Abstract
This work represents the results from the experimental investigation conducted on the utilization of ferrochrome slag (FCS) as a replacement of Portland cement (PC) in cement composites. A total of seven mix compositions were made and the corresponding performance of the mix were evaluated in terms of its strength, durability and microstructural properties. Results from this study showed that the incorporation of FCS as partial replacement of the PC resulted in an increase in the set times and a decrease in the water content for normal consistency. The compressive strength of the mortar mixes was decreased with incorporation of FCS into the mortar mixtures. However, mortar mixtures up to 30% of PC replacement with FCS exhibited compressive strength higher than 30 MPa. In terms of durability, 30% FCS replacement resulted in 18% reduction in water absorption along with lower loss in the compressive strength and improved resistance to acid attack. Microstructural investigation showed that the use of FCS as a replacement to PC resulted in an increment in the formation of calcium silicate hydrate which results in a compact and densified microstructure of the mortars. Further, to ensure the environmental soundness of FCS mortars, a leaching test was conducted for the first 14 days of curing, and it was found that the Cr leaching values are under the permissible limits and can be used in general construction applications.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The increasing demand and production of Portland cement (PC) for the manufacturing of various cementitious composites has resulted in an imminent need to search other alternatives to replace the PC. The need to replace PC is due to its high consumption of natural resources coupled with the high carbon dioxide emission during its production [1, 2]. Extensive research and development over the years have resulted in the recycling of various waste products as a partial substitute to PC in cementitious composites [3,4,5,6]. Waste products such as fly ash [7,8,9], glass powder [10, 11], slag [12, 13], rice husk ash [14,15,16], coconut shell ash [17], sawdust ash [18] etc., have been recycled successfully into cementitious composites as supplementary cementitious materials (SCMs). In addition to the reduction in carbon dioxide emissions and conservation of the environment, with the use of these wastes materials, there is a reduction in the overall cost of cementitious materials [19]. It is further noticed that the incorporation of such wastes into cementitious composites help enhancing the durability properties due to their pore filling effects and pozzolanic reactions [20]. The materials used as a replacement of PC are referred to as SCMs and can be used as high as 80% replacement of PC when water is used as the mixing solution. In certain types of composites where alkali activators are used, these SCMs can be used as a complete replacement of PC [5, 21,22,23]. However, more focus is now placed on the possible use of these SCMs as replacement of PC as the conventional mixing methods can still be used and the production process is safer due to the non-use of corrosive solutions as activator. Though the use of SCMs such as fly ash and blast furnace slag has been extensive studied and used for various applications [10, 24], there is limited study on the possible use of ferrochrome slag (FCS) as possible replacement of PC in cementitious composites. As the name implies, FCS is the waste product of ferrochrome production in steel industries. The production of any amount of Ferrochrome has been found to produce an equivalent or even a higher amount of FCS [25]. The current annual production of FCS as waste is estimated to be 15 million tonnes, South Africa, Kazakhstan and India are the major producers of ferrochrome and thereby these countries generates a significant proportion of the global FCS production [26]. Majority of these FCS dumped in landfills where they occupy valuable land space and contaminate their immediate environment. Therefore, like other industrial wastes; finding ways to incorporate FCS into cementitious composites will be an effective way to utilize these wastes. Most studies on the use of FCS has utilized it as a replacement of the conventional natural aggregates in cementitious concrete composites [27,28,29,30]. Al-Jabri et al. [30] used FCS to replace natural fine aggregate up to 20% in mortar mixtures. The study showed that the use of FCS into the mixtures resulted in 35% and 42% improvement in the mechanical and shrinkage resistance respectively, of the mortar mixtures. However, considering the highest embodied carbon and energy values of PC in synthesis of cementitious composites, incorporation of FCS as replacement of PC will result in a significant reduction in both the carbon footprint and embodied energy of cementitious composites. However, as the chemical composition of the FCS differs from other types of slag, it is imperative to investigate how the incorporation of FCS as binder constituent in cementitious composites will affect the corresponding properties of the composites. Therefore, this preliminary study is part of an extensive study undertaken to evaluate how FCS the performance of cementitious composites. In this study, seven mortar mixtures with varying content of FCS up to 30% replacement of PC were made and the corresponding mechanical, microstructural and durability properties were evaluated.
