contamination during the propagation process such as use of resistant rootstocks, hot water treatment of cuttings, cultural practices in nurseries and vineyards, and the management of fungal diseases by chemical or biological control (Gramaje et al. 2018).
The intensive use of fungicides leads to an increased risk of contamination of the environment and harmful effects on food security as well as selection of chemical-resistant strains in the pathogen population (Boubakri et al. 2015). The use of biocontrol agents, when proven technical and cost-effective, is an attractive alternative for which these issues are not of concern (Shafi et al. 2017). Moreover, use of chemical pesticides in nurseries is complicated by the difficulty of traditional techniques such as spraying or immersion to reach the target pathogen that reside inside the xylem and phloem (Waite et al. 2015).
Antagonistic bacteria often act mainly by antibiosis mechanism and occasionally by parasitism and competition. Microorganisms of the former group usually have a broad spectrum of action, being the production of toxic substances more effective than other mechanisms involved in inhibition of pathogen growth (Santos et al. 2015). Studies have shown the efficacy of B. subtilis in the control of vine fungal pathogen including Eutypa lata (Ferreira et al. 1991; Kotze et al. 2011), Botrytis cinerea and Colletotrichum gloeosporioides (Furuya et al. 2011; Boubakri et al. 2015), Phaeomoniella chlamydospora, Phomopsis viticola, Diplodia seriata, Lasiodiplodia theobromae, Neofusicoccum australe and N. parvum (Kotze et al. 2011; Rezgui et al. 2016), Plasmopara viticola (Furuya et al. 2011; Boubakri et al. 2015) and Dactylonectria macrodidyma (Santos et al. 2016). Furthermore, B. subtilis works as a plant growth-promoting rhizobacterium that stimulates the growth and development of plants, through the bioavailability of nutrients and the stimulus of hormonal biosynthesis, increasing the yield, reducing biotic and abiotic stresses (Compant et al. 2010; Kejela et al. 2017). This study evaluated the antifungal activity and plant growth promotion of a B. subtilis strain F62 against black foot pathogens inoculated on micropropagated or cutting-derived vine rootstocks.
Material and methods
Pathogens and antagonistic microorganisms
Three isolates of black foot pathogens were collected from grapevines showing symptoms of black foot disease and stored in the fungal collection of IFRS (Instituto Federal de Educação, Ciência e Tecnologia do Rio Grande do Sul Bento Gonçalves, RS, Brazil). The isolates were identified based on their sequences of 5.8S ribosomal RNA (rRNA) genes which were deposited in GenBank (Table 1).
The antagonistic rhizobacterium used in this study is a soil-borne B. subtilis strain F62 obtained in Caxias do Sul, Brazil. The amplification of the 16S rDNA region (Sterky and Lundeberg 2000) showed 100% similarity to a pre-existing sequence in NCBI of B. subtilis at accession number NR 102783.2.
In vitro antifungal assay
The conidia were obtained from 10-day old colonies incubated in Potato Dextrose Agar (PDA) at 25 °C in 12 h light/12 h dark and the spore concentration was adjusted to 105 conidia mL−1 with distilled and sterile water. The bacterium was incubated in Potato Dextrose broth (PD) at 28 °C for 48 h with shaking at 150 rpm. The cells were centrifuged at 3500×g for 5 min at 23 °C, the pellet was washed three times with sterilized water, resuspended in 0.85% NaCl solution and the concentration was adjusted to 106 CFU mL−1. The cell-free filtrate was obtained from the bacterial culture supernatant after 0.22 μm membrane filtration.
The effect of B. subtilis F62 on mycelial growth was evaluated in two different trials. In the dual culture assay (antagonism by diffusible compounds), a 6 mm diameter agar disc of mycelium was placed on the center of a Petri dish containing PDA. After 24 h, it was applied 25 μl of suspension of B. subtilis F62 at four equidistant points around fungal mycelium. The volatile compounds assay was assessed using two Petri dishes containing PDA overlaid and sealed with parafilm: on the upper dish was inoculated a 6 mm diameter agar disc of mycelium and on the lower dish was spread 100 μL of B. subtilis F62 suspension. As a negative control, dishes were inoculated only with pathogens. Each treatment was replicated ten times, and the dishes were incubated for 21 days at 25 °C in dark. The mycelial growth was measured using a digital caliper, and the data was converted into mycelial growth inhibition
where dc and dt represent the mean colony diameters of control and treated groups, respectively. The mycelial growth speed index (MGSI) was determined according to the formula
where d represents the mean colony diameter at the present day, dp represents mean colony diameter from the previous day and N represents number of days after dish incubation (Oliveira et al. 2016).
