Abstract
Phosphor powder sample was characterized, and the leaching kinetics of yttrium, and europium in hydrochloric acid were investigated. Under optimized leaching conditions, 98% Y and 97% Eu were extracted, and a reaction curve was generated using the percentage of extraction as a function of time and temperature. Based on R2 values, shrinking spherical particle and shrinking core model were not applicable. SEM analysis also confirmed shrinking core behavior was not applicable due to the lack of core–shells in the leach residue. The kinetic data were best fitted by a logarithmic rate expression of the empirical model. Activation energy was calculated to be 77.49 kJ/mol for Y and 72.75 kJ/mol for Eu in the temperature range of 298–343 K.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Rare earth elements (REEs) are used in nearly all high-technology applications ranging from green energy to defense-related equipment. Rare earth–phosphor mixture makes up 2 wt% of a typical 40 W fluorescent lamp [1]. Thus, several processes have been investigated for recovery of the REEs in phosphor powder.
Takahashi and co-workers performed a series of hydrometallurgy-based separation methods to recover of rare earths from phosphors [2, 3, 4]. Rabah proposed a process for the recovery of europium, yttrium, and other valuable rare earth salts from waste fluorescent lamp phosphor using pressure leaching [5]. Wang et al. conducted leaching experiments on spent trichromatic phosphor mixtures and recovered 96.28% of yttrium with 4 M hydrochloric acid with 4.4 g/L hydrogen peroxide [6]. Yang et al. developed a two-stage leaching process with sulfuric and nitric acid in the first and second stages, respectively, to recover 99% yttrium and 95% europium from phosphor dust [7]. De Michelis et al. produced yttrium oxalate of 99% purity after leaching yttrium from waste phosphor powder followed by precipitation with oxalic acid [8]. Eduafo et al. developed a three-stage leaching and precipitation process for selective extraction and recovery of the REEs from waste phosphor powder to produce 99.57% pure, mixed oxides with yttrium to europium ratio of 1:21 [9, 10]. Tunsu et al. extracted rare earth metals (REMs) from fluorescent lamp waste leachates using solvent extraction process with Cyanex 572 and 923 [11, 12].
While there is increasing attention on recovering REMs from spent fluorescent lamps due to the criticality of these metals, little effort has been placed in investigating the mechanism and kinetics of leaching REEs from waste lamp phosphors. This paper addresses the kinetics of leaching of yttrium and europium.
Materials and Methods
Materials
Waste phosphor powder from the waste fluorescent lamps was provided by Veolia ES Technical Solutions, L.L.C (WI, USA). Solutions of known concentration were prepared with deionized water and diluted with 2% HNO3 before chemical analysis with ICP-OES. All chemicals used were of analytic grade reagents (Sigma Aldrich, USA).
Procedure
Waste phosphor powder from lamps were sieved to 75 μm to separate any broken glass particles present [9]. A weighed sample of the sieved phosphor powder was added to a heated solution and stirred with a magnetic stirrer for the desired time. The amount of sample added was governed by the needed solid–liquid ratio (S/L) or pulp density. After leaching, the mixture was filtered, and samples were taken from the leachate and analyzed for REEs and other elements.
Analysis
The concentration of ions in the leach liquor was determined by using inductively coupled plasma-optical emission spectrometer (ICP-OES, Optima 5300 DV Perkin Elmer Co., MA, USA) and chemical composition of the phosphor powder was determined with X-ray fluorescence spectrometer (XRF). Phosphor powder samples were obtained and characterized for specific mineralogical and metallurgical parameters using Microtrak particle analyzer, X-ray powder diffraction analysis (XRPF), and scanning electron microscope analysis with energy-dispersive X-ray spectroscopy (SEM–EDX, JEOL JSM-7000F).
