Abstract
The influence of the lulo fruit maturity stage on the secretion of polygalacturonase (PG) and pectate lyase (PL) enzymes by Colletotrichum acutatum was determined. The study was performed using in vitro assays in the presence of exocarp and cell walls (CW) of the fruit at three different ripening stages (unripe, semi-ripe and ripe). The results showed that PG behaves as a constitutive enzyme and is only induced by semi-ripe CW, while PL is induced with the exocarp of lulo fruits at the three maturation stages, although its activity was highest with semi-ripe fruit. This enzymatic behavior might be related to the quiescent stage of the pathogen in the fruit, as C. acutatum remains quiescent until maturation begins. The profiles of PL induction were different in the presence of the CW and the exocarp inducers, whereas PG levels barely varied, indicating that the process of obtaining the CWs may eliminate a compound that contributes to the induction of PL secretion.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The genus Colletotrichum contains many species that cause one of the most destructive plant diseases, called anthracnose, in a wide range of plants, including vegetables, fruits, legumes and perennial trees, worldwide (Sharma and Kuishrestha 2015; Gan et al. 2016; De Silva et al. 2017). Species from this genus have been used in many studies concerning fungus-plant interactions. Colletotrichum acutatum is a main fruit pathogen in important crops such as strawberry (Guidarelli et al. 2011), citrus (Timmer and Peres 2015), apple (Jurick et al. 2011), olive (Talhinhas et al. 2011), blueberry (Miles et al. 2011; Miles et al. 2012) and hazelnut (Sezer and Dolar 2012).
Solanum quitoense Lam (lulo) is a tropical fruit native to the Andes, cultivated and consumed mainly in Ecuador, Colombia and Central America with great potential for international markets. It is characterized by a refreshing and intense aroma, high nutritional value and the presence of several bioactive compounds (Forero et al. 2016; González Loaiza et al. 2014).
Anthracnose is considered one of the most important diseases of S. quitoense and leads to severe economic losses. C. acutatum was identified as the main pathogen in lulo fruits in some areas of Colombia (Cerón 2005; Ochoa et al. 2016). This pathogen exhibits both infection strategies described for Colletotrichum species: intracellular hemibiotrophy, in which nutrients are obtained from living host cells and large primary hyphae grown intracellularly through epidermal cells with no appearance of tissue damage (quiescent infection), and subcuticular-intramural necrotrophy, in which nutrients are obtained from dead host cells that have been killed by the fungus when thin secondary hyphae colonize host tissue both inter- and intra-cellularly (at which point anthracnose symptoms, such as tissue maceration, become visible) (Thrower 1966; Perfect et al. 1999; Wharton and Diéguez-Uribeondo 2004; Guidarelli et al. 2011; Cannon et al. 2012; Sezer and Dolar 2012; Baroncelli et al. 2017). Penetration of the cell wall during the biotrophic phase and tissue maceration during the necrotrophic phase indicate that fungal cell wall-degrading enzymes (CWDEs) are active (Wei et al. 1997; Reignault et al. 2008; Bellincampi et al. 2014). Comparative analyses of the genome reveal that Colletotrichum species have adapted the profiles of their cell wall-degrading enzymes according to their infection lifestyles (Gan et al. 2016).
Plant cell walls represent a formidable barrier against microbial invasion which must be overcome by any successful pathogen. Cell walls consist mainly of polysaccharides (cellulose, hemicelluloses and pectins) that play an important role in defending plants against pathogens, and these walls are dynamic reservoirs of antimicrobial proteins and secondary metabolites that inhibit the growth of many pathogens (Roncero et al. 2000; Vorwerk et al. 2004; Underwood 2012; Bellincampi et al. 2014).
Most phytopathogenic microorganisms produce enzymes that can degrade cell wall polymers (Walton 1994; Underwood 2012; Gan et al. 2016). CWDEs are particularly important for fungal pathogens lacking specialized penetration structures and for necrotrophic pathogens during late invasion stages (Roncero et al. 2000; Kubicek et al. 2014; Lo Presti et al. 2015; Lyu et al. 2015). Polygalacturonases (PG: endo-PG, E.C.3.2.1.15 and exo-PG, E.C.3.2.1.67 and E.C.3.2.1.82) are pectinolytic enzymes that catalyze the hydrolytic cleavage of polygalacturonic acid chains by introducing water across the oxygen bridge (De Lorenzo and Ferrari 2002; Jayani et al. 2005; Kubicek et al. 2014). Pectate lyases (PL: endo-PL, E.C.4.2.2.2 and exo-PL, E.C.4.2.2.9) cleave uronic acid-containing polysaccharide chains via a β-elimination mechanism to generate an unsaturated hexenuronic acid residue and a new reducing end (Marín-Rodríguez et al. 2002; Kubicek et al. 2014; Dubey et al. 2016). Pectin lyases (Endo-PNL, E.C.4.2.2.10) act on the pectic substances that occur as structural polysaccharides in the middle lamella and primary cell walls of higher plants by eliminative cleavage of (1→4)-alpha-D-galacturonan methyl ester to give oligosaccharides with 4-deoxy-6-O-methyl-alpha-D-galact-4-enuronosyl groups at their non-reducing ends (Bugbee 1990; Aleandri et al. 2007; Yadav et al. 2009; Lara-Márquez et al. 2017).
Since pectinases are the first enzymes secreted by most fungal pathogens when they attack plant cell walls (followed by hemicellulases and cellulases), they have been studied in several plant-pathogen interactions (Jones et al. 1972; Gómez-García and Martínez 2005; Kramer-Haimovich et al. 2006; Ramos et al. 2010; Zhang et al. 2014). It has been demonstrated that pectinases are involved in pathogen penetration and colonization of plants (Shih et al. 2000; Zhang et al. 2014; Ramos et al. 2016; Dubey et al. 2016; Yarullina et al. 2016).
Fruit ripening involves changes in the composition and organization of pectin, hemicellulose and cellulose polysaccharides in the cell wall, which occur as a coordinated series of assembly and disassembly steps (Goulao et al. 2007). Host maturity plays an important role in the defense mechanism of fruits, and softening facilitates the growth of many pathogens (Prusky et al. 2013).
Environmental conditions, including carbon source, level of nitrogen, osmolarity and pH can affect the production of enzymes and their secretion in several organisms (Fernández-Acero et al. 2010; Prusky et al. 2016). Ambient pH levels or factors determining host pH are important because they determine the ability of the pathogen to successfully colonize, invade and kill the target host with the help of secreted pathogenicity factors (Prusky et al. 2001; Prusky and Yakoby 2003; Akimitsu et al. 2004; Prusky et al. 2013; Zhang et al. 2014; Ramos et al. 2016). A large number of fungal plant pathogens modify the pH of the host in several ways. In the case of the genus Colletotrichum, several species have been found that alkalinize the host, for example, C. gloeosporioides, C. coccodes and C. higginsianum (Drori et al. 2003; Prusky et al. 2013).