The conidial germination assay was carried out in flasks containing 5 mL PD broth with shaking at 130 rpm at 28 °C for 24 h in three different treatments: control = 105 conidia mL−1; Bac + Pat = 105 CFU mL−1 of bacterial suspension +105 conidia mL−1 of pathogen suspension; Fil + Pat = 1 mL of bacterial filtrate +105 conidia mL−1 of pathogen suspension. The germination rate was evaluated by observing 100 conidia per replicate in an optical microscope. Each treatment was repeated three times.
In vivo antagonism: rootstock cuttings
Vegetative material of grapevine rootstocks 1103P and SO4 was collected in the field at the Embrapa’s experimental station, in Bento Gonçalves, Brazil. Rootstock cuttings (30 cm length and 12 mm diameter) were treated with sterilized water for 24 h and then submitted to hot water treatment (Bleach et al. 2013). Cuttings were arranged in a growth chamber at 28 °C and 70% relative humidity for 15 days, acclimated at room temperature for 5 days and, subsequently, transferred to plastic flasks containing 250 mL autoclaved substrate (90% sphagnum peat and 10% vermiculite) with 5 g L−1 of gradual release fertilizer (5–6 months). In the in vivo assay, the conidia suspension of D. macrodidyma TD1110 (5 105 conidia g−1 of substrate) was prepared according to Santos et al. (2016), and B. subtilis F62 was inoculated at the concentration of 104 CFU g−1 of substrate. Each rootstock were submitted to four different treatments in a completely randomized design, with application of 10 mL of suspension onto substrate: control = distilled and autoclaved water; Bac = B. subtilis F62 (1st and 14th days); Bac + Pat = B. subtilis F62 (1st and 14th days) + TD1110 (7th day) and Pat = TD1110 (7th day). Thirty cuttings were used per treatment of each rootstock.
The experiment was conducted in a greenhouse for 160 days as described (Gramaje et al. 2016). Afterwards, the following parameters were assessed: length of primary shoot (LPS, cm), number of nodes in the primary shoot (NNPS), total number of shoots in the plant (TNS), total number of nodes in the plant (TNN), shoot dry weight (SDW, g), root dry weight (RDW, g) and frequency of pathogen re-isolation (RI, %). Dry weight was determined after drying plant material in forced ventilation at 60 °C until constant weight. Pathogen re-isolation was carried out employing eight fragments from basal ends of the cuttings distributed in two Petri dishes, disinfested and incubated as described (Santos et al. 2016).
In vivo antagonism: micropropagated rootstocks
Initially, shoots were collected from cuttings of 1103P and SO4 and submitted to hot water treatment, then they were immersed in 70% alcohol for 1 min followed by immersion in 1% sodium hypochlorite containing 0.02% Tween 20 for 20 min and in the end washed three times by soaking in sterile water. The propagules were inoculated in tubes containing 12 mL of half concentration MS medium (Murashige and Skoog 1962), supplemented with 30 g L−1 sucrose, 6 g L−1 agar, 1 mg L−1 BAP (6-benzylaminopurine) and the pH was adjusted to 5.8. The explants were submitted to two subcultures, and the plantlets were rooted in the same medium, supplemented with 15 g L−1 sucrose, 6 g L−1 agar and 0.1 μg L−1 NAA (α-naphthaleneacetic acid). Growth was performed at 25 ± 2 °C, 70% relative humidity, in 16 h light/8 h dark, with a light intensity of 72 μmol m−2 s−1 provided by fluorescent lamps. Plantlets with approximately four leaves and suitable root systems were transferred to plastic cups containing 180 mL autoclaved substrate (90% sphagnum peat and 10% vermiculite) and acclimatized for 30 days at 23–28 °C, relative humidity higher than 60% and light intensity of 400 μmol m−2 s−1.
The in vivo assay with micropropagated plants of SO4 and 1103P was performed in triplicate with 30 replicates per treatment in a completely randomized design. The inoculum concentrations were the same described in Section 2.3. Plants were submitted to four treatments, drenching 4 mL of suspension in the substrate: control = sterile water; Bac = B. subtilis F62 (1st and 14th days); Bac + Pat = B. subtilis F62 (1st and 14th days) + TD1110 (7th day) and Pat = TD1110 (7th day).
The plants were evaluated in three distinct periods: beginning of the assay; 30 days later: variation of leaf number (ΔLeaf1), variation of shoot length (ΔLength1, cm); 160 days later: variation of leaf number (ΔLeaf2), variation of shoot length (ΔLength2, cm), shoot dry weight (SDW, g), root dry weight (RDW, g) and pathogen re-isolation (RI, %).