Experimental
Materials
Portland cement (PC) supplied by M/s Mahashakti Cement was taken as the main binder for all the mix compositions evaluated in this study. FCS was obtained from FACOR, Odisha, India, and further it has been beneficiated to extract the metal contents for economic benefits. The obtained FCS was pulverized and sieved. Only the FCS particles less than 75 µm were taken in this investigation to replace the PC.
Mixture Design
The detailed mixture composition of the mortar mixtures investigated in this investigation is provided in Table 1. The compositions are presented in terms of the mass ratio to PC. The ratio (by wt%) of PC to fine aggregate was kept at 1:3 and FCS used to replace PC up to 30%. (by wt%) The number in the mixture ID signifies the amount (in %) of PC replaced with FCS. However, for the fresh property’s evaluation, only the paste (i.e. binder and water only) was evaluated.
Sample Preparation and Curing
The mortar mixtures presented in Table 1 were prepared by mixing the binder part (PC and FCS) and the fine aggregate as per IS 4031-6 [31]. The PC and FCS were used as binder component while the natural river sand was as fine aggregate. At first the dry mixing was done of all the materials followed by the addition of water and mixed thoroughly in order to obtain a homogeneous mixture. The mixture was then poured into steel moulds and vibrated for two minutes for optimum compaction. After the casting process, the cubes were demoulded approximately after 24 h and cured in water until the day of testing.
Test Methods
Fresh Properties
For the fresh properties, the evaluation was carried out only on the binder component (i.e., no aggregates). The setting times of the binder pastes were evaluated with a Vicat needle as per the standard test procedures of IS 4031-5 [32]. The amount of water required to achieve a normal consistency of the binder pastes was evaluated as per the standard test protocols of IS 4031-4 [33].
Hardened Properties
Compressive Strength
To evaluate the compressive strength of the PC mortar mixtures along with FCS in different percentage, the Indian standard IS 4031-6 [31] is followed. A compressive testing machine of capacity 2000 kN (HEICO) and mortar cubes of 70.6 mm were used for the evaluation. For each mixture, a total number of three samples were tested and the average of the three sample’s compressive strength result were presented.
Water Absorption
The water absorption of the mortars specimens were estimated as per the standard test procedures mentioned in ASTM C 642 [34]. The mortar samples were oven dried at a temperature of 110 °C for approximately 24 h and then allowed to cool down. After the samples have been cooled down, the dry mass of the samples was taken followed by the complete immersion of the samples in water with approximately 21 °C for 48 h. After 48 h of immersion, the samples were taken out of water and surface dry to remove the excess water on the samples. The mass of the saturated surface dry was measured as the wet mass, and the absorption of each mixture calculated using Eq. 1. For each mix composition, a total of three samples were used for the water absorption evaluation.
Acid Resistance
The acid resistance of the mortar mixtures was assessed by first curing the samples in water for 28 days after which it was immersed in a 2% H2SO4 solution for 28 days. After 28 days of immersion of the taken specimens in the acidic solution, the acid resistance of the samples was evaluated in terms of the visual appearance, loss in compressive strength and loss in mass. For each mix composition, triplicate of each mixture was used and the results represented for both the loss in compressive strength and mass signify the average obtained from three samples of the same mix.
Microstructural Properties
A scanning electron microscope (SEM) was used to study both the morphology of the FCS and the microstructure of some of the mixtures. SEM was used to study the microstructural properties of mortar samples after 90 days of curing of samples from some of the mixtures. The hardened mortar samples were cut in sections, mounted using a polymer based resin and polished using silicon carbide papers before analysis, and FCS was analysed as powder. A Zeiss SUPRA 55-VP was used for SEM observations. X-ray powder diffraction (XRD) was carried out (Rigaku-Ultima-IV) on the FCS and mortars to identify the compounds present in the FCS and its corresponding composites. Electron probe microanalysis was also carried out on the FCS in order to have more understanding on the chemical composition of FCS.