Knowledge of the molecular and metabolic events responsible for the onset of the necrotrophic stage, occurring in both the host and fungi, is important in order to develop strategies to enhance fruit defense and decrease fungal virulence, which ultimately will result in increased fruit quality. This knowledge can be considered in breeding programs, pre- and post-harvest treatments or alternatively provide a framework for biotechnological approaches (Alkan and Fortes 2015).
Currently, only a few reports have addressed the perception of and response to fungally secreted molecules in fruit ripening (Prusky et al. 2013). The objective of this study was to evaluate, by using in vitro assays, the influence of pH, carbon source (cell wall and exocarp) and lulo fruit ripening state (unripe, semi-ripe and ripe) on the induction of pectinolytic PG and PL enzymes secreted by C. acutatum.
Materials and methods
Fungal material
A C. acutatum strain was obtained from the Mycology and Plant Pathology Laboratory of Universidad de los Andes (LAMFU). The fungal isolate was maintained in a solid medium of potato dextrose agar (PDA; Merck) at 30 °C in the dark. The inoculum was prepared in sterile 25% potato broth and grown at 25 °C for 8 days with agitation. The number of conidia was determined with the aid of a hemacytometer.
Origin of the fruits
The plants of lulo used in the study were grown in Campo Hermoso, Boyacá (Colombia) at 1300 m elevation, 5°01′52”N and 73°06′12”W with an average temperature of 21 °C and annual precipitation of 2450 mm. The fruits were collected from plants at three different points of the crop.
Maturity stages were determined by the following physicochemical parameters: hardness (lbf/pg2), soluble solids (°Brix), pH, total acidity (g citric acid/100 g of fresh pulp) and maturity index (IM), according to the Colombian Technical Standards NTC 4592 (1999a), xNTC 4623 (1999b) and NTC 4624 (1999c). The maturity index (MI) was evaluated as the ratio between soluble solids and total acidity (González Loaiza et al. 2014). Each of these parameters was evaluated for each state of maturity in a sample of three fruits, in triplicate.
Obtention of lulo fruit exocarps
Lulo fruits (unripe, semi-ripe and ripe) were washed in a 1% sodium hypochlorite solution for 15 min and then with distilled water for 5 min. The exocarp was taken, ground in liquid nitrogen, dried at 37 °C overnight and then kept in an ultraviolet laminar flow cabin for 2 h before inoculation assays (Rodríguez and Restrepo 2011). The extraction of the exocarp was performed for each collected group independently.
Obtention of lulo fruit cell walls
Lulo fruit exocarp (unripe, semi-ripe and ripe) was washed twice using 7 mL of 10 mM sodium phosphate buffer solution (pH 7) per g of exocarp and centrifuged at 4000 g for 20 min at 4 °C. The insoluble material was washed twice with 4 mL of 1% SDS per g of exocarp and centrifuged at 4000 g for 15 min at 4 °C. The pellet was washed three times with 3 mL of distilled deionized water and centrifuged at 4000 g for 20 min at 4 °C. The residue remaining after the water extraction, which contains the cell walls used in this study, was dried at 37 °C overnight. Immediately preceding their use, the cell walls were placed in an ultraviolet laminar flow cabin for 2 h (Gómez-García and Martínez 2005). The extraction of the cell wall was performed for each collected group independently.
Enzyme production and growth culture
First, 50 mL Erlenmeyer flasks containing 15 mL of 25% sterile potato broth were supplemented with 0.2% (w/v) of different carbon sources: lulo fruit CW and exocarp samples at three maturity stages (unripe, semi-ripe and ripe). Three flasks were used, one for each of the three groups. Each flask contained the CW or exocarp from a group of collected fruits. These were inoculated until a suspension of 3–4 × 105 conidia/mL was obtained. A control of culture medium that lacked the added carbon source was used. Cultures were agitated on a rotary shaker at 150 rpm and 20 °C, and triplicate flasks were harvested on days 0, 2, 4, 6, 7, 8, 9, 10, 11, 12 and 13 for assays. CW or exocarp, as well as fungal mycelia, were removed from the growth medium by centrifugation at 5000 rpm and 4 °C for 15 min. An aliquot of the supernatant (culture filtrate) was used to determine the pH, while the remainder was stored at −20 °C (Martínez et al. 2012).
Determining reaction conditions for PG and PL culture filtrates
Reaction conditions were optimized to achieve maximum enzymatic efficiency. For PG reactions, the following ranges were employed: temperature from 20 to 80 °C; polygalacturonic acid (PGA) (from citrus fruit; Sigma) from 0.1 to 0.6% (w/v); reaction time from 15 to 120 min. For pH assays, buffers consisting of 0.1 M acetic acid-sodium acetate with 75 mM EDTA (pH 4.0–5.5), and 0.1 M NaH2PO4-Na2HPO4 with 75 mM EDTA (pH 6.0–7.0) were used. The same ranges in temperature, polygalacturonic acid and reaction time were used for PL reactions. Buffers consisting of 0.1 M NaH2PO4-Na2HPO4 (pH 6.5), 0.1 M Tris-HCl (pH 7.0–9.0) and 0.1 M glycine-NaOH (pH 10.0) were used. PLs enzymes require CaCl2 as a cofactor, therefore 0.2–0.7 mM CaCl2 was used in the assays. Control samples were subjected to the same treatment conditions but without enzyme. Each determination of the evaluated parameters was carried out in triplicate.
Polygalacturonase (PG) activity
PG activity was determined by measuring the number of reducing groups released from polygalacturonic acid. Reducing groups were measured by adapting the technique described by Nelson (1944) as modified by Somogyi (1952). The reaction mixture, containing 190 μL of 0.3% (w/v) polygalacturonic acid in 100 mM acetate, 75 mM EDTA buffer pH 5.0 and 60 μL from the culture filtrate, was incubated at 30 °C for 1.5 h. Reactions were stopped by adding Nelson-Somogyi alkaline copper reagent. Controls without either enzyme or substrate were run simultaneously. Reducing group formation was estimated by measuring the absorbance at 500 nm and comparing the absorbance with a calibration curve obtained using D-glucose as standard. PG activity was expressed in nanokatals (nkat) per mL of culture filtrate, defined as the amount of enzyme that releases 1 nmol of D-galacturonic acid/s/mL under the above conditions. The measurement of activity was carried out in triplicate in each of the Erlenmeyer flasks, three flasks per day of test. The results were expressed as the average of three biological replicates and three technical replicates.