Data analysis
All data were subjected to Kolmogorov-Smirnov test to evaluate the normality of the data. The in vitro antagonism of B. subtilis by volatile and diffusible compounds and conidia germination were analyzed according to Factorial ANOVA. Parametric data were analyzed by ANOVA followed by Tukey test, and non-parametric data were analyzed by Kruskal Wallis followed by Dunn-Bonferroni test. In the in vivo assays, it was used the ratio for Factorial ANOVA analyses, as a dimensionless variable, calculated as [(C-T)/T]*100, where C is the control and T is the treatment. Factors that did not interacted significantly were analyzed by Kolmogorov-Smirnov test for rootstock and Kruskal-Wallis followed by Dunn-Bonferroni test for treatments. Data from significant interactions were analyzed by Kruskal-Wallis followed by Dunn-Bonferroni. The analyses were performed in SPSS 22.0 software (SPSS Inc. Chicago, IL), and the alpha level for statistical significance was set at p ≤ 0,05.
Results
In vitro antifungal assay
The analysis of mycelial growth assay data showed a significant interaction between isolates and treatments for both conditions (Table 2, Table 3 and Supplementary Material 1). B. subtilis F62 reduced significantly the mycelial growth speed index (MGSI) of I. liriodendri TD1117 and TD176, and D. macrodidyma TD1110 in the dual culture assay (Table 2 and Supplementary Material 2). Moreover, isolates submitted to antagonism by volatile compounds did not show any difference in the mycelial growth among them (Table 2 and Supplementary Material 3). The inhibition occurred mainly due to diffusible compounds and varied from 61.1% (TD1110) to 69.0% (TD1117) relative to the control. On the other hand, the volatile compounds reduced fungal mycelium diameter ranging from 2.9% (TD1110) to 14.2% (TD176) (Table 2) with sparse growth and morphological abnormalities in the development of fungal mycelium compared to the control (Supplementary Material 4).
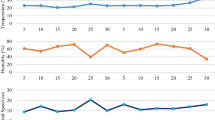
Conidia germination of the three pathogen isolates was evaluated in order to characterize the action of B. subtilis F62 and its compounds, and the statistical analyses indicated an interaction between isolates and treatments (Fig. 1 and Supplementary Material 1). In general, the number of conidia germinated was lower in the treatments with B. subtilis cells and cell-free filtrate compared to the control that presented all the conidia germinated (100) after 24 h of incubation. In the treatment Bac + Pat, the number of conidia germinated varied from 100 in the isolate of D. macrodydima (TD1110) to 74.1 (TD176) and 55.5 (TD1117) in both I. liriodendri isolates (Fig. 1). Moreover, Fil + Pat treatment resulted in the number of conidia germinated varying from 28.7 in the isolate of D. macrodydima (TD1110) to 7.4 and 72 in the isolates of I. liriodendri TD176 and TD1117, respectively (Fig. 1).
In vivo antagonism: rootstock cuttings
The isolate of D. macrodidyma (TD1110) was selected for the in vivo assays for presenting the lowest response to the antagonism with B. subtilis F62. All parameters evaluated presented none interaction between treatment and rootstock factors, and just the treatment showed significantly affect the responses (Table 3 and Supplementary Material 1). The inoculation of B. subtilis F62 had a significant positive effect on the growth promotion of cuttings of SO4, with an increase of length of primary shoot, number of nodes in the primary shoot, and total number of nodes in the plant concerning the control, while other responses were not affected. Similarly, the bioagent inoculation played a significant role in growth promotion in cuttings of 1103P with an increase of length of primary shoot compared to control, number of nodes in the primary shoot compared to pathogen inoculation treatment and total number of nodes in the plant compared to pathogen inoculation and B. subtilis + pathogen inoculation treatment (Table 3). Cuttings of SO4 infected with the pathogen showed similar responses to the control and 1103P had a reduction of length of primary shoot compared to control and B. subtilis treatment (Table 3). Moreover, B. subtilis + pathogen inoculation treatment did not alter the plant growth of SO4 and 1103P cuttings. The bioagent inoculation reduced the frequency of D. macrodidyma re-isolation in SO4 cuttings from 91.3% in pathogen inoculation treatment to 65.8% in B. subtilis + pathogen inoculation treatment, and in 1103P cuttings from 89.2% in pathogen inoculation to 80.0% in B. subtilis + pathogen inoculation treatment, however, none of these results were statistically different (Table 3).