Results and Discussion
Characterization of Phosphor Powder
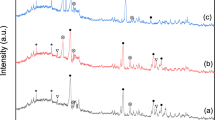
Cumulative particle size analysis of the phosphor powder ranges from 1 to 700 μm (Fig. 1). The REE bearing are less than or equal to 30 μm. Table 1 shows CaO and P2O5, which come from the white halophosphate phosphor, and are present in higher concentrations. There are also oxides—SiO2, Na2O, and K2O—which are from glass splinters introduced into the waste phosphor powder during lamp recycling. Yttrium is the predominant REE, while terbium has the lowest concentration. XRD analysis shows the major phases identified in the powder were fluoroapatite, wakefieldite, calcite, quartz (crystalline SiO2), yttrium oxide, and monazite (Fig. 2) [9].
The surface morphology of powder particles was detected with SEM imaging with backscattered electron detector (BSE). The micrograph shows the particles have different shapes and sizes (Fig. 3). The lighter fraction is heavier by atomic weight and represents the REEs, while the lower atomic weight darker fraction represent the non-REEs such as calcium, phosphorus, aluminum, and silicon. Table 2 shows the different phosphors used in lamps with calcium halophosphate phosphor at point 6; red, blue, and green rare earth phosphors at points 1, 5, and 8, respectively. Point 4 shows silica, while points 2 and 7 show a combination of calcite and alumina.
Leaching of REEs
The phosphor powder was sieved to 75 μm to remove coarse glass and pretreated with hydrochloric acid at room temperature to remove the bulk of the non-REEs according to the flowsheet developed by Eduafo et al. [9]. Yttrium and europium were then extracted from the leach residue at varying times and temperatures using hydrochloric acid and under the optimized leaching conditions—2 M HCl, 180 g/L pulp density, and 600 rpm stirring speed. From these experiments, a maximum yields of 98% Y and 97% Eu were recovered under the optimal leaching conditions of 2 M HCl, 180 g/L, 600 rpm, 80 °C, and a reaction rate curve was generated to determine the leaching model. The proposed chemical reaction for the leaching of yttrium or europium (\( {\text{Re}} \)) with HCl is as follows (Eq. 1):
The proton destabilizes the rare earth oxide crystal lattice by attacking the oxygen to form water. The chloride ion from HCl is a strong complexing ligand and forms an aquo-complex with the unbounded rare earth cation to form rare earth chlorides. The reaction can go forward or backward depending on the concentration and solubility of rare earth chlorides.
The reaction curve in Fig. 4 shows that the extents of leaching of Y and Eu increased with the increasing time and temperature. The rate of the leaching reaction is very fast as the reaction curve starts to flatten after 7.5 min.
Leaching Model
Data obtained in the leachings of Y and Eu as a function of temperature and time were used in a kinetics study to determine the leaching model. Chemical- and film diffusion-controlled models for shrinking spherical particle model were tested but were not applicable to the experimental data as can be seen by the \( R^{2} \) values in Table 3. Chemical-, ash-diffusion-, and film-diffusion-controlled models for the shrinking core models also showed a poor fit at 70 °C. This assessment seems to be confirmed by SEM which showed the lack of core-shells to support shrinking core behavior (Fig. 5). The micrograph and EDS data in Table 4 shows the predominant elements in the leach residue is mainly silicon, aluminum, and the green phosphor—lanthanum, cerium, and terbium.
The kinetic data were also analyzed using the empirical model of leaching governed by the following logarithmic equation (Eq. 2) [13]:
where \( E \) is the percentage of particle leached into the solution at time \( t \) and \( k_{I} \) is the specific rate constant for the empirical model. Table 3 shows that the kinetic data relatively show a better fit to the logarithmic equation than the shrinking spherical particle and shrinking core models, and this is evident from the high \( R^{2} \) values.
The activation energies for leaching of yttrium and europium from the phosphor powder were calculated using the specific rate constant, \( k_{I} \) to generate the Arrhenius plot (Fig. 6). Activation energies were calculated to be 77.49 kJ/mol for yttrium and 72.75 kJ/mol for europium between 298 K and 343 K, and these values are within the range for metal dissolution following the logarithmic equation [14]. Therefore, the empirical model explains the mechanism of leaching of yttrium and europium from waste lamp phosphor powder. Himanshu et al. investigated the kinetics and mechanism of leaching of rare earth metals from phosphor powder, and their experimental data also followed empirical model of leaching. They found the activation energies to be 76 kJ/mol and 61 kJ/mol for dissolution of Y and Eu, respectively, in the temperature range of 343–368 K [14].