Pectate lyase (PL) activity
PL activity was measured by monitoring the unsaturated oligogalacturonates accumulated after the enzymatic cleavage of polygalacturonic acid by measuring the increase in absorbance at 232 nm (Collmer et al. 1988). The substrate used for this assay was 0.3% (w/v) polygalacturonic acid in 100 mM Tris-HCl buffer pH 8.5 and 0.5 mM CaCl2. The standard reaction mixtures contained 250 μL of substrate and 100 μL of culture filtrate. After incubation at 45 °C for 1 h, reactions were stopped by heating the samples in boiling water for 5 min. Appropriate controls (without either enzyme or substrate) were run simultaneously. Enzyme activity was expressed in nanokatals (nkat) per mL of culture filtrate, defined as the amount of enzyme that forms 1 nmol of 4,5-unsaturated compound/s/mL with a molar extinction coefficient (ε) of 4.6 L/mol/cm (Brühlmann 1995) under the above conditions. The results were expressed as the average of three biological replicates and three analytical replicates.
Experimental design and statistical methods
In the assay for determining reaction conditions for PG and PL culture filtrates, each determination of the evaluated parameters was carried out in triplicate and the data were expressed as the means ± standard deviations (SDs).
In vitro assays were conducted in a completely randomized design that evaluated two carbon sources (CW and exocarp) and three different ripening states during 13 days. For each day of the enzymatic activity assay, three independent biological replicates with controls were used to evaluate PG and PL activity in culture filtrates. The results of final activity of PG and PL were expressed as the average of three biological replicates and three analytical replicates.
Statistical analysis was performed for each enzymatic activity in the different treatments in triplicate, and the data were expressed as the means ± standard deviations (SDs). Analysis of variance and differences between means were analyzed using one-way ANOVA with Statgraphics software version 5.1. Different mean values used for the inductor were compared and grouped by the Tukey test. Differences with P values lower than 0.05 were considered statistically significant.
Results
Maturity stage determination
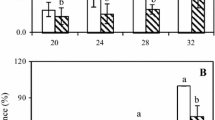
The fruits were classified as being unripe, semi-ripe and ripe based on their hardness, °Brix, pH, titratable acidity and maturity index (Table 1).
Optimal conditions for PG and PL enzyme activity in culture filtrates
The best reaction conditions found for enzymes in the culture filtrates are listed in Table 2.
PG activity in culture filtrates
PG activity was detected in culture filtrates with two carbon sources (unripe exocarp and CW) beginning at day 4, and no enzyme induction was observed throughout the assays (13 days). Culture filtrates containing unripe CW, compared to controls, showed a statistically significant decrease (α = 0.05) in enzyme activity over the 13 days. From day 8, a decrease in PG activity of culture filtrates with exocarp compared to the controls was found (α = 0.05) (Fig. 1a). These results indicate that unripe lulo fruits may contain inhibitors for PGs secreted by C. acutatum.
The assays with semi-ripe exocarp and CW of lulo fruit indicated that PG activity was present from day 6 onward, and enzyme induction was observed between days 8–11 (Fig. 1b). During these days, the assays showed a statistically significant increase (α = 0.05) in PG activity levels compared to the control, and this state of maturation was the only one that demonstrated the induction of this enzyme. This result indicates that when the process of fruit maturation begins, the changes that occur in the cell wall lead to an increase in the secretion of the enzyme.
Enzyme activity was present from day 2 onwards during the assays with lulo ripe exocarp and CWs (Fig. 1c), and a maximum was observed between days 7 and 9. However, no PG induction was found when comparing these results with the controls (α = 0.05).
Considering the results obtained for the control assays and the assays carried out with two carbon sources (exocarp and CW) at the three maturation stages of the lulo fruits (where this enzyme was secreted from early times to day 13), this enzyme might be important in the lulo-C. acutatum interaction.
PL activity in culture filtrates
Enzyme activity was present from day 10 onwards in the control assays. In vitro assays using unripe lulo exocarp induced PL activity from day 4 onwards (α = 0.05) (Fig. 2a). Assays with unripe CWs revealed no induction of this enzyme activity and presented low activity only during the final day of the assays (day 13). This result indicates that the treatment to obtain the cell wall eliminates compounds that activate PL secretion.
A noticeable induction of this enzyme was found from day 8 onwards in the assays on lulo fruit semi-ripe exocarp (Fig. 2b). The maximum activity was obtained between days 8 and 10, being 3–6 times greater than that at the other two maturity stages (α = 0.05). In this case, enzyme induction was maintained until day 13 and began to decrease from day 11. Induction was only present in assays on CW between days 8 and 10 (α = 0.05), but the activity values were 10–20 times smaller than those obtained with exocarp.
PL activity was induced in assays with ripe exocarp or CW of lulo fruits from day 6 onwards (α = 0.05) (Fig. 2C). However, the activity was lower than that obtained with unripe or semi-ripe CWs and exocarp of lulo fruits.
Together, these results indicate that the highest levels of activity of PL enzyme secreted by C. acutatum were found in the culture media containing semi-ripe CWs and exocarp.
Culture medium pH variation
The pH values in the culture filtrates were similar and independent of the carbon source and the ripening stage. The data obtained from semi-ripe fruits assays (Fig. 3) is similar to those obtained for assays with unripe and ripe fruits (data not shown). The medium pH increased from 5.5 (day 0) to 8.5 (day 13) in every assay as time progressed. This shows that the fungus alkalized the culture medium while growing, which is a natural process that was not affected by the carbon sources employed under the conditions in which these assays were carried out.
Discussion
The changes in the physicochemical properties of lulo (Solanum quitoense Lam) were evaluated in the present research as a function of the three different degrees of maturity. The content of soluble solids was similar to that reported by González Loaiza et al. (2014), Mejía et al. (2012) and Caicedo and Higuera (2007). Soluble solids content in lulo increased (p < 0.05) as the fruit reached the highest degree of maturity (Table 1). According to Gómez et al. (2002) and Fisk et al. (2006), the increase in soluble solids during the ripening of climacteric fruits is the result of the activity of the sucrose phosphate synthase enzyme (SPS), which is responsible for hydrolyzing starch granules. Conversely, cell wall protopectins are hydrolyzed to soluble pectins and contribute to the increased soluble solid concentration during the ripening process (Prasanna et al. 2007). The MI increased (p < 0.05) with the degree of maturity of the fruits (Table 1) according to the findings reported previously for lulo (González Loaiza et al. 2014; Mejía et al. 2012; Caicedo and Higuera 2007). This behavior is a consequence of the reduction of acidity and the increase in soluble solids during fruit ripening.