In vivo antagonism: micropropagated rootstocks
Morphophysiological parameters and re-isolation of pathogen were evaluated in micropropagated rootstocks to characterize the role of B. subtilis F62 and statistical analyses resulted in an interaction between isolates and treatments for variation of shoot length at 30 days and root dry weight, and showed that treatment influenced variation of leaf number at 30 and 160 days, variation of shoot length at 160 days later, shoot dry weight and re-isolation and rootstock influenced just the variation of leaf number at 30 days (Table 4 and Supplementary Material 1). In interaction results, B. subtilis treatment in SO4 rootstock resulted in significantly longer plants at 30 days (ΔLength1), and B. subtilis treatment in SO4 and 1103P with B. subtilis + pathogen inoculation treatment in 1103P resulted in plants with higher root dry weight. The B. subtilis + pathogen inoculation treatment presented higher variation of shoot length at 30 days and root dry weight than pathogen inoculation treatment for 1103P. The rootstock analyses showed that 1103P had a higher number of leaves at 30 days than SO4. The treatment with B. subtilis F62 inoculation (Bac) presented an increase in variation of shoot length at 160 days later and shoot dry weight compared to control for SO4 and promoted higher variation of leaf number at 30 and 160 days, variation of shoot length at 160 days later, and shoot dry weight compared to control for 1103P. The treatment B. subtilis + pathogen inoculation treatment presented results similar to control and higher than pathogen inoculation treatment in variation of leaf number at 30 and 160 days for SO4 (Table 4). The frequency of fungal re-isolation was significantly reduced from 66.7% in pathogen inoculation treatment to 42.1% in B. subtilis + pathogen inoculation treatment (24.6% reduction) for SO4, and from 81.9% in pathogen inoculation treatment to 52.4% in B. subtilis + pathogen inoculation treatment (difference of 29.5% in the incidence of the pathogen) for 1103P (Table 4). Taking the results altogether, B. subtilis F62 minimized the negative impacts of D. macrodidyma infection in plants from B. subtilis + pathogen inoculation treatment when compared to pathogen inoculation treatment in SO4 and 1103P micropropagated rootstocks.
Discussion
The rhizobacterium B. subtilis has been used in the in vitro and in vivo biocontrol of different fungal diseases. In this work, the in vitro antagonism of B. subtilis against causal pathogens of black foot diseases was confirmed by a diffusible compound assay. Similarly, studies developed by Alfonzo et al. (2009) indicated that different strains of B. subtilis inhibited the in vitro growth of four phytopathogenic fungi of the vine (Phaeoacremonium aleophilum, Phaemoniella chlamydospora, Fomitiporia mediterranea and Lasiodiplodia theobromae). Also, Ferreira et al. (1991) reported a reduction of 88% in the mycelial growth of Eutypa lata using a B. subtilis strain isolated in pruning wounds of the vine. Inhibition of mycelial growth over 50% of Botryosphaeriaceous species causing grapevine trunk diseases were verified by Wicaksono et al. (2017) by diffusible compounds of Pseudomonas sp. I2R21 and Burkholderia sp. W6R12A and W4R11. The maximum inhibition percentage (64.7%) was verified using Pseudomonas sp. I2R21 in the biocontrol of Diplodia seriata. Similar results were reported by Santos et al. (2016) which had inhibition of mycelial growth from 20.9% to 50.6% in D. macrodidyma, using commercial products containing B. subtilis (Rizos and Rizolyptus). Another study evaluating the action of 19 bacterial strains belonging to the genera Pantoea, Pseudomonas, Curtobacterium and Bacillus on the growth of fungi causing stem disease in vines (Lasidiodiplodia theobromae, Neofusicoccum parvum and Schizophyllum commune) showed inhibition varying from 2.5% to 81.5%, with a mean of 24.9% in the paired culture (Rezgui et al. 2016).
The antagonism by volatile compounds of B. subtilis F62 was not expressive in this study and did not differ statistically from the control of each isolate. Differently, Santos et al. (2016) reported that the volatiles compounds of B. subtilis of commercial products (Rizos and Rizolyptus) inhibited the mycelial growth of D. macrodidyma, ranging from 29.5% to 69.1%. Wicaksono et al. (2017) also reported inhibition of mycelial growth by volatile compounds higher than 30% in Neofusicocumum ribis employing Pseudomonas sp. W1R33, W7R11, and W7R13. Although no inhibition by volatile compounds in mycelial growth was observed in our study, physiological abnormalities in the mycelium were detected, consistent with the observations of Chaurasia et al. (2005) which reported hyphae lysis, vacuolization, and granulation of mycelial structures on Fusarium oxysporum induced by B. subtilis volatile and diffusible compounds.