Leaching Study of Cr from FCS Powder and FCS Based Cement Concrete (CC)
The Cr leaching from both the FCS used in the experimentation and the FCS-based CC were checked through a leaching test. Distilled water with a pH of 6.5 is used for the leaching procedure and a solid to liquid ratio of 1:10 is mainlined throughout the experiments. At desired time period, the leachates were collected and filtered using 42 No., 15 cm filter paper for further analysis. An Inductive Couple Plasma Optical Emission Spectroscopy (ICP-OES) was used to detect the leached chromium from the leachate samples.
Results and Discussion
Raw Material Characterization
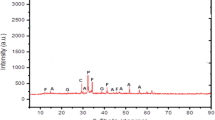
Table 2 and Fig. 1 presents the chemical composition and size distribution, respectively of the PC and FCS. Figure 2 presents the X-ray powder diffraction (XRD) spectrum of the FCS. It can be seen from Fig. 2 that the main mineral phases present in FCS are Spinel (MgAl2O4) and Forsterite (Mg2SiO4). The JCPDS file number of MgAl2O4 and Mg2SiO4 are 01-082-2424 and 01-085-136, respectively. The corresponding mineral compounds identified on diffractogram quite match with its chemical composition reported in Table 2, which is mainly composed of Mg, Al and Si oxides. In addition, the diffractogram exhibited a small hump between 15 and 35 2 theta range corresponding to the glassy or reactive phases of Si, Al and Mg contained in FCS powder. In the literature, the amorphous phase leads to the improvement of reactivity and strength of cementitious materials. Hence, partial replacement of Portland cement by FCS would provide high strength by modifying the interfacial zone (between binder phase and aggregates) leading to the formation of a less permeable cement matrix [35]. The fine aggregates used have a specific gravity and maximum aggregate size of 2.24 and 2 mm, respectively. All mixtures were mixed with potable water that is free from impurities.
Electron probe microanalysis was conducted to determine the chemical composition of different elements present in FCS as shown in Fig. 3. Figure 3 indicates that the major elements present in the slag matrix are Si, Mg and Al and however it contains a low amount of Fe, Cr along with a small amount of Ca. The elements were visible in different colours in Fig. 3 with the magnifying intensity of 50 µm and while the metallic content of iron and chromium were dispersed in a very low amount on the slag surface. Correlations between elements in the slag on the surface were examined using elemental mapping and finalized that silicon, magnesium and aluminum are spread frequently part of the FCS slag surface. The major oxides (Si, Mg and Al) that are in amorphous phase in FCS when incorporated in Portland mortars could be expected to react with CaO (main component) from Portland cement in presence of water to form C–S–H, C–(A)–S–H and Mg(OH)2/M–S–H type binder phases through pozzolanic reaction by reinforcing the structure of the mortar with time [36,37,38].
Fresh Properties
As mentioned earlier, the fresh properties were evaluated only on the paste (i.e. binder and water). Setting time and consistency are some of the major properties that are related to the practical mixing and utilization of cementitious composites. Setting times of cementitious composites is an indication of the practical time available for mixing, transporting and placing of the fresh mixtures. The setting times can be classified into two namely the initial and final setting time. The setting times of the mortar mixtures evaluated in this study are presented in Fig. 4. It can be seen from Fig. 4 that the setting times (i.e. initial and final) of the mixtures increased with a higher content of FCS. The initial and final setting time of mortar mixtures incorporating 30% of FCS as partial replacement of PC is 42% and 16% respectively, higher than the mixture made with only PC as the binder. The increase in the setting times of the mixtures with increasing FCS content can be associated with their lower calcium oxide content which resulted in lower strength gain. The dilution effect of the FCS on the PC will also result in lower availability of calcium hydroxide in the matrix resulting in lower strength gain. The initial setting times of all mixtures evaluated in this study was greater than 30 min satisfying the requirement of Indian Standards.