The reaction conditions of enzymes secreted by microorganisms can vary for a specific pathogen, so we determined the optimal conditions in the culture filtrate for the activity of enzymes secreted by C. acutatum before determining the enzymatic PG and PL activity in in vitro assays. PGs isolated from different microbial sources differ from each other with respect to their physicochemical and biological properties and their mode of action. Most of the PGs obtained from different microbial sources have an optimal pH range of 3.5–5.5 and an optimal temperature range of 30–50 °C (Jayani et al. 2005). PG secreted by C. acutatum has an optimal pH value and temperature of 5.0 and 30 °C, respectively (Table 2). PLs absolutely require Ca2+ ions (Jayani et al. 2005), which is evident for the enzyme secreted by C. acutatum because it requires a concentration of 0.5 mM to reach its optimal activity. Most of the lyases have their pH optimum in the alkaline range (7.5–10.0) and a temperature optimum from 40 to 50 °C (Jayani et al. 2005), and the PL secreted by C. acutatum has optimum conditions within these ranges (pH 8.5 and 45 °C, Table 2).
In the present study, the exocarp or CWs of unripe fruits significantly affected the activity of PG and PL enzymes, being more evident for PL which exhibited no activity resulting from the addition of unripe CW. Decreasing PL and PG enzyme activity in cultures in which CWs or exocarp from fruit in the unripe stage has been added can be related to the presence of antifungal compounds (Prusky et al. 1988), polygalacturonase-inhibiting proteins (De Lorenzo and Ferrari 2002), phenols (Hunter 1974; Wattad et al. 1994), and phytoalexins (Wharton and Diéguez-Uribeondo 2004). The possible presence of antifungal compounds in unripe lulo could explain the inhibition observed in the pectic enzymes tested. Our results showed a decrease in PG activity and an inhibitory effect on PL from the culture filtrates with CW or exocarp from unripe lulo fruits.
The induction of both PG and PL enzymes in assays using CWs or exocarp of semi-ripe lulo fruits was relevant. Higher induction levels of PL enzyme during this maturity stage was found, and these levels were three times higher than those in ripe fruit and six times those in unripe fruit. PG enzyme was only induced during the semi-ripe maturity stage. Since fruit CWDEs are normally activated during ripening, it has been commonly assumed that fruit softening contributes to the transition to susceptibility to pathogens (Alkan and Fortes 2015). Semi-ripe lulo fruit was most sensitive to C. acutatum pathogen attack, followed by ripe and unripe fruit (Cerón 2005). This pattern matches the secretion profiles found for the enzymes studied in our work.
Assays using exocarp or CWs of ripe fruits showed neither induction nor inhibition of PG. In contrast, PL was induced with two carbon sources used in this work, but these levels were lower than those in semi-ripe fruit. Fruit maturity involves biochemical changes such as the conversion of reserve polysaccharides into soluble sugars (Wharton and Diéguez-Uribeondo 2004). The pectinolytic enzymatic activation of the fruit is therefore necessary, but it seems that the pathogen does not require high-level secretion of its own enzymes during this maturity stage. Cell wall disassembly and softening may affect the embedded plant protein that modulates pathogen recognition and plant responses (Prusky et al. 2013).
The chemical composition of CW polysaccharides is very important for the plant-pathogen interaction (Vorwerk et al. 2004; Bellincampi et al. 2014). CWs of fruits are a complex and dynamic barrier that changes during ripening, and its interaction with fungal pathogens plays a major role in the defense response against pathogens. Pathogens respond to the changes that they induce in the host by altering their own enzymes and compounds, which allows them to infect and breakdown or grind fruit tissue (Blanco-Ulate et al. 2014; Agudelo-Romero et al. 2015; Alkan and Fortes 2015). Signals for release from quiescence probably occur during fruit ripening and may include disassembled cell wall substrates, alterations in cuticles and other signals (Cantu et al. 2008a; b; Mengiste 2012; Alkan and Fortes 2015).
The activity of PG and PL enzymes secreted by C. acutatum in the presence of CWs and exocarp in different stages of maturation can be related to C. acutatum’s hemibiotrophic behavior, keeping it quiescent and secreting PG until the fruit starts ripening (Prusky et al. 2013). The activation of quiescent infection is facilitated by large gene families of CWDEs. This behavior has been observed for the endoPG families of B. cinerea (Van der Cruyssen et al. 1994; Wubben et al. 1999; Wubben et al. 2000) and glucanases in A. alternata (Eshel et al. 2002). Secretome analysis of B. cinerea-infected fruit (Shah et al. 2012) has revealed that quiescent infections of unripe fruit activate many more plant defense-related proteins than infections of ripe fruit, suggesting that impaired cellular responsiveness in ripe fruit could be a quality of decreased resistance.
Constitutive expression of PGs occurs in fungi, and this basal level of activity is important during the early phases of infection to release metabolites that in turn trigger the synthesis of inducible pg genes (Fraissinet-Tachet et al. 1995; ten Have et al. 2001). Our results show that PG enzyme had similar secretion and induction levels in the presence of CWs and exocarp in all stages of maturation. PG was also secreted by control groups from early times, meaning that PGs is constitutive. Endo and exo-polygalacturonases have long been proposed to play an important role in the fungal pathogenicity of plants by depolymerizing homogalacturonan, a major component in the CW (Walton 1994; Bellincampi et al. 2014), and they are the first enzymes to be secreted by pathogens when encountering CWs (Jones et al. 1972). Nevertheless, the role of PGs depends on the studied model (Isshiki et al. 2001). For example, F. oxysporum f. sp. lycopersici produces an array of pectinolytic enzymes during growth on pectic substrates (Roncero et al. 2000; de Sain and Rep 2015). It has been found that this array can facilitate biotrophic development without excessive maceration of infected tissue (Sherwood 1966; Prusky et al. 2013).
Our results showed that the fruit’s three stages of maturation induced PL secretion by C. acutatum, but the highest levels were observed during the semi-ripe stage. This result suggests that this enzyme plays a fundamental role in the C. acutatum-lulo interaction. PLs are primary virulence factors in various plant-pathogen interactions (Barras et al. 1994; Rogers et al. 2000; Marín-Rodríguez et al. 2002; Prusky et al. 2013; Sharma and Kuishrestha 2015; de Sain and Rep 2015). This enzyme degrades the plant cell wall, and its expression can be easily seen in the necrotrophic phase of infection. The pel-B gene encodes for pectate lyase (Yakoby et al. 2001), and the expression of this gene also induces a host defence mechanism. Crawford and Kolattukudy (1987) concluded that PL may be important since antibodies against a PL from F. solani inhibit the development of disease symptoms on pea stems.
The responses to the addition of CWs or exocarp to the culture medium were different for each enzyme, which showed how the first contact with the CW of fruit influenced the pathogen’s secretion of enzymes used for attacking the host. PG activity was similar for both carbon sources, CW or exocarp, while PL activity was higher in the presence of exocarp in all ripening stages. The treatment to obtain CW involves washing with phosphate buffer and SDS, and these steps can eliminate some proteins or lipids. Jones et al. (1972) reported a method for obtaining CW from tomato fruit using 100 or 500 mM potassium phosphate buffers at pH 7.0. They observed that the 500 mM buffer was able to extract the inducer from the exocarp. Something similar could have occurred in our case, and the inducer could have been eliminated during the process of extracting the CW. Influence from pectin changes during different maturity stages has not been ruled out (Vorwerk et al. 2004). Pectic solubilization is considered a universal feature of pectin modification, and depolymerization of pectins seems to occur in some species (Goulao et al. 2007).