In the conidia germination assay, the inhibition occurred by bacterial suspension and cell-free filtrate of B. subtilis F62 culture, with some differences between the different pathogen isolates. Interestingly, Sotoyama et al. (2016) described that B. amyloliquefaciens IUMC7 showed antagonistic activity in the conidial suspension of F. oxysporum f. sp. lycopersici and that the bacterial culture supernatant, containing metabolites, inhibited the elongation and generated abnormalities in the germ tube. Also, the antagonism of B. amyloliquefaciens LBM 5006 on conidia germination and development in Aspergillus spp., Fusarium spp., and Bipolaris sorokiniana was reported by Benitez et al. (2010). Similarly, Cao et al. (2012) found that B. subtilis strain SQR 9 inhibited the mycelial growth and conidial germination of F. oxysporum f. sp. cucumerinum. Metabolites produced by B. subtilis, such as bacilomycin D, cause severe injury to the membrane, hyphae cell wall, and fungal spores (Gong et al. 2014). Studies developed by Romero et al. (2007) demonstrated that lipopolypeptides such as iturin and fengicin produced by B. subtilis reduced the germination of mildew conidia in Podosphaera fusca. Gong et al. (2014) studied the effect of purified bacilomycin on conidia and found inhibition of 96.63% on spore germination and 98.10% on sporulation of Aspergillus flavus.
The grapevine rootstocks 1103P and SO4 showed different responses in the in vivo trials that could be related to genetic and physiological characteristics. While SO4 is a rootstock originated from the crossing V. berlandieri x V. riparia and characterized by medium vigor, Paulsen 1103 came from the cross V. berlandieri x V. rupestris and is characterized by high vigor (Ollat et al. 2016). Besides, a factor that influences the growth promotion in different species is the way that bacteria colonize roots since they can develop on the entire surface or in some rhizodermal cells, establishing themselves as microcolonies or biofilms. Furthermore, the bacterial growth rate and the ability to migrate to the aerial part of the plant are other important factors involved in the different responses expressed in plants (Compant et al. 2010).
In the in vivo assay with cuttings was verified growth promotion in SO4 on treatment with B. subtilis F62 for half of the parameters evaluated when compared to control, while in 1103P there was no significant effect, except for the length of the primary shoot. Differently, Santos et al. (2016) verified a consistent increase in plant growth troughs the parameters evaluated in one-year-old grapevine cv. Merlot grafted on 1103P when treated with B. subtilis concerning the control. These different responses may be due to the host-bacterium-plant interaction that involves the recognition process and specific interactions, such as the production of exudates that is linked to the rhizosphere and rhizoplane colonization (Lugtenberg and Kamilova 2009). Moreover, the composition of the exudate depends on exposure to stress conditions, genotype, and stage of plant development (Haichar et al. 2008).
Micropropagated plants of 1103P and SO4 showed a significant growth when treated with B. subtilis F62 and compared to control. Similarly, studies developed by Nain et al. (2012) demonstrated that B. subtilis strain RM-2 had positive effects on seed germination, fresh and dry weight, leaf area, shoot and root length, and an increase in bean yield. Lopez-Valdez et al. (2011) also observed growth promotion, with an increase in root length, and fresh and dry root and shoot weight one month after inoculation of B. subtilis in sunflower. This growth promotion is a result of the increase in nutrient uptake, reduction in the biosynthesis of ethylene, and the production of phytohormones as 3-indoleacetic acid and gibberellic acid. While 3-indoleacetic acid plays an essential role in adventitious root emission, gibberellic acid acts in combination with auxins in plant elongation, and leaf bud formation (Zahir et al. 2003, Ahemad and Kibret 2014). Bioagent inoculation had a positive effect even in rootstocks infected with D. macrodidyma, resulting in growth promotion similar to healthy plants. It is known that the exudate composition in roots is potentially influenced by phytopathogens, which may have affected the bacterium activity (Compant et al. 2010). Moreover, having efficient root colonization requires competition between pathogenic and non-pathogenic microorganisms for space and nutrients (Shafi et al. 2017).