Conclusion
The kinetics of leaching of yttrium and europium have been studied in the temperature range of 25–70 °C, and this present work shows the experimental data best fit the logarithmic equation of the empirical model than the shrinking spherical particle and shrinking core models using the R2 values. SEM further confirms the inapplicability of the shrinking core model. Data should have been taken between 0 and 7.5 min to effectively analyze the reaction kinetics. Kinetic data after 7.5 min measured the thermodynamic effect since the extent of reaction did not change much with time. The activation energies required for dissolution of yttrium and europium in the temperature range from 298 K to 343 K were found to be 77.49 kJ/mol and 72.75 kJ/mol, respectively.
References
Tunsu C, Petranikova M, Ekberg C, Retegan T (2016) A hydrometallurgical process for the recovery of rare earth elements from fluorescent lamp waste fractions. Sep Purif Technol 161:172–186
Takahashi T, Tomita K, Sakuta Y, Takano A, Nagano N (1996) Report of Hokkaido Industrial Research Institute, no. 295. Hokkaido Industrial Research Institute, Hokkaido
Takahashi T, Takano A, Saito T, Nagano N (1999) Reports of Hokkaido Industrial Research Institute, no. 298. Hokkaido Industrial Research Institute, Hokkaido
Takahashi T, Takano A, Saitoh T, Nagano N (2003) Reports of Hokkaido Industrial Research Institute, no. 302. Hokkaido Industrial Research Institute, Hokkaido
Rabah MA (2008) Recyclables recovery of europium and yttrium metals and some salts from spent fluorescent lamps. Waste Manag 28(2):318–325
Wu Y, Yin X, Zhang Q, Wang W, Mu X (2014) The recycling of rare earths from waste tricolor phosphors in fluorescent lamps: a review of processes and technologies. Resour Conserv Recycl 88:21–31
Yang F, Kubota F, Baba Y, Kamiya N, Goto M (2013) J Hazard Mater 254–255:79–88
De Michelis I, Ferella F, Varelli EF, Vegliò F (2011) Selective extraction and recovery of rare earth metals from phosphor powders in waste fluorescent lamps using an ionic liquid system. Waste Manag 31(12):2559–2568
Eduafo PM (2016) ProQuest number: 10019488, Colorado School of Mines, ProQuest Dissertations Publishing
Rabah MA (2016) Waste. In: Strauss M, Eduafo P and Mishra B (eds) 4843-148-PROV
Tunsu C, Lapp JB, Ekberg C, Retegan T (2016) Selective separation of yttrium and europium using Cyanex 572 for applications in fluorescent lamp waste processing. Hydrometallurgy 166:98–106
Tunsu C, Ekberg C, Foreman M, Retegan T (2014) Solvent Extr Ion Exch 32(6):650–668
Kim E, Kim M, Lee J, Jeong J, Pandey BD (2011) Studies on the solvent extraction of rare earth metals from fluorescent lamp waste using Cyanex 923. Hydrometallurgy 107:124–132
Verma HR, Sahu SK, Meshram P, Pandey BD, Mankhand TR (2013) Kinetics of hydrometallurgical extraction of rare earth metals from waste phosphor. Int J Res Eng Technol (IJRET) 2(5):251–255
Author information
Authors and Affiliations
Corresponding author
Additional information
The contributing editor for this article was T. Hirato.
Rights and permissions
About this article
Cite this article
Eduafo, P.M., Mishra, B. Leaching Kinetics of Yttrium and Europium Oxides from Waste Phosphor Powder. J. Sustain. Metall. 4, 437–442 (2018). https://doi.org/10.1007/s40831-018-0189-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40831-018-0189-x