Figure 5 presents the water to binder (i.e., PC and FCS) requirement for normal consistency. It can be seen from Fig. 5 that the water to the binder for normal consistency decreases with increasing content of FCS as partial replacement of PC in mortar mixtures. The water to binder requirement to achieve normal consistency for 0FCS, 10FCS, 20FCS and 30FCS is 0.30, 0.28, 0.27 and 0.26, respectively. The reduction in the water to binder requirement to achieve a normal consistency with the introduction of FCS can be attributed to its low water demand due to the absence of hydraulic phases. This observation is similar to that of Rahman et al. [39] where ferronickel slag was used to replace PC.
Hardened Properties
Compressive Strength
Figure 6 depicts the compressive strength results of the seven mortar mixes studied. The compressive strength of all mixes increased with age regardless of the binder concentration as seen in the graph. The increasing compressive strength can be associated with the progression of the hydration reaction and the formation of a corresponding product with time. It can also be seen from the figure that the compressive strength of the mixtures decreased with a higher content of FCS. The decrease in the compressive strength at an early age (i.e., 7 days) is more significant compared to that at a later age (i.e., 28 days). The compressive strength of 30FCS at 7 days and 28 days is 63% and 31% lower than that of 0FCS. The decrease in strength of the mixtures incorporating FCS can be attributed to the dilution effect of the PC and the lower calcium oxide content in FCS. Nonetheless, as all mixtures exhibited a high compressive strength at 28 days (i.e., greater than 25 MPa), they can be used for a variety of structural and non-structural purposes as per Indian standard requirements [40].
Water Absorption
The tendency of cementitious composites to absorb water is a strong indicator of their overall durability. Most deleterious ions that deteriorate cementitious composites use water as a pathway to penetrate the composite. Therefore, the higher the water absorption value of cementitious composites, the more susceptible they are to these deleterious ions. The water absorption of the mix compositions was evaluated in this study is presented in Fig. 7. It can be observed from the figure that the use of FCS as partial replacement of PC in mortar mix compositions resulted in a decrease in the water absorption. The water absorption of mortar mixtures with 5%, 10%, 15%, 20%, 25% and 30% FCS as replacement of PC is 10%, 9%, 26%, 42%, 17% and 18% lower than the mortars made with 100% PC as the binder. However, the optimum content of FCS for water absorption reduction was found to be 20%. The reduction in the water absorption of the mixtures with the addition of FCS can be attributed to the pore filling effect of the FCS which results in a compact and more densified microstructure of the mortars.
Acid Attack Resistance
The acid resistance of the mixtures was evaluated in terms of the visual appearance, change in mass and loss in compressive strength. The pictures of some of the samples before and after immersion in the acidic solution is presented in Fig. 8. It can be seen from Fig. 8 that samples with and without FCS undergoes some form of physical deterioration. However, the physical deterioration is lower in mortar samples incorporating FCS. Mortar samples incorporating FCS as a 30% replacement of PC exhibited the best resistance to the physical deterioration due to the acid attack. Figure 9 presents the loss in mass of the samples due to acid attack. In agreement with the physical deterioration observed on the samples, the loss in mass of the samples reduced with a higher content of FCS. The loss in mass of 10FCS, 20FCS and 30FCS is 24%, 62% and 76% lower than the mortar samples made with only PC as the binder. The higher acid attack resistance of mixtures incorporating FCS can be attributed to low Ca/Si ratio due to replacement of Portland cement mortar which limited the formation portlandite (Ca(OH)2) compared to that of the mixture with only PC as the binder [41]. Hence the breaking down of Si–O–Si, and Ca–Si belonging to the gel network (responsible to good cohesion between commentating phase and aggregates justifying the losses recorded in weight and compressive strength) would be more pronounced in 0FCS sample [42]. Similarly, it can be seen from Fig. 10 that the loss in strength of mortars incorporating FCS is lower. The loss in compressive strength of mixtures made with 5%, 15%, 25% and 30% FCS as partial replacement of PC is 19%, 55%, 74% and 78% lower than the mortar mixture made with only PC as the binder (i.e. 0FCS).
