Abstract
During the zinc electrowinning process, fluorine (F) and chlorine (Cl) ions can cause corrosion of the anode plate and difficulty in stripping the zinc cathode sheet. Because of the increasing use of F/Cl in zinc concentrate in industrial production and the stricter requirements of large-size cathode technology (LARCATH), it is very important to remove F and Cl fully from the zinc sulfate solution. In this paper, tests for removing F/Cl in zinc sulfate solution from zinc plant are carried out by the goethite method. The effects of several factors on the removal of Fe, F, and Cl are studied, including the temperature, flow rate of the zinc sulfate solution, pH value, initial iron concentration, and F and Cl concentrations in the zinc sulfate solution. It can be determined from the experimental results that the removal efficiency of F and Cl can reach 26.4 and 38.6%, respectively, under the conditions of 90 °C, pH value 2.75, and flow rate 3.8 mL/min. The removal rate of F ions is mainly related to the amount of lime milk, whereas the pH value has the greatest impact on the removal rate of Cl. The concentration of negative ions on the surface of goethite increases with increasing pH, which is harmful to the absorption of Cl.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Currently, 85% of the metallic zinc in the world is produced by the hydrometallurgical method. Normally, the zinc sulfide ore is first roasted and then treated by the wet method. In the process of roasting, 70% of the F and Cl in the zinc concentrate enter the flue gas in the form of compounds with low boiling points, while the rest of F and Cl enter the zinc sulfate solution, a zinc-ore acid leaching solution containing iron, fluorine, and chlorine, following the roasted zinc oxide [1]. F and Cl are accumulated in the solution, and their concentrations increase gradually.
The harm caused by F and Cl ions is a common problem faced by all zinc plants. It is mainly demonstrated that F ions can destroy aluminum oxide films on the surface of the aluminum plate cathode in the zinc electrowinning process, which causes the zinc and aluminum surfaces to form a zinc–aluminum alloy and further leads to difficult divestiture of zinc. On the other hand, Cl ions react with the anode, which can prevent separation of the zinc plate and shorten the service life of the anode. Moreover, Cl ions are oxidized into Cl2 by manganese dioxide in the acidic environment, which increases the level of air pollution [2].
In the zinc sulfate solution, the methods to remove F primarily include adsorption [3, 4], chemical precipitation [5], electrocoagulation, and anion exchange [6]; for Cl, they mainly include chemical precipitation, the electrochemical method, and the oxidation method [7, 8]. In particular, the chemical precipitation methods include the widely used Cu2Cl2 precipitation [9, 10], BiOCl precipitation [11], and AgCl precipitation.
Because F and Cl are both halogen elements, their chemical properties are very similar. The process can be greatly simplified if F and Cl can be removed simultaneously from the zinc sulfate solution. Some methods could be applied to remove F and Cl simultaneously, such as the goethite method, hydrotalcite adsorption method [12, 13], extraction method [14], and hydrogen volatilization method [15, 16]. However, most of these methods have some limitations in practice. The goethite method that relies on the iron removal process without any additional processes is different from the other methods, and iron removal is a necessary process in zinc hydrometallurgy. The iron removal method is widely used, which can greatly simplify the zinc hydrometallurgy process, as well as remove fluorine and chlorine simultaneously.
The purpose of the present work is to investigate the effects of simultaneous removal of fluoride and chlorine from goethite, and the impact of related factors on its effectiveness. The iron removal tests by goethite are carried out in zinc sulfate solution containing iron, fluorine, and chlorine. The experimental setup facilitates performing the precipitation reaction under different control conditions. It is hoped that fluorine and chlorine will be effectively removed during the zinc hydrometallurgy process, so that their impact on the process can be reduced to within an allowable range.
Materials and Methods
Experimental Materials and Principles
The iron removal tests are mainly performed in zinc sulfate solution from a zinc plant, and its main chemical components and information on reagents used in the tests are shown in Tables 1 and 2, respectively.
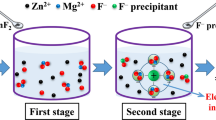
The principle of removing F and Cl is to utilize coprecipitation in the goethite process. In this process, F will precipitate in the form of CaF2 if lime milk is used as a neutralizing agent.
Coprecipitation can be realized by adsorption, occlusion, or the mixed crystal method. Among these methods, surface adsorption is the most common type because of the incomplete balance of ionic charge on the crystal surface. The surface adsorption of goethite is related to the surface charge and specific surface area, which are mainly affected by grain size, surface hydroxyl content, and pH value.
