Abstract
This study involved the geochemical assessment of the occurrence and distribution of heavy metals in available agricultural soils and stream sediments in the lead–zinc mining areas of Abakaliki, Southeastern Nigeria. A total area of about 794.2 km2 was assessed, with major communities covered including Enyigba, Mkpuma Akpatakpa, Ameka, Amorie, Amanchara and Alibaruhu where active and abandoned mines are located. One hundred and sixteen soil/stream sediments were collected and subjected to geochemical analysis using the atomic absorption spectrophotometric method. The result shows concentration of Cd > Pb > As > Zn > Cu > Ag > Hg > Cr, especially in the areas close to the active mines. The contamination percentage of metal in samples shows Cd (40.51%), Pb (36.20%), As (23.27%), Zn (17.24), Cu (8.62%) and Cr (0%). This implies that arable soils around the mining areas are not suitable for food crop production as biomagnification can occur in the food chain. The consumption of food produce in these areas can lead to potential health risk as they accumulate. However, the Mkpuma Akpatakpa and Ameka areas show higher distribution of these metals than the Enyigba and Amanchara areas. Seasonal analysis shows a decreased concentration of chemical constituents in the rainy season relative to the dry season except for Cd, Cr and Hg.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The effects of mineral deposits’ weathering and degeneration may be deleterious on soil and water quality due to soil–water interaction (Obasi and Akudinobi 2019b; Loska and Wiechula 2003; Lucassen et al. 2002). This may not only lead to challenges in sustainable water quality; pollutants and contaminants from mining sources constitute a major threat to human health, aquatic lives, land use and agriculture, and other aspects of ecosystem imbalance (Zhiyuan et al. 2014; Yousefi et al. 2017). Obasi (2017) noted that soils and environmental degradation problem are obvious in mining areas. Many authors including (Vanek et al. 2005; Akoto et al. 2008) have shown that the major pathways for heavy metals are by sedimentation of particles and by the sorption of dissolved metals from surface water or groundwater seepages in contact with stream sediments. Kishe and Machwa (2003), and Lech et al. (2007) noted that geochemical stream sediment surveys are employed almost invariably for reconnaissance studies in drainage basins, because if properly collected, these stream sediments represent the best composite materials from catchments areas upstream from sampling sites. Therefore, by sampling and analysing stream sediments and soils, it is possible to recognize geochemical anomalies within a catchment area and trace them to their source.
Geochemical soil survey, according to the dictionary of Earth Science Ailsa and Michael (1999), is the process of collecting and analysing unconsolidated soil sediments in order to locate geochemical anomalies in the underlying rock and to use these to find ore bodies. In weathering environments, many minerals, particularly sulphide minerals, are unstable and will break down as a result of oxidation or other chemical reactions, and this is the underlying rationale behind stream sediments and soil geochemical surveys (AusIMM 2011). Their breakdown often results in dispersion of both ore and indicator elements in solution, in run-off and in ground water for relatively long distances within the drainage basin (Levinson 1974; Al-Momani 2009).
Oti and Nwabue (2013), Chibuike and Obiora (2014), and Obiorah et al. (2016) have shown that plants’ intake of heavy metals in mining areas can have adverse effects on man. Kamau (2001), Asaah et al. (2006), Ezeh et al. (2007), Dikinya and Areola (2010), Li et al. (2014), Harikumar and Jisha (2010), Casper et al. (2004); Mendoza et al. (1996), Nriagu and Pacyna (1988), Obasi and Akudinobi (2019a), Obiorah et al. (2018), and Alexakis (2016) have evaluated the concentration of heavy metals in soils and sediments in mining areas in different parts of the world. These works have shown that mining activities have effects on the surrounding soils in the areas.
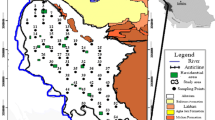
Rural communities of Enyigba, Mkpuma Akpatakpa, Ameka, Amorie, Amanchara, Agbaja and Alibaruhu where active and abandoned mines are located comprise the lead–zinc mining areas of Abakaliki. These communities are endowed with lead–zinc mineralization which occurs as lodes and veins (Obiorah et al. 2016; Obasi and Akudinobi 2015; Obasi 2017; Obasi et al. 2018a, b; Obasi and Akudinobi 2019a, b). The mineralization in the area has facilitated small-scale mining in the area with the consequent random citing of excavations and waste dumps, and unplanned disposal of mine wastes (see Fig. 1). All these affect the hydrogeochemistry of the area. Also, these are agrarian communities with peasant farming as their major occupation. The villagers cultivate the farmlands in the rainy season and the river banks and alluvial plains in the dry season. The purpose of this work, therefore, is to establish the concentration and distribution of Pb, Zn, Cu, Cd, Hg, Cr, Ag and As in the soils/sediments of the mining areas of Abakaliki. Emphasis shall be placed on qualitative assessment of various soils in the area. Hence, providing valuable information in this regard may constitute a vital planning tool to healthcare providers and agro-allied industries on possible bioaccumulation of heavy metals in the food chain.