Microstructural Properties
The scanning electron microscope (SEM) image of 0FCS and 20FCS is presented in Figs. 11 and 12 respectively. It can be seen that the micrograph of Portland cement without FCS appears almost homogeneous compact and dense (Fig. 11). This results from an important production of the main binder phase C–S–H which embed sand particles in the matrix justifying the high strength achieved at early as well as old ages. In addition, there is not observed the interfacial transition zone due to a lack of binder phase required to develop a strong matrix. This suggests that the water to binder ratio was beneficial for the binder phase development and quite matches the trend of mechanical strength and microstructure. When 20 wt% of FCS is added the macrograph is similar to that of the specimen with only PC (Fig. 12). However, the presence of angular particles in the matrix is attributed to unreacted FCS particles. When FCS is incorporated into Portland mortars it could improve the pozzolanic reaction resulting from the reaction between calcium oxides with silicon, magnesium and aluminum oxides (contained in FCS) leading to the additional M–S–H/Mg(OH)2 type, C–S–H and C–(A)–S–H binder phases which are responsible for the strength development achieved in all samples at 28 days [38, 43, 44]. These new-formed binding phases contributed to densify the microstructure and allowed the reduction in water absorption explained earlier. Even though from 5 to 20 wt% the compressive increased with curing time (from 3 to 28 days) and remain almost constant. The presence of pores in the matrix (Fig. 10) allowed the penetration of acid in the matrix. Thus the presence of binder phases such as C–S–H, C–A–S–H, Ca(OH)2 and M–S–H/Mg(OH)2 in contact of acidic attack would induce the acid–base reaction leading to breaking down of Ca–O, Mg–O, Al–Si–O and Si–O–Si chemical bonds resulting in loss of weight and compressive most observed in Portland mortars containing FCS due to higher amount of portlandite (Ca(OH)2) which could not well resist compared to others replacing by FCS at different contents (5, 10, 15, 20, 25 and 30 wt%) [42]. These additives seem to reduce the weakening of the structural properties (losses in strength and weight) when samples are immersed in acidic medium. This could be due to the low Ca/Si ratio in the whole system considering the fact that FCS also contains the reactive particles which can be consumed by portlandite from Portland cement resulting in a low amount of Ca(OH)2 [41]. This would contribute to good resistance of Portland cement mortar specimens containing FCS in acid medium.
The XRD patterns of mixtures incorporating FCS as 0%, 20% and 30% replacement of the PC is presented in Fig. 13. It can be seen from Fig. 13 that some reflection peaks of portlandite CH (Ca(OH)2, PDF N° 44-1481) were reduced leading to the formation of calcium silicate hydrate (CSH) suggesting the consumption of CH in the system through pozzolanic reaction [45]. The peak of CSH in the mix with 20% FCS at around 29° (2 theta) has the highest intensity suggesting contribution of FCS in CSH formation. This observation has been reported by several authors [38, 45] where they combined PC, lime and slag for the synthesis of cementitious materials. Moreover, the XRD pattern of PC without additive is dominated by CH followed by a minor of calcium silicate (CS) and Ettringite (E) Ca6Al2(SO4)3 (OH)12 26H2O PDF N° 41-1451). The formation of the CSH phase in PC mortar justified the development in compressive strength.
Environmental Soundness
The use of FCS as a partial replacement to PC in cement composites is found suitable considering all the essential requirements that include the material’s fresh, hardened, and durability properties. This study is a preliminary work planned to evaluate FCS’s feasible utilization as a cement replacement in cementitious composites. Thus, leaching aspects of the produced composites were not extensively assessed. However, a novice attempt has been made to understand the leaching behaviour of Cr from the FCS-based CC in the early days of curing through the leaching test. Table 3 displays the leaching values of Cr from both FCS and the optimum FCS-based CC, i.e., 20FCS after 14 days. From the results, it can be observed that there is a significant reduction in the leaching potential of Cr from the FCS-based CC in comparison to the raw FCS. As per U.S. Environmental Protection Agency, the permissible total Cr leaching is 5.0 ppm and the values obtained in the case of 20FCS, the leaching values for Cr(total) is 0.071 ppm which is significantly lower than the acceptable standards [42]. This behaviour is attributed to the dense CC matrix that has encapsulated the Cr, preventing it from leaching.