The amount of hydroxyl on the surface depends on the nature of goethite, and the grain size of precipitation is related to the ionic concentration in the precipitation reaction. Huai’s (Von Weimarn) empirical formula [17] is obtained according to the related experimental phenomena. It indicates that the dispersion of precipitation reflecting the size of the precipitation particle is associated with the relative supersaturation degree of the solution as follows:
where D represents the degree of dispersion, C Q denotes the concentration of sediment, S denotes the solubility of the precipitate, C Q − S denotes the supersaturation, which is the driving force of precipitation, (C Q − S)/S is the relative supersaturation, and K is a constant related to the properties of the precipitation medium and temperature.
It is known that the greater the relative supersaturation degree of the solution, the greater the number of formed nuclei, which is positively associated with the degree of dispersion.
Occlusion is a coprecipitation phenomenon, in which the impurity ions have insufficient time to exchange with the reaction ions and are eventually trapped in the precipitate. In the mixed crystal method, impurity ions enter the precipitation lattice, due to similar charge and radius, during the process of crystallization. Because the ionic radii of F and Cl ions (0.136 and 0.181 nm) are close to that of the hydroxide ion (0.140 nm), they can enter the lattice easily in the formation of goethite.
Experimental Procedure
The goethite method [18] has been used successfully in the zinc hydrometallurgy industry as a readily filterable method to remove iron from zinc sulfate solutions. The necessary condition to form the precipitate is to maintain a ferric iron concentration of no more than 1 g/L by controlling the addition rate of ferric iron solutions into the precipitation vessel. All experiments are carried out in the apparatus shown in Fig. 1. The iron removal tests are conducted according to the following procedures. First, the 300 mL solution after iron removal is added to a three-neck round-bottom flask and is heated in a constant-temperature water bath. Second, the zinc sulfate solution and neutralizing agent (e.g., lime milk) are added into the flask, and the latter is used to maintain a stable pH, as the acidity of the solution can increase with the formation of goethite. Then, after a period of reaction time, the reaction liquid is filtered, and the filter residue is washed with a certain amount of dilute sulfuric acid. Finally, the solution after iron removal is sent to chemical assay to test the ion concentration.
The determination of the ion concentration in the test process is carried out by the state nonferrous metal quality supervision and inspection center. The steam distillation and ion chromatography method is used to determine the amounts of fluorine and chlorine in the zinc sulfate solution. The potassium dichromate titration method is used for the iron ion test, and inductively coupled plasma atomic emission spectrometry (ICP-AES) is used for the zinc ion test.
The removal rates of F and Cl ions can be calculated in the goethite experiment by the following formula:
where φ is the removal rate of ions, C 0 is the iron concentration of the zinc sulfate solution, C 1 is the iron concentration of the reaction solution, V 0 is the zinc sulfate solution volume, and V 1 is the reaction solution volume.
Results and Discussion
In this paper, the effects of the reaction temperature, addition rate of the zinc sulfate solution, pH value, and concentrations of F, Cl, and Fe ions on the removal rates of F and Cl have been investigated. A summary of the iron removal test parameters studied is given in Table 3.
Influence of Reaction Temperature
The temperature has a significant impact on the removal rates of F and Cl in the process of goethite formation. The related results are displayed in Fig. 2. The removal rate of F changes in the range of 39.3–44.7% with increasing temperature and reaches ~ 50% when the temperature is higher than 90 °C. Moreover, with increasing temperature, the removal rate of Cl decreases from 24.6 to 14.9%.
With increasing temperature, the solubility of goethite increases, and the relative supersaturation of the solution decreases, which promotes the formation of goethite with a large grain size. As the specific surface area of the large-grain goethite is small, it is not conducive to the adsorption of F and Cl ions. However, the lime milk is added quickly, and the formation of the calcium fluoride precipitate is also fast at higher temperatures, which is helpful for the removal of F.
It can be observed from Fig. 3 that the removal rate of iron fluctuates between 97 and 99%. A temperature of 90 °C is sufficiently high to reach equilibrium, and there is no significant increase in the removal rate of iron with further increases in temperature.
Effect of Addition Rate of Zinc Sulfate Solution
The addition rate is an important factor in the goethite process. Within a certain range, the relative supersaturation of goethite increases with increasing addition rate, which is closely related to the removal of F and Cl. When the addition rate is beyond this range and the iron concentration is greater than 1 g/L in the solution, it will result in the formation of iron hydroxide rather than goethite. The effect of the addition rate of leaching liquid is investigated between 2.0 and 20.0 mL/min, and the results for the removal rates of F and Cl are shown in Fig. 4.