Regarding the biocontrol, B. subtilis F62 reduced the percentage of re-isolation of D. macrodidyma only in micropropagated plants of 1103P and SO4 rootstocks. The different response between cuttings and micropropagated plants to B. subtilis F62 inoculation should be related to plant developmental stage, origin of material, culture conditions and aseptic condition, the last in the case of micropropagated plants. Also, the antagonistic activity against the pathogen may be explained by the bioagent ability to develop on soil and the broad spectrum of action against fungi according to previous studies (data not published). Santos et al. (2016) also observed a reduction in the re-isolation frequency of D. macrodidyma, varying from 17.87% to 40.32% in vines cv. Merlot grafted on 1103P, though it was not different from pathogen inoculated plant. Likewise, Baumgartner and Warnock (2006) found that B. subtilis inhibited the growth of Armillaria mellea, which causes root rot in vines. However, field results indicated that the treatments did not prevent infection but may assist in maintaining productivity in infected vines. Another study carried out on vine pruning injuries of Merlot and Chenin Blanc cultivars demonstrated that B. subtilis reduced the incidence of N. australe, N. parvum, D. seriata, L. theobromae, E. lata, P. chlamydospora and P. viticola eight months after starting the experiment (Kotze et al. 2011). Also corroborating our results, Rezgui et al. (2016) reported that B. subtilis B6 inhibited in 35% the percentage of necrosis caused by N. parvum in cv. Muscat d’Italie grafted on 1103P when compared to control inoculated only with phytopathogenic fungus. The black foot disease usually manifests in mature grapevines, but its causal agent can be frequently isolated from symptomatic and asymptomatic rooted rootstock cuttings (Agustí-Brisach and Armengol 2013). That could explain the cuttings and micropropagated plants of SO4 and 1103P rootstocks with a positive presence of the pathogen but absent of symptoms. Moreover, these plants were evaluated 160 days after inoculation, and that could be not enough to have the development of symptoms, or the bioagent could be permitting the plant to tolerate the pathogen occurrence. The last could be possible as plants inoculated with the pathogen and treated with B. subtilis F62 bioagent presented a growth similar to control plants, but further studies should be carried out for a longer time to evaluate if symptoms would appear.
Different studies have demonstrated a positive relationship between the application of Bacillus spp. and the induction of resistance against phytopathogenic microorganisms. These rhizobacteria may promote the formation of pores in the fungal cell wall, the disintegration of organelles and nucleic acids (Zhao et al. 2013), the production of siderophores, lytic and oxidative enzymes that reduce the phytopathogens growth in the rhizosphere (Compant et al. 2010). The mechanism of action of B. fortis and B. subtilis against Fusarium sp. in tomatoes was investigated by Akram and Anjum (2011) which found an increase in the concentration of phenylalanine ammonia-lyase, polyphenol oxidase and peroxidase enzymes involved in systemic resistance. The use of B. subtilis suspension in ginseng induced the formation of periderm in wounds on roots challenged with C. destructans (Jang et al. 2011). Similar results were obtained by Gupta et al. (2000) using cell-free filtrate of B. subtilis strain FZB-G that acted on signal transduction and activation of defense-related genes in tomato plants.
Activation of defense mechanisms is the main effect of rhizobacteria on plant disease suppression (Iniguez et al. 2005). Besides, chemical and structural modifications are involved in the reduction of infection by pathogens such as increased cell wall deposition, obstruction of intercellular spaces and epidermal cells with osmotic compounds that hinder fungal colonization (Shafi et al. 2017). Thus, different mechanisms of action of Bacillus spp. reduce the incidence of phytopathogens.
In summary, B. subtilis F62 assisted in the plant growth of cuttings and micropropagated plants and promoted in vitro and in vivo biocontrol of D. macrodidyma in micropropagated plants of 1103P and SO4. It showed to be a potential bioagent to be used in the vine propagation process, mainly in micropropagated rootstocks, aiming at protection against D. macrodidyma. Further studies should be carried out to evaluate the induction of resistance in rootstocks and to understand the mechanisms of action in the biocontrol of the black foot disease.
References
Abreo E, Martinez S, Bettucci L, Lupo S (2010) Morphological and molecular characterization of Campylocarpon and Cylindrocarpon spp. associated with black foot disease of grapevines in Uruguay. Australasian Plant Pathology 39:446–452