Though minimal Cr leaching is observed at 14 days of the leaching procedure, considering the presence of Cr in the FCS, there is a possibility of chromium leaching to the environment from concrete structures made with FCS-based cement concrete in long-term exposure to different environmental conditions. Thus, more investigations are encouraged to understand the effect of hydration on the oxidation state of the present Cr in FCS and long-term exposure to different conditions.
Conclusions
This study conducted the use of ferrochrome slag (FCS) as a cement replacing material in PC mortar. Results from this study showed that the incorporation of FCS in PC in mortar resulted in increase in the setting times and decrease in consistency limit due to the absence of hydraulic phase content and low reactivity. A slight decrease in the compressive strength was also observed when FCS was used up to 30% replacement of PC. Nonetheless, the compressive strength of all mixtures incorporating FCS are still suitable for various structural applications. In terms of durability, the incorporation of FCS as partial replacement of PC was found to reduce the water absorption and acid attack resistance of the mortar. The enhancement of the durability of mortar mixtures incorporating FCS can be attributed to the pore filling effect of the FCS coupled with its pozzolanic properties that consumed the available CH in the matrix. The leaching of Cr from FCS-based CC is negligible compared to the raw FCS at 14 days, and thus it can be concluded that FCS-based PC encapsulates the leachable chromium at the initial days. However, extensive leaching studies are required with time and other parameters to establish FCS-based PC as environmentally safe. Further, as FCS is a relatively new replacement for the binder in CC, thus, more future studies are recommended in areas such as shrinkage, freeze and thaw resistance etc. before its practical application.
References
Andrew RM (2018) Global CO2 emissions from cement production. Earth Syst Sci Data. https://doi.org/10.5194/essd-10-195-2018
Purnell P (2013) The carbon footprint of reinforced concrete. Adv Cem Res. https://doi.org/10.1680/adcr.13.00013
Das SK, Mishra J, Mustakim SM, et al (2022) Sustainable utilization of ultrafine rice husk ash in alkali activated concrete: characterization and performance evaluation. J Sustain Cem Based Mater 11(2):100–112. https://doi.org/10.1080/21650373.2021.1894265
Das SK, Mishra S, Das D et al (2021) Characterization and utilization of coal ash for synthesis of building materials. In: Jyothi RK, Parhi PK (eds) Clean coal technologies. Springer International Publishing, Cham, pp 487–509
Mishra J, Das SK, Krishna RS, Nanda B (2020) Utilization of ferrochrome ash as a source material for production of geopolymer concrete for a cleaner sustainable environment. Indian Concr J 94:40–49
Mustakim SM, Das SK, Sahu T, Adesina A (2022) Development of greener lightweight aggregates from industrial waste products for use in construction composites. Innov Infrastruct Solut 8:25. https://doi.org/10.1007/s41062-022-00997-4
Flower DJM, Sanjayan JG (2007) Green house gas emissions due to concrete manufacture. Int J Life Cycle Assess. https://doi.org/10.1007/s11367-007-0327-3
Das SK, Dan AK, Behera U et al (2021) A novel approach on leaching study for removal of toxic elements from thermal power plant-based fly ash using natural bio-surfactant. Case Stud Chem Environ Eng 4:100156. https://doi.org/10.1016/J.CSCEE.2021.100156
Behera U, Das SK, Mishra DP et al (2021) Sustainable transportation, leaching, stabilization, and disposal of fly ash using a mixture of natural surfactant and sodium silicate. ACS Omega. https://doi.org/10.1021/acsomega.1c03241