The addition rate has a great effect on the removal rates of F and Cl, as indicated in Fig. 4. The removal rates of F and Cl increase significantly with the increasing addition rate from 2 to 3.8 mL/min. With the increase of the flow rate of the solution, the relative supersaturation of the solution increases and the grain size of goethite becomes small, which benefits the adsorption of F and Cl ions. The removal rates of F and Cl are almost unchanged when the addition rate increases from 3.8 to 9.2 mL/min. This is due to the saturated adsorption of goethite on F and Cl ions. As the addition rate continues to increase, the removal rates of F and Cl ions increase greatly. A high addition rate can cause high iron concentration locally, which will result in the formation of ferric hydroxide colloids. Colloids with a high specific surface area have more adsorption sites than goethite crystals [15], which is beneficial to the removal of F and Cl ions. However, under this condition, it is difficult to filter the liquid. Finally, the removal rate of F increases slowly and that of Cl decreases gradually with the continuous increase in addition rate. The latter is mainly attributed to the short reaction time, further reducing the coprecipitation of Cl ions.
When the addition rate is less than 9.2 mL/min, the removal rate of iron decreases slowly with increasing liquid addition rate, as shown in Fig. 5. When the addition rate is greater than 9.2 mL/min, the iron removal rate decreases rapidly, and the iron ions are not completely precipitated due to the slow reaction rate during the formation of ferric hydroxide colloids and the short reaction time.
Influence of Reaction pH
In Fig. 6, the curves show the significant influence of the pH value on the removal rates of F and Cl. The removal rate of F increases gradually at first, but it increases significantly when the pH value is larger than 3.0. Moreover, with the increasing solution pH, the removal rate of Cl increases first and then decreases. When the pH value is low, goethite with a large grain size is easily formed, and the adsorption of goethite is less, which is not beneficial to the removal of Cl ions. However, the low pH is favorable to the protonation of the hydroxyl group on the surface of goethite, which causes more positive charges on the surface, thereby promoting the adsorption of anions. Considering the above different effects of pH, the maximum removal rate of Cl is 38.6% at a pH value of ~ 2.7.
The formation [17] of surface hydroxyl groups of goethite can be expressed as follows:
The protonation and deprotonation reactions [15] on the surface of goethite can be expressed, respectively, as follows:
It can be observed from Fig. 7 that the iron removal rate increases gradually with the increase in pH value. This is mainly caused by the reducing iron concentration in this process. When the zinc concentration is 120 g/L, the pH value of zinc precipitation is ~ 4.0. The adsorbed zinc ion on the goethite surface is active, and the precipitation pH is less than 4.0. Therefore, when the pH value is greater than 3.0, the zinc content in the slag increases sharply. In order to obtain a high efficiency of iron removal and low zinc content in slag, the pH value should be controlled at ~ 3.0.
Influence of Iron Concentration
The higher the iron concentration, the greater the amount of lime milk added. The high concentration of calcium ions can reduce the equilibrium concentration of F in the zinc sulfate solution. As shown in Fig. 8, the removal rate of F increases with the increasing iron concentration. When the iron content is greater than 20 g/L, the removal rate of F is greater than 80%.
The removal of F can be accomplished by two methods: CaF2 precipitation and goethite precipitation. The solubility of lime milk is too small to provide enough calcium ions to reduce the F ion concentration to 15 mg/L in the solution [19]. In the experiment, the concentration can be as low as 2–4 mg/L, so the other mechanism exists, i.e., goethite precipitation. Zinc oxide is applied as a neutralization reagent in the goethite test. At a temperature of 90 °C and a pH value of 3.0, the removal rate of F reaches 20.4%, which indicates that a portion of the F ions can be removed in the form of goethite, in addition to the form of CaF2 precipitation.
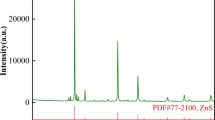
Figure 9 presents the X-ray diffraction pattern of the iron slag sample from the above tests. The presence of goethite phases can clearly be seen in this figure. The diffraction peak is wide, which illustrates that the crystallinity of goethite is not high.
As shown in Fig. 8, the removal rate of Cl increases slowly with the increasing iron concentration. This can be explained by an increase in the formation of goethite with the increasing iron concentration, and the removal of Cl increases with the coprecipitation of goethite. The adsorption capacity of goethite to Cl is limited; therefore, the adsorption amount of Cl increases slowly with the increasing goethite formation.
With the increase of the iron concentration, more iron needs to be removed from the solution. However, it can be observed from Fig. 10 that when the iron concentration changes from 5 to 30 g/L, the removal rate of iron also changes from 95 to 98%. Therefore, the iron removal efficiency of the goethite method is high.
Influence of F/Cl Ion Concentration
It can be observed from Fig. 11 that the removal rate of Fe is less influenced by the F content in the zinc sulfate solution. As the F concentration in the zinc sulfate solution increases, the removal rate increases from 39.3 to ~ 50.0%.
Moreover, the curves in Fig. 12 show that the iron removal rate is rarely affected by the change of the Cl concentration in the leaching liquid and always remains in the range of 95.9–96.9%. As the Cl concentration of the leaching liquid increases, the removal rate increases from 20.5 to 27.5% and eventually tends to be steady.