Agustí-Brisach C, Armengol J (2013) Black-foot disease of grapevine: an update on taxonomy, epidemiology and management strategies. Phytopathologia Mediterranea 52:245–261
Ahemad M, Kibret M (2014) Mechanisms and applications of plant growth promoting rhizobacteria: current perspective. Journal of King Saud University - Science 26:1–20
Akram W, Anjum T (2011) Quantitative changes in defense system of tomato induced by two strains of Bacillus against Fusarium wilt. ndian Journal of Fundamental and Applied Life Sciences 1:7–13
Alfonzo A, Conigliaro G, Torta L, Burruano S, Moschetti G (2009) Antagonism of Bacillus subtilis strain AG1 against vine wood fungal pathogens. Phytopathologia Mediterranea 48:155–158
Aroca A, Gramaje D, Armengol J, García-Jiménez J, Raposo R (2010) Evaluation of grapevine nursery process as a source of Phaeoacremonium spp. and Phaeomoniella chlamydospora and occurrence of trunk disease pathogens in rootstock mother vines in Spain. European Journal of Plant Pathology 126:165–174
Baumgartner K, Warnock AE (2006) A soil inoculant inhibits Armillaria mellea in vitro and improves productivity of grapevines with root disease. Plant Disease 90:439–444
Benitez LB, Velho RV, Lisboa MP, Medina LF, Brandeli A (2010) Isolation and characterization of antifungal peptides produced by Bacillus amyloliquefaciens LBM5006. Journal of Microbiology 48:791–797
Berlanas C, López-Manzanares B, Gramaje D (2017) Estimation of viable propagules of black-foot disease pathogens in grapevine cultivated soils and their relation to production systems and soil properties. Plant Soil 417:467–479
Bleach C, Jones E, Ridgway H, Jaspers M (2013) Hot water treatment to reduce incidence of black foot pathogens in young grapevines grown in cool climates. Phytopathologia Mediterranea 52:347–358
Boubakri H, Hadj-Brahim A, Schmitt C, Soustre-Gacougnolle I, Mliki A (2015) Biocontrol potential of chenodeoxycholic acid (CDCA) and endophytic Bacillus subtilis strains against the most destructive grapevine pathogens. New Zealand Journal of Crop and Horticultural Science 4:261–274
Cao Y, Xu Z, Ling N, Yuan Y, Yang X, Chen L, Shen B, Shen Q (2012) Isolation and identification of lipopeptides produced by B. subtilis SQR 9 for suppressing Fusarium wilt of cucumber. Scientia Horticulturae 135:32–39
Chaurasia B, PandeyA PLMS, Trivedi P, Kumar B, Colvin N (2005) Diffusible and volatile compounds produced by antagonistic Bacillus subtilis strain cause structural deformations in pathogenic fungi in vitro. Microbiological Research 160:75–81
Compant S, Clement C, Sessitsch A (2010) Plant growth promoting bacteria in the rhizo and endosphere of plants. Their role, colonization, mechanisms involved and prospects for utilization. Soil Biology and Biochemistry 42:669–678
Ferreira JHS, Matthee FN, Thomas AC (1991) Biological control of Eutypa lata on grapevine by antagonistic strain of Bacillus subtilis. Phytopathology 81:283–287
Furuya S, Mochizuki M, Aoki Y, Kobayashi H, Takayanagi T, Shimizu M, Suzuki S (2011) Isolation and characterization of Bacillus subtilis KS1 for the biocontrol of grapevine fungal diseases. Biocontrol Science and Technology 21:705–720
Garrido LR, Sônego OR, Gomes VN (2004) Fungos associados com odeclínio e morte de videiras no estado do Rio Grande do Sul. Fitopatologia Brasileira 29:322–324
Gong Q, Zhang C, Lu F, Zhao H, Bie X, Lu Z (2014) Identification of bacillomycin D from Bacillus subtilis fmbJ and its inhibition effects against Aspergillus flavus. Food Control 36:8–14
Gramaje D, Armengol J (2011) Fungal trunk pathogens in the grapevine propagation process: potential inoculum sources, detection, identification, and management strategies. Plant Disease 95:1040–1055
Gramaje D, Alaniz S, Abad-Campos P, García-Jiménez J, Armengol J (2016) Evaluation of grapevine rootstock against soilborne pathogens associated with trunk diseases. Acta Horticulturae 1136:245–250
Gramaje D, Úrbez-Torres JR, Sosnowski MR (2018) Managing grapevine trunk diseases with respect to etiology and epidemiology: current strategies and future prospects. Plant Disease 102:12–39
Gupta V, Bochow H, Dolej S (2000) Plant growth-promoting Bacillus subtilis strain as potential inducer of systemic resistance in tomato against Fusarium wilt. Journal of Plant Diseases and Protection 107:145–154
Haichar FZ, Marol C, Berge O, Rangel-Castro JI, Prosser JI, Balesdent J, Heulin T, Achouak W (2008) Plant host habitat and root exudates shape soil bacterial community structure. ISME Journal 2:1221–1230
Halleen F, Fourie PH (2016) An integrated strategy for the proactive management of grapevine trunk disease pathogen infections in grapevine nurseries. South African Journal for Enology and Viticulture 37:104–114