Adesina A, Awoyera P (2019) Overview of trends in the application of waste materials in self-compacting concrete production. SN Appl Sci. https://doi.org/10.1007/s42452-019-1012-4
Shayan A, Xu A (2004) Value-added utilisation of waste glass in concrete. Cem Concr Res. https://doi.org/10.1016/S0008-8846(03)00251-5
Shariq M, Prasad J, Masood A (2010) Effect of GGBFS on time dependent compressive strength of concrete. Constr Build Mater. https://doi.org/10.1016/j.conbuildmat.2010.01.007
Das SK, Mishra J, Singh SK et al (2020) Characterization and utilization of rice husk ash (RHA) in fly ash—blast furnace slag based geopolymer concrete for sustainable future. Mater Today Proc. https://doi.org/10.1016/j.matpr.2020.02.870
Das SK, Mishra J, Mustakim SM (2018) Rice husk ash as a potential source material for geopolymer concrete: a review. Int J Appl Eng Res 13:81–84
Das SK, Singh SK, Mishra J, Mustakim SM (2020) Effect of rice husk ash and silica fume as strength-enhancing materials on properties of modern concrete—a comprehensive review. In: Lecture notes in civil engineering. pp 253–266
Alomayri T, Adesina A, Das S (2021) Influence of amorphous raw rice husk ash as precursor and curing condition on the performance of alkali activated concrete. Case Stud Constr Mater 15:e00777. https://doi.org/10.1016/J.CSCM.2021.E00777
Ikponmwosa EE, Ehikhuenmen S, Emeshie J, Adesina A (2020) Performance of coconut shell alkali-activated concrete: experimental investigation and statistical modelling. Silicon. https://doi.org/10.1007/s12633-020-00435-z
Ikponmwosa EE, Falade FA, Fashanu T et al (2020) Experimental and numerical investigation of the effect of sawdust ash on the performance of concrete. J Build Pathol Rehabil 5:15. https://doi.org/10.1007/s41024-020-00081-3
Sivakrishna A, Adesina A, Awoyera PO, Rajesh Kumar K (2019) Green concrete: a review of recent developments. Mater Today Proc. https://doi.org/10.1016/j.matpr.2019.08.202
Thomas M (2011) The effect of supplementary cementing materials on alkali-silica reaction: a review. Cem Concr Res 41(12):1224–1231. https://doi.org/10.1016/j.cemconres.2010.11.003
Kumar Das S, Adediran A, Rodrigue Kaze C et al (2022) Production, characteristics, and utilization of rice husk ash in alkali activated materials: an overview of fresh and hardened state properties. Constr Build Mater 345:128341. https://doi.org/10.1016/J.CONBUILDMAT.2022.128341
Mishra J, Nanda B, Patro SK et al (2022) Strength and microstructural characterization of ferrochrome ash- and ground granulated blast furnace slag-based geopolymer concrete. J Sustain Metall 8:156–169. https://doi.org/10.1007/s40831-021-00469-6
Kaze CR, Tome S, Lecomte-Nana GL et al (2021) Development of alkali-activated composites from calcined iron-rich laterite soil. Materialia (Oxford) 15:101032. https://doi.org/10.1016/j.mtla.2021.101032
Das SK, Mustakim SM, Adesina A et al (2021) Solid wastes: alternative materials for cementitious composites production. In: Hussain CM, Velasco-Muñoz JF (eds) Sustainable resource management. Elsevier, Amsterdam, pp 199–220
Niemelä P, Kauppi M (2007) Production, characteristics and use of ferrochromium slags. In: Innovations in the ferro alloy industry—proceedings of the XI international conference on innovations in the ferro alloy industry, Infacon XI
Das SK, Tripathi AK, Kandi SK et al (2023) Ferrochrome slag: a critical review of its properties, environmental issues and sustainable utilization. J Environ Manag 326:116674. https://doi.org/10.1016/J.JENVMAN.2022.116674
Adesanya E, Karhu M, Ismailov A et al (2020) Thermal behaviour of ladle slag mortars containing ferrochrome slag aggregates. Adv Cem Res. https://doi.org/10.1680/jadcr.19.00040
Acharya PK, Patro SK (2016) Utilization of ferrochrome wastes such as ferrochrome ash and ferrochrome slag in concrete manufacturing. Waste Manag Res. https://doi.org/10.1177/0734242X16654751