Developmental conditions in the host tissue determine the outcome of the fruit-pathogen interaction. The ability of postharvest pathogens to affect the host environment by enhancing fruit ripening and/or modulating ambient pH levels is a key aspect of a complex set of characteristics of a fungus and controls its transition from quiescent to actively infecting (Prusky et al. 2001; Prusky et al. 2013).
Ambient pH is important for pathogenic fungi because it determines a pathogen’s ability to successfully colonize and invade a targeted host with the aid of secreted pathogenicity factors. Ammonium secretion by developing mycelia was detected in association with the pathogenicity of many Colletotrichum species, including C. gloeosporioides, C. acutatum, C. higginsianum, C. graminicola, and C. coccodes (Alkan et al. 2008; Diéguez-Uribeondo et al. 2008; Miyara et al. 2008; O'Connell et al. 2012). Our results showed that C. acutatum alkalized the medium from an initial pH of 5.5 to the final value of 8.5 as the incubation period advanced. This result was independent of inducer presence and maturity stage. The pH value of the culture medium affected the induction of the two pectinolytic enzymes. However, PL was preferably produced at alkaline pH, and its optimal pH value was also found in this region. PG was produced earlier when the pH was acidic, and its activity was observed during the assay until the medium pH increased to 8.0 on day 13. This behavior can be attributed to an enzyme’s wide pH tolerance range or to the presence of isoenzymes. The importance of pH in plant-pathogen interactions has been highlighted by several research groups (Yakoby et al. 2000; Prusky et al. 2001; Manteau et al. 2003; Drori et al. 2003; Aleandri et al. 2007; Prusky and Lichter 2008; Sharma and Kuishrestha 2015) whose results have shown that a pathogen can modulate its host pH, inducing tissue alkalization or acidification by ammonium concentration or organic acid production, respectively. These changes activate transcription and fungal enzyme production, thereby promoting host tissue maceration (Prusky and Yakoby 2003; Prusky et al. 2013).
Colletotrichum spp. use ammonium secretion as an invasion strategy, and the pH will therefore increase at the infection site (Yakoby et al. 2000; Prusky et al. 2001; Drori et al. 2003; Sharma and Kuishrestha 2015). A relationship has been reported between this phenomenon and the activation of enzymes related to pectin degradation for C. gloeosporioides in avocado (Yakoby et al. 2000), C. coccodes in tomato (Alkan et al. 2008; Alkan et al. 2009), C. acutatum in almond (Diéguez-Uribeondo et al. 2008) and C. musae in banana (De Costa and Chandima 2014). For C. gloeosporioides in avocado, ammonia accumulated in the infected area, wherein the pH increased from 7.5 to 8 and PL activity was optimal (Prusky et al. 2001).
In vitro assays using different carbon sources from fruit, such as CWs and exocarp, allow better approximation of the response to the interaction. The results from the present study revealed differences in PG and PL enzyme activity in assays with different CW and exocarp maturity stages in lulo fruit. PG works as a constitutive enzyme and is only induced during the semi-ripe maturity stage, while PL works as an induced enzyme during all maturity stages. It was also found that fruit CW and exocarp can affect the production of these enzymes in different ways and have no direct relationship to the pH under assay conditions. These results contribute to the knowledge of the interaction and highlight the importance of pectic enzymes in the infection of a quiescent pathogen such as C. acutatum during different stages of the ripening of the lulo fruit.
References
Agudelo-Romero P, Erban A, Rego C, Carbonell-Bejerano P, Nascimento T, Sousa L, Martínez-Zapater JM, Kopka J, Fortes AM (2015) Transcriptome and metabolome reprogramming in Vitis vinifera cv. Trincadeira berries upon infection with Botrytis cinerea. Journal of Experimental Botany 66:1769–1785
Akimitsu K, Isshiki A, Ohtani K, Yamamoto H, Eshel D, Prusky D (2004) Sugars and pH: a clue to the regulation of fungal cell wall-degrading enzymes in plants. Physiological and Molecular Plant Pathology 65:271–275
Aleandri MP, Magro P, Chilosi G (2007) Modulation of host pH during the wheat-Fusarium culmorum interaction and its influence on the production and activity of pectolytic enzymes. Plant Pathology 56:517–525
Alkan N, Fortes AM (2015) Insights into molecular and metabolic events associated with fruit response to post-harvest fungal pathogens. Frontiers in Plant Science 6:889
Alkan N, Fluhr R, Sherman A, Prusky D (2008) Role of ammonia secretion and pH modulation on pathogenicity of Colletotrichum coccodes on tomato fruit. Molecular Plant-Microbe Interactions 21:1058–1066
Alkan N, Davydov O, Sagi M, Fluhr R, Prusky D (2009) Ammonium secretion by Colletotrichum coccodes activates host NADPH oxidase activity enhancing host cell death and fungal virulence in tomato fruits. Molecular Plant-Microbe Interactions 22:1484–1491
Baroncelli R, Talhinhas P, Pensec F, Sukno SA, Le Floch G, Thon MR (2017) The Colletotrichum acutatum species complex as a model system to study evolution and host specialization in plant pathogens. Frontiers in Microbiology 8:2001
Barras F, Van Gijsegem F, Chatterjee AK (1994) Extracellular enzymes and pathogenesis of soft-rot Erwinia. Annual Review of Phytopathology 32:201–234
Bellincampi D, Cervone F, Lionetti V (2014) Plant cell wall dynamics and wall-related susceptibility in plant-pathogen interactions. Frontiers in Plant Science 5:1–8
Blanco-Ulate B, Morales-Cruz A, Amrine KC, Labavitch JM, Powell AL, Cantu D (2014) Genome-wide transcriptional profiling of Botrytis cinerea genes targeting plant cell walls during infections of different hosts. Frontiers in Plant Science 5:435
Brühlmann F (1995) Production and characterization of an extracellular pectate lyase from an Amycolata sp. Applied and Environmental Microbiology 61:3580–3585