Iniguez AL, Dong Y, Carter HD, Ahmer BMM, Stone JM, Triplett EW (2005) Regulation of enteric endophytic bacterial colonization by plant defenses. Molecular Plant-Microbe Interactions 18:169–178
Jang Y, Kim SG, Kim YH (2011) Biocontrol efficacies of Bacillus species against Cylindrocarpon desctructans causing ginseng root rot. The Plant Pathology Journal 27:333–341
Kejela T, Thakkar VR, Patel RR (2017) A novel strain of Pseudomonas inhibits Colletotrichum gloeosporioides and Fusarium oxysporum infections and promotes germination of coffee. Rhizosphere 4:9–15
Kotze C, Van Niekerk J, Mostert L, Halleen F, Fourie P (2011) Evaluation of biocontrol agents for grapevine pruning wound protection against trunk pathogen infection. Phytopathologia Mediterranea 50:S247–S263
Lopez-Valdez F, Fernandez-Luqueno F, Ceballos-Ramírez J (2011) A strain of Bacillus subtilis stimulates sunflower growth (Helianthus annuus L.) temporarily. Scientia Horticulturae 128:499–505
Lugtenberg B, Kamilova F (2009) Plant growth promoting rhizobacteria. Annual Review of Microbiology 63:541–556
Murashige T, Skoog FA (1962) A revised medium for a rapid growth and bioassays with tobacco tissues cultures. Journal of Plant Physiology 15:473–479
Nain ML, Yadav RC, Saxena J (2012) Characterization of multifaceted Bacillus sp. RM-2 for its use as plant growth promoting bioinoculant for crops grown in semi-arid deserts. Applied Soil Ecology 59:124–135
Oliveira TAS, Blum LEB, Duarte EAA, Moreira ZPM, Luz EDMN (2016) Variability of aggressiveness and virulence of Phytophthora palmivora influencing the severity of papaya fruit rot in postharvest in Bahia, Brazil. Científica 44:185–195
Ollat N, Bordenave L, Tandonnet JP, Boursiquot JM, Marguerit E (2016) Grapevine rootstocks: origin and perspectives. Acta Horticulturae 1136:11–22
Rezgui A, Ben Ghnaya-Chakroun A, Vallance J, Bruez E, Hajlaoui MR, Sadfi-Zouaoui N, Rey P (2016) Endophytic bacteria with antagonistic traits inhabit the wood tissues of grapevines from Tunisian vineyards. Biological Control 99:28–37
Romero D, Vicente A, Rakotoaly RH, Dufour SE, Veening JW, Arrebola E, Cazorla FM, Kuipers OP, Paquot M, Perez-Garcia A (2007) The iturin and fengycin families of lipopeptides are key factors in antagonism of Bacillus subtilis toward Podosphaera fusca. Molecular Plant-Microbe Interactions 20:430–440
Santos RF, Durigon MR, Blume E (2015) Aggressiveness of Ilyonectria spp. and Cylindrocarpon pauciseptatum associated with black foot disease of grapevine. Revista Brasileira de Ciências Agrárias 10:49–53
Santos RF, Heckler LI, Lazarotto M, Garrido LR, Rego C, Blume E (2016) Trichoderma spp. and Bacillus subtilis for control of Dactylonectria macrodidyma in grapevine. Phytopathologia Mediterranea 55:293–300
Shafi J, Tian H, Ji M (2017) Bacillus species as versatile weapons for plant pathogens: a review. Biotechnology and Biotechnological Equipment 31:446–459
Sotoyama K, Akutsu K, Nakajima M (2016) Biological control of Fusarium wilt by Bacillus amyloliquefaciens IUMC7 isolated from mushroom compost. Journal of General Plant Pathology 82:105–109
Sterky F, Lundeberg J (2000) Sequence analysis of genes and genomes. Journal of Biotechnology 76:1–31
Waite H, Whitelaw-Weckert M, Torley P (2015) Grapevine propagation:principles and methods for the production of high-quality grapevine plant material. New Zealand Journal of Crop and Horticultural Science 43:144–161
Wicaksono WA, Jones EE, Mon J, Ridgway HJ (2017) Using bacterial endophytes from a New Zealand native medicinal plant for control of grapevine trunk diseases. Biological Control 114:65–72
Zahir ZA, Arshad M, Frankenberger WT (2003) Plant growth promoting Rhizobacteria: applications and perspectives in agriculture. Advances in Agronomy 81:97–168
Zhao X, Zhou Z-J, Han Y (2013) Isolation and identification of antifungal peptides from Bacillus BH072, a novel bacterium isolated from honey. Microbiological Research 168:598–606
Acknowledgements
The funding for this research was provided by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).
Author information
Authors and Affiliations
Corresponding author
Additional information
Section Editor: Flávio H. V. Medeiros
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Supplementary Material 1
(DOCX 20 kb)
Supplementary Material 2
(DOCX 32 kb)
Supplementary Material 3
(DOCX 30 kb)
Supplementary Material 4
(DOCX 105 kb)
Rights and permissions
About this article
Cite this article
Russi, A., Almança, M.A.K., Grohs, D.S. et al. Biocontrol of black foot disease on grapevine rootstocks using Bacillus subtilis strain F62. Trop. plant pathol. 45, 103–111 (2020). https://doi.org/10.1007/s40858-019-00319-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40858-019-00319-7