Al-Jabri KS (2018) Research on the use of Ferro-Chrome slag in civil engineering applications. In: MATEC web of conferences. p 1017
Al-Jabri K, Shoukry H, Khalil IS et al (2018) Reuse of waste ferrochrome slag in the production of mortar with improved thermal and mechanical performance. J Mater Civ Eng 30:04018152. https://doi.org/10.1061/(asce)mt.1943-5533.0002345
IS: 4031 (Part 6) (2005) Methods of physical tests for hydraulic cement part 6 determination of compressive strength of hydraulic cement other than masonry cement (first revision)
IS 4031—Part V (1988) Methods of physical tests for hydraulic cement. Part V—determination of initial and final setting times. India
IS 4031—Part IV (1988) Methods of physical tests for hydraulic cement. Part IV—determination of consistency of standard cement paste
ASTM C 642-2006 (2006) Standard test method for density, absorption, and voids in hardened concrete. ASTM International, West Conshohocken, pp 1–3. https://doi.org/10.1520/C0642-13.5
Sunayana S, Barai SV (2021) Partially fly ash incorporated recycled coarse aggregate based concrete: microstructure perspectives and critical analysis. Constr Build Mater 278:122322. https://doi.org/10.1016/j.conbuildmat.2021.122322
Wild S, Khatib JM (1997) Portlandite consumption in metakaolin cement pastes and mortars. Cem Concr Res. https://doi.org/10.1016/S0008-8846(96)00187-1
Deschner F, Winnefeld F, Lothenbach B et al (2012) Hydration of Portland cement with high replacement by siliceous fly ash. Cem Concr Res. https://doi.org/10.1016/j.cemconres.2012.06.009
Vance K, Aguayo M, Oey T et al (2013) Hydration and strength development in ternary Portland cement blends containing limestone and fly ash or metakaolin. Cem Concr Compos. https://doi.org/10.1016/j.cemconcomp.2013.03.028
Rahman MA, Sarker PK, Shaikh FUA, Saha AK (2017) Soundness and compressive strength of Portland cement blended with ground granulated ferronickel slag. Constr Build Mater. https://doi.org/10.1016/j.conbuildmat.2017.02.023
IS 456 (2000) Plain and reinforced concrete—code of practice
Pacheco-Torgal F, Ding Y, Miraldo S, et al (2012) Are geopolymers more suitable than Portland cement to produce high volume recycled aggregates HPC? Constr Build Mater 36:1048–1052. https://doi.org/10.1016/j.conbuildmat.2012.07.004
Wang W, Noguchi T (2020) Alkali-silica reaction (ASR) in the alkali-activated cement (AAC) system: a state-of-the-art review. Constr Build Mater 252:119105. https://doi.org/10.1016/j.conbuildmat.2020.119105
Jaritngam S, Prachasaree W, Somchainuek O, Taneerananon P (2012) An investigation of lateritic soil cement for sustainable pavements. Indian J Sci Technol: Trans of Civil Eng 38:275–284
Phummiphan I, Horpibulsuk S, Sukmak P et al (2016) Stabilisation of marginal lateritic soil using high calcium fly ash-based geopolymer. Road Mater Pavement Des. https://doi.org/10.1080/14680629.2015.1132632
Hosan A, Shaikh FUA (2020) Influence of nano-CaCO3 addition on the compressive strength and microstructure of high volume slag and high volume slag-fly ash blended pastes. J Build Eng. https://doi.org/10.1016/j.jobe.2019.100929
Acknowledgements
The authors are thankful the CSIR-Institute of Minerals and Materials Technology, Bhubaneswar, India for giving permission to publish this research.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
The contributing editor for this article was Mansoor Barati.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Das, S.K., Rajput, P., Mustakim, S.M. et al. Towards Sustainable Construction: Utilization of Ferrochrome Slag as Portland Cement Replacement in Cementitious Composites. J. Sustain. Metall. 9, 329–340 (2023). https://doi.org/10.1007/s40831-023-00653-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40831-023-00653-w