Bugbee WM (1990) Purification and characteristics of pectin lyase from Rhizoctonia solani. Physiological and Molecular Plant Pathology 36:15–25
Caicedo O, Higuera BL (2007) Inducción de polifenoloxidasa en frutos de lulo (Solanum quitoense) como respuesta a la infección con Colletotrichum acutatum. Acta Biológica Colombiana 12:41–54
Cannon PF, Damm U, Johnston PR, Weir BS (2012) Colletotrichum: current status and future directions. Studies in Mycology 73:181–213
Cantu D, Vicente AR, Greve LC, Dewey FM, Bennett AB, Labavitch JM, Powell ALT (2008a) The intersection between cell wall disassembly, ripening, and fruit susceptibility to Botrytis cinerea. Proceedings of the National Academy of Sciences, USA 105:859–864
Cantu D, Vicente AR, Labavitch JM, Bennett AB, Powell AL (2008b) Strangers in the matrix: plant cell walls and pathogen susceptibility. Trends in Plant Science 13:610–617
Cerón GS (2005) Detección de Colletotrichum (Penz) Sacc en estructuras vegetativas y reproductivas de Lulo Solanum quitoense Lam. M.Sc. Thesis. Universidad Nacional de Colombia, Bogotá
Collmer A, Ried JL, Mount MS (1988) Assay methods for pectic enzymes. Methods in Enzymology 161:329–335
Crawford MS, Kolattukudy PE (1987) Pectate lyase from Fusarium solani f. sp. pisi: purification, characterization, in vitro translation of the mRNA, and involvement in pathogenicity. Archives of Biochemistry and Biophysics 258:196–205
De Costa DM, Chandima AAG (2014) Effect of exogenous pH on development and growth of Colletotrichum musae and development of anthracnose in different banana cultivars in Sri Lanka. Journal of the National Science Foundation of Sri Lanka 42:229–240
De Lorenzo G, Ferrari S (2002) Polyalacturonase-inhibiting proteins in defense against phytopathogenic fungi. Current Opinion in Plant Biology 5:1–5
De Silva DD, Crous PW, Ades PK, Hyde KD, Taylor PW (2017) Life styles of Colletotrichum species and implications for plant biosecurity. Fungal Biology Reviews 31:155–168
Diéguez-Uribeondo J, Förster H, Adaskaveg JE (2008) Visualization of localized pathogen-induced pH modulation in almond tissues infected by Colletotrichum acutatum using confocal scanning laser microscopy. Phytopathology 98:1171–1178
Drori N, Kramer-Haimovich H, Rollins J, Dinoor A, Okon Y, Pines O, Prusky D (2003) External pH and nitrogen source affect secretion of pectate lyase by Colletotrichum gloeosporioides. Applied and Environmental Microbiology 69:3258–3262
Dubey AK, Yadav S, Kumar M, An G, Yadav D (2016) Molecular biology of microbial pectate lyase: a review. British Biotechnology Journal 13:1–26
Eshel D, Lichter A, Dinoor A, Prusky D (2002) Characterization of Alternaria alternata glucanase genes expressed during infection of resistant and susceptible persimmon fruits. Molecular Plant Pathology 3:347–358
Fernández-Acero FJ, Colby T, Harzen A, Carbú M, Wieneke U, Cantoral JM, Schmidt J (2010) 2-DE proteomic approach to the Botrytis cinerea secretome induced with different carbon sources and plant-based elicitors. Proteomics 10:2270–2280
Fisk CL, McDaniel MR, Strik BC, Zhao Y (2006) Physicochemical, sensory, and nutritive qualities of hardy kiwifruit (Actinidia arguta) ‘Ananasnaya’ as affected by harvest maturity and storage. Journal of Food Science 71:S204–S210
Forero DP, Carriazo JG, Osorio C (2016) Effect of different drying methods on morphological, thermal, and biofunctional properties of lulo (Solanum quitoense Lam.) fruit powders. Drying Technology 34:1085–1094
Fraissinet-Tachet L, Reymond-Cotton P, Fèvre M (1995) Characterization of a multigene family encoding an endopolygalacturonase in Sclerotinia sclerotiorum. Current Genetics 29:96–99
Gan P, Narusaka M, Kumakura N, Tsushima A, Takano Y, Narusaka Y, Shirasu K (2016) Genus-wide comparative genome analyses of Colletotrichum species reveal specific gene family losses and gains adaptation to specific infection lifestyles. Genome Biology and Evolution 8:1467–1481
Gómez M, Lajolo F, Cordenunsi B (2002) Evolution of soluble sugars during ripening of papaya fruit and its relation to sweet taste. Journal of Food Science 67:442–447
Gómez-García L, Martínez ST (2005) Induction of two pectolitic enzymes during the model Fusarium oxysporum f.sp. Dianthi-carnation. Revista Colombiana de Química 34:25–34
González Loaiza DI, Ordóñez Santos LE, Vanegas Mahecha P, Vásquez Amariles HD (2014) Changes in physicochemical properties of the fruit of lulo (Solanum quitoense Lam.) harvested at three degrees of maturity. Acta Agronómica 63:11–17
Goulao LF, Santos J, de Sousa I, Oliveira CM (2007) Patterns of enzymatic activity of cell wall-modifying enzymes during growth and ripening of apples. Postharvest Biology and Technology 43:307–318
Guidarelli M, Carbone F, Mourgues F, Perrotta G, Rosati C, Bertolini P, Baraldi E (2011) Colletotrichum acutatum interactions with unripe and ripe strawberry fruits and differential responses at histological and transcriptional levels. Plant Pathology 60:685–697
ten Have A, Breuil WO, Wubben JP, Visser J, van Kan JA (2001) Botrytis cinerea endopolygalacturonase genes are differentially expressed in various plant tissues. Fungal Genetics and Biology 33:97–105
Hunter RE (1974) Inactivation of pectic enzymes by polyphenols in cotton seedlings of different ages infected with Rhizoctonia solani. Physiological Plant Pathology 4:151–159
Isshiki A, Akimitsu K, Yamamoto M, Yamamoto H (2001) Endopolygalacturonase is essential for citrus black rot caused by Alternaria citri but not brown spot caused by Alternaria alternata. Molecular Plant-Microbe Interactions 14:749–757
Jayani RS, Saxena S, Gupta R (2005) Microbial pectinolytic enzymes: a review. Process Biochemistry 40:2931–2944
Jones TM, Anderson AJ, Albersheim P (1972) Host-pathogen interactions. Physiological Plant Pathology 2:153–166
Jurick WM, Janisiewicz WJ, Saftner RA, Vico I, Gaskins VL, Park E, Forsline P, Fazio G, Conway WS (2011) Identification of wild apple germplasm (Malus spp.) accessions with resistance to the postharvest decay pathogens Penicillium expansum and Colletotrichum acutatum. Plant Breeding 130:481–486