Conclusions
In this study, the purpose is to explore the removal effects on F and Cl ions during the iron removal of goethite. We analyze some chief affecting factors, such as the reaction temperature, addition rate of the leaching liquid, pH value, and the F, Cl, and Fe concentrations. This research is based on the coprecipitation of F and Cl in the iron removal process. In addition, lime milk is used as a neutralization reagent to form CaF2 and remove some of the F. The experimental results demonstrate that the goethite method can remove F and Cl effectively. The removal rates of F and Cl are up to 80 and 38.6%, respectively. The method is mainly based on the goethite coprecipitation, which is primarily affected by the pH of the solution. The removal rate of Cl ions is relatively small when the pH is high. The main reason for this is that the negative charge on the goethite surface increases with increasing pH, which is not beneficial to the adsorption of anions. In the process of zinc hydrometallurgy, the concentrations of F and Cl in the zinc sulfate solution can be effectively reduced by controlling the conditions of iron removal in the goethite method. Because iron removal is a necessary process in zinc hydrometallurgy, the removal of F and Cl can not only simplify the process, but also does not induce any additional investment in the goethite method. Therefore, this method is considered to have good economic benefit as well as an effective capacity for removing F and Cl.
References
Liao Y (2008) Discussion about the movement direction and distribution of fluorine and chlorine and influences of them to the production process in the zinc production of some company. Hunan Nonferrous Metals 24(1):20–23
Tang D, Mao X, Huang B (2004) Study on F−, Cl− purification processes from zinc sulfate solution. J Guizhou Univ Technol 33(1):15–18
Li Y, Meng F, Yao R (2010) Development and application of fluoride removal in drinking water treatment. CNKI J 36(7):10–13
Sairam Sundaram C, Viswanathan N, Meenakshi S (2009) Defluoridation of water using magnesia/chitosan composite. J Hazard Mater 163(2–3):618–624
Xie W (1996) Study on the removal of F- from electrolytic zinc solution used for zinc-making in wet method. J Guangxi Univ Nationalities 2(2):26–30
Song Z (2009) Technology of fluorine and chlorine removal by ion exchange method in zinc for industrialization. China Patent 101492772A
Angleur FJJ (1979) Process for the removal of impurities contained in a zinc and cadmium sulfate solution. US Patent 4156711
Bolton GL, Saskatchewan F, Verner B (1983) Removal of manganese and chloride ions from aqueous acidic zinc sulphate solutions. US Patent 4379037
Yang J, Niu H (2010) Dechlorination test of copper scale in zinc hydrometallurgy system and the industrialization research. YunNan Metal 39(6):21–25
Li C, Li Z, Zhang Y (2002) Chloride removing from zinc neutral leachate with nascent copper by cuprous chloride precipitation. Nonferrous Metals 54(1):30–33
Wen J (2008) Study on the choice of dechlorination in the production of electric zinc of Jinshi Metallurgy Chemical Plant. Hunan Nonferrous Metals 24(6):34–36
Jing H (2005) Adsorption behaviors of calcined Mg-Al-CO3-LDH towards removal of chloride from aqueous solution. Zhejiang University, Hangzhou
Yu Y (2010) Modification and granulation of Mg/Al layered double hydroxides and adsorption of halide anions. Ocean University of China, Qingdao
Luo Y (2009) A method of removing chloride from zinc sulfate solution. China Patent 103060561A
Jinfa X (1980) Gas to remove fluorine in zinc sulfate solution. Nonferrous Metals 1:61
Fugleberg SP, Poijarvi JI (1987) Hydrometallurgical method for treating valuable metal raw materials containing chloride and fluorides. US Patent 4698139
Wei J, Daqing W (2000) Surface ionization and surface complexation models at mineral/water interface. Adv Earth Sci 15(1):90–96
Davey PT, Scott TR (1976) Removal of iron from leach liquors by the “Goethite” process. Hydrometallurgy 2:25–33
Wang Q, Wu Y (2006) Defluorination with chemical precipitation method from wastewater. In: Proceedings of the eighth symposium on water treatment chemistry and symposium of China Chemical Society
Acknowledgements
This work was supported by the National Natural Science Foundation of China (grant no. 51574034), the Innovative Research Team in Beijing General Research Institute of Mining & Metallurgy.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
The contributing editor for this article was Brajendra Mishra.
Rights and permissions
About this article
Cite this article
Hu, Y., Wang, H., Wang, Y. et al. Simultaneous Removal of Fluorine and Chlorine from Zinc Sulfate Solution in Iron Precipitation Process. J. Sustain. Metall. 4, 95–102 (2018). https://doi.org/10.1007/s40831-017-0154-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40831-017-0154-0