Kramer-Haimovich H, Servi E, Katan T, Rollins J, Okon Y, Prusky D (2006) Effect of ammonia production by Colletotrichum gloeosporioides on pelB activation, pectate lyase secretion and fruit pathogenicity. Applied and Environmental Microbiology 72:1034–1039
Kubicek CP, Starr TL, Glass NL (2014) Plant cell wall-degrading enzymes and their secretion in plant pathogenic fungi. Annual Review of Phytopathology 52:427–451
Lara-Márquez A, Oyama K, Zavala-Páramo MG, Villa-Rivera MG, Conejo-Saucedo U, Cano-Camacho H (2017) Evolutionary analysis of pectin lyases of the genus Colletotrichum. Journal of Molecular Evolution 85:120–136
Lo Presti L, Lanver D, Schweizer G, Tanaka S, Liang L, Tollot M, Zuccaro A, Reissmann S, Kahmann R (2015) Fungal effectors and plant susceptibility. Annual Review of Plant Biology 66:513–545
Lyu X, Shen C, Fu Y, Xie J, Jiang D, Li G, Cheng J (2015) Comparative genomic and transcriptional analyses of the carbohydrate-active enzymes and secretomes of phytopathogenic fungi reveal their significant roles during infection and development. Scientific Reports 5:15565
Manteau S, Abouna S, Lambert B, Legendre L (2003) Differential regulation by ambient pH of putative virulence factor secretion by the phytopathogenic fungus Botrytis cinerea. FEMS Microbiology Ecology 43:359–366
Marín-Rodríguez MC, Orchard J, Seymour GB (2002) Pectate lyases, cell wall degradation and fruit softening. Journal of Experimental Botany 53:2115–2119
Martínez ST, Martínez P, González VC, Higuera BL (2012) Determinación in vitro de los patrones de inducción de una endoxilanasa (EC 3.2.1.8) secretada por Fusarium oxysporum f. sp. dianthi. Purificación y caracterización parcial. Revista Colombiana de Química 41:359–376
Mejía CM, Gaviria DA, Duque AL, Rengifo RM, Aguilar EF, Hernán AA (2012) Physicochemical characterization of the lulo (Solanum quitoense Lam.) castilla variety in six ripening stages. Vitae 19:157–165
Mengiste T (2012) Plant immunity to necrotrophs. Annual Review of Phytopathology 50:267–294
Miles TD, Day B, Schilder AC (2011) Identification of differentially expressed genes in a resistant versus a susceptible blueberry cultivar after infection by Colletotrichum acutatum. Molecular Plant Pathology 12:463–477
Miles TD, Hancock JF, Callow P, Schilder AMC (2012) Evaluation of screening methods and fruit composition in relation to anthracnose fruit rot resistance in blueberries. Plant Pathology 61:555–566
Miyara I, Shafran H, Haimovich HK, Rollins J, Sherman A, Prusky D (2008) Multi-factor regulation of pectate lyase secretion by Colletotrichum gloeosporioides pathogenic on avocado fruits. Molecular Plant Pathology 9:281–291
Nelson N (1944) A photometric adaptation of the Somogyi method for determination of glucose. Journal of Biological Chemistry 153:378–380
NTC 4592 (1999) Instituto Colombiano de Normas Técnicas. Productos de Frutas y Verduras. Determinación del pH. Icontec, Bogotá
NTC 4623 (1999) Instituto Colombiano de Normas Técnicas. Productos de Frutas y Verduras. Determinación de la acidez titulable. Icontec, Bogotá
NTC 4624 (1999) Instituto Colombiano de Normas Técnicas. Jugos de Frutas y Hortalizas. Determinación del contenido de sólidos solubles. Método refractométrico. Icontec, Bogotá
Ochoa J, Clements C, Barrera V, Dominguez JM, Ellis MA, Alwang J (2016) IPM packages for naranjilla: sustainable production in an environmentally fragile region. In: Muniappan R, Heinrichs EA (eds) Integrated pest management of tropical vegetable crops. Springer Netherlands, Amsterdam, pp 209–221
O'Connell RJ, Thon MR, Hacquard S, Amyotte SG, Kleemann J, Torres MF, Damm U, Buiate EA, Epstein L, Alkan N, Altmüller J, Alvarado-Balderrama L, Bauser CA, Becker C, Birren BW, Chen Z, Choi J, Crouch JA, Duvick JP, Farman MA, Gan P, Heiman D, Henrissat B, Howard RJ, Kabbage M, Koch C, Kracher B, Kubo Y, Law AD, Lebrun M-H, Lee Y-H, Miyara I, Moore N, Neumann U, Nordström K, Panaccione DG, Panstruga R, Place M, Proctor RH, Prusky D, Rech G, Reinhardt R, Rollins JA, Rounsley S, Schardl CL, Schwartz DC, Shenoy N, Shirasu K, Sikhakolli UR, Stüber K, Sukno SA, Sweigard JA, Takano Y, Takahara H, Trail F, van der Does HC, Voll LM, Will I, Young S, Zeng Q, Zhang J, Zhou S, Dickman MB, Schulze-Lefert P, Ver Loren van Themaat E, Ma L-J, Vaillancourt LJ (2012) Lifestyle transitions in plant pathogenic Colletotrichum fungi deciphered by genome and transcriptome analyses. Nature Genetics 44:1060–1065
Perfect SE, Hughes HB, O'Connell RJ, Green JR (1999) Colletotrichum: a model genus for studies on pathology and fungal-plant interactions. Fungal Genetics and Biology 27:186–198
Prasanna V, Prabha TN, Tharanathan RN (2007) Fruit ripening phenomena an overview. Critical Reviews in Food Science and Nutrition 47:19
Prusky D, Lichter A (2008) Mechanisms modulating fungal attack in post-harvest pathogen interactions and their control. European Journal of Plant Pathology 121:281–289
Prusky D, Yakoby N (2003) Pathogenic fungi: leading or led by ambient pH ? Molecular Plant Pathology 4:509–516
Prusky D, Kobiler I, Yakoby B (1988) Involvement of epicatechin in cultivar susceptibility of avocado fruits to Colletotrichum gloeosporioides after harvest. Phytopathology 123:140–146
Prusky D, McEvoy JL, Leverentz B, Conway WS (2001) Local modulation of host pH by Colletotrichum species as a mechanism to increase virulence. Molecular Plant-Microbe Interactions 14:1105–1113
Prusky D, Alkan N, Mengiste T, Fluhr R (2013) Quiescent and necrotrophic lifestyle choise during postharvest disease. Annual Review of Phytopathology 51:155–176
Prusky D, Barad S, Ment D, Bi F (2016) The pH modulation by fungal secreted molecules: a mechanism affecting pathogenicity by postharvest pathogens. Israel Journal of Plant Sciences 63:22–30
Ramos AM, Gally M, García MC, Levin L (2010) Pectinolytic enzyme production by Colletotrichum truncatum, causal agent of soybean anthracnose. Revista Iberoamericana de Micología 27:186–190
Ramos AM, Gally M, Szapiro G, Itzcovich T, Carabajal M, Levin L (2016) In vitro growth and cell wall degrading enzyme production by Argentinean isolates of Macrophomina phaseolina, the causative agent of charcoal rot in corn. Revista Argentina de Microbiología 48:267–273
Reignault P, Valette-Collet O, Boccara M (2008) The importance of fungal pectinolytic enzymes in plant invasion, host adaptability and symptom type. European Journal of Plant Pathology 120:1–11
Rodríguez JM, Restrepo LP (2011) Activity of pectic enzymes involved in the ripening process of lulo (Solanum quitoense Lam.) Agronomía Colombiana 29:63–71
Rogers LM, Kim Y, Guo W, Gonzales-Candelas L, Li D, Kolattukudy PE (2000) Requirement of either a host- or pectin- induced pectate lyase for infection of Pisum sativum by Nectria hematococca. Proceedings of the National Academy of Sciences, USA 97:9813–9818
Roncero MI, Di Pietro A, Ruiz-Roldán MC, Huertas-González MD, Garcia-Maceira FI, Méglecz E, Jiménez A, Caracuel Z, Sancho-Zapatero R, Hera C, Gomez-Gomez E, Ruiz-Rubio M, Cl G-V, Páez MJ (2000) Role of cell wall-degrading enzymes in pathogenicity of Fusarium oxysporum. Revista Iberoamericana de Micología 17:S47–S53
de Sain M, Rep M (2015) The role of pathogen-secreted proteins in fungal vascular wilt diseases. International Journal of Molecular Sciences 16:23970–23993
Sezer A, Dolar FS (2012) Colletotrichum acutatum, a new pathogen of hazelnut. Journal of Phytopathology 160:428–430
Shah P, Powell ALT, Orlando R, Bergmann C, Gutierrez-Sanchez G (2012) Proteomic analysis of ripening tomato fruit infected by Botrytis cinerea. Journal of Proteome Research 11:2178–2192
Sharma M, Kuishrestha S (2015) Colletotrichum gloeosporioides: an anthracnose causing pathogen of fruits and vegetables. Biosciences, Biotechnology Research Asia 12:1233–1246
Sherwood RT (1966) Pectin lyase and polygalacturonase production by Rhizoctonia solani and other fungi. Phytopathology 56:279–286
Shih J, Wei Y, Goodwin P (2000) A comparison of the pectate lyase genes, pel-1 and pel-2, of Colletotrichum gloeosporioides f. sp. malvae and the relationship between their expression in culture and during necrotrophic infection. Gene 243:139–150
Somogyi M (1952) Notes on sugar determination. Journal of Biological Chemistry 195:19–23
Talhinhas P, Mota-Capitão C, Martins S, Ramos AP, Neves-Martins J, Guerra-Guimarães L, Várzea V, Silva MC, Sreenivasaprasad S, Oliveira H (2011) Epidemiology, histopathology and aetiology of olive anthracnose caused by Colletotrichum acutatum and C. gloeosporioides in Portugal. Plant Pathology 60:483–495
Thrower LB (1966) Terminology for plant parasites. Journal of Phytopathology 56:258–259
Timmer LW, Peres NA (2015) Where have all the flowers gone? Postbloom fruit drop of citrus in the Americas. Journal of Citrus Pathology 2:1–6
Underwood W (2012) The plant cell wall: a dynamic barrier against pathogen invasion. Frontiers in Plant Science 3:85
Van der Cruyssen G, de Meester E, Kamoen O (1994) Expression of polygalacturonase of Botrytis cinerea in vitro and in vivo. Mededelingen-Faculteit Landbouwkundige en Toegepaste Biologische Wetenschappen Universiteit Gent 59:895–905
Vorwerk S, Somerville S, Somerville C (2004) The role of plant cell wall polysaccharide composition in disease resistance. Trends in Plant Science 9:203–209
Walton JD (1994) Deconstructing the wall cell. Plant Physiology 104:1113–1118
Wattad C, Dinoor A, Prusky D (1994) Purification of pectate lyase produced by Colletotrichum gloeosporioides and its inhibition by epicatechin: a possible factor involved in the resistance of unripe avocado fruits to anthracnose. Molecular Plant-Microbe Interactions 7:293–297
Wei YD, Byer KN, Goodwin PH (1997) Hemibiotrophic infection of round-leaved mallow by Colletotrichum gloeosporioides f. sp. malvae in relation to leaf senescence and reducing reagents. Mycological Research 101:357–364
Wharton PS, Diéguez-Uribeondo J (2004) The biology or Colletotrichum acutatum. Anales del Jardin Botánico de Madrid 61:3–22
Wubben JP, Mulder W, ten Have A, Van Kan JAL, Visser J (1999) Cloning and partial characterization of endopolygalacturonase genes from Botrytis cinerea. Applied and Environmental Microbiology 65:1596–1602
Wubben JP, ten Have A, van Kan JAL, Visser J (2000) Regulation of endopolygalacturonase gene expression in Botrytis cinerea by galacturonic acid, ambient pH and carbon catabolite repression. Current Genetics 37:152–157
Yadav S, Yadav PK, Yadav D, Yadav KDS (2009) Pectin lyase: a review. Process Biochemistry 44:1–10
Yakoby N, Kobilier I, Dinoor A, Prusky D (2000) pH regulation of pectate lyase secretion modulates the attack of Colletotrichum gloeosporioides on avocado fruits. Applied and Environmental Microbiology 66:1026–1030
Yakoby N, Beno-Moualem D, Keen N, Dinoor A, Pines O, Prusky D (2001) Colletotrichum gloeosporioides pelB is an important virulence factor in avocado fruit-fungus interaction. Molecular Plant-Microbe Interactions 14:988–995
Yarullina LG, Akhatova AR, Kasimova RI (2016) Hydrolytic enzymes and their proteinaceous inhibitors in regulation of plant–pathogen interactions. Russian Journal of Plant Physiology 63:193–203
Zhang J, Bruton BD, Biles CL (2014) Cell wall-degrading enzymes of Didymella bryoniae in relation to fungal growth and virulence in cantaloupe fruit. European Journal of Plant Pathology 139:749–761
Acknowledgements
This research was supported by DIB, Universidad Nacional de Colombia, 8003173, Quipu 201010010541.
Author information
Authors and Affiliations
Corresponding author
Additional information
Section Editor: Leandro Dallagnol
Rights and permissions
About this article
Cite this article
Martínez-González, A.P., Higuera-Mancipe, B.L. & Martínez-Peralta, S.T. The influence of lulo (Solanum quitoense Lam) fruit maturity stage on polygalacturonase and pectate lyase secretion by Colletotrichum acutatum. Trop. plant pathol. 43, 218–229 (2018). https://doi.org/10.1007/s40858-017-0209-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40858-017-0209-6


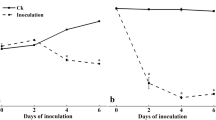




 ), lulo fruit cell wall inducer during semi-ripe stage (
), lulo fruit cell wall inducer during semi-ripe stage ( ) and control (
) and control ( )
)