Geology and physiography
Geologically, the study area is underlain by the Asu River Group. The sediment is a product of the earliest documented marine transgression in Nigeria (Obage 2009; Nwajide 2013). According to Kogbe (1976), the group consists largely of olive-brown sandy shales, fine-grained micaceous sandstones and micaceous mudstones. Bluish-grey or olive-brown shales, which weather to a rusty brown colour, are also present. Nwachukwu (1975) described the sediments as structurally folded, particularly in the southern area of Abakaliki with fold axes stretching in the NE-SW direction. Locally, the shales are fissile at Enyigba, Amanchara and Mkpuma Akpatakpa area, while the indurated, hard and baked facies are prominent in the Abakaliki urban. Lead–zinc mineralizations (veins and lodes) were observed variously at Mkpuma Akpatakpa, Ameka, Enyigba, Amorie, Ekweburu Village and Agbaja. The area is mainly drained by the Ebonyi River and other tributaries like River Ewe, River Ogbogbo and Ikenyi River. All these rivers have major flow in the N-S direction in the tabular plain of the area (Fig. 2).
Material and method
Sample collection and preparation
A total of one hundred and sixteen (116) stream sediments and soil samples were collected in two seasons, fifty-eight (58) from each season, at various locations from the major tributaries and distributaries of Ebonyi River (which controls the drainage of the area), mine sites and agricultural farmlands (Fig. 2). Pretest systematic sample preparation was done for each soil sample analysed, including drying, screening and digestion. At the point of collection, the soil was dug about 10 cm to avoid undue concentration from top soil. Since the sediments were damp, they were firstly dried and disaggregated prior to sieving. The samples were laid out in pre-numbered evaporating dishes and sun-dried for three days. Each sample was disaggregated and homogenized by the use of agate pestle and mortar. Ukpong (1991) noted that for sediments from Benue Trough, the optimum grain size to achieve best geochemical contrast is 120mesh. The nylon screen was used to avoid contamination. After screening, 2.0 g of each sample was weighed for digestion. The samples were digested by using aqua regia, which was prepared by the mixture of HNO3 and HCl in the ratio 1:3. The heavy metal analysis was conducted using Varian AA240 atomic absorption spectrophotometer in line with American Public Health Association (APHA) (2005) specification. Electrical conductivity (EC) and pH were carried out using electrical conductivity meter (DDS 307 model) and pH meter (Hanna model H1991300), respectively.
Laboratory analysis
Laboratory analysis for the concentration of As, Cr, Zn, Ni, Pb, Cu, Hg, Cr, Cd and Mn2+ was done using fast sequential (FS) (Varian 2400 AA) atomic absorption spectrophotometer. Agilent FS2400AA has an accuracy level of 99.8%, precision of 97.6% and detection limit of 0.0003 ppm. All sampling steps and data analysis were performed according to standard methods for water and wastewater APHA (1995).
Data analysis
Microsoft Excel and Suffer 11 software were used to show the distribution of the contaminants in the area. The results were discussed and compared with the World Health Organization (WHO) standards for water quality with respect to the associated health risk.
Results and discussion
The results of geochemical analysis of soils/stream sediments are shown in Table 1, while Table 2 is a statistical summary with average abundance.
Concentration and distribution of various elements
Zinc (Zn)
Zinc is well dispersed in the study area, and all the samples recorded concentrations for zinc. The concentration of zinc ranged from 6.01 to 203 mg/kg, with a mean concentration of 48.07 mg/kg, from the dry season samples, while concentration of rainy season samples ranges from 0.08 mg/kg to 186 mg/kg. The standard deviation is 29.81. It also has a geochemical background value that has been set at 70 mg/kg in the Earth’s crust and 90 mg/kg for shale (Table 2). The highest concentrations of zinc were recorded at the areas around Enyigba, Ameka and Mkpuma Akpatakpa, where active mining is ongoing (Fig. 3a). This result shows higher concentrations in soils of the derelict mines than stream sediments in the area. The lowest concentration was recorded in River Abe Odomoke.
The distribution and transport of zinc in water, sediments and soils are dependent upon the species present and characteristics of the environment, especially pH, redox potential (Eh), salinity, nature and concentrations of complexing ligands, cation exchange capacity, and the concentration of zinc (Mcqueen 2008; Gundersen and Steinnes 2003). Sorption is the dominant reaction, resulting in the enrichment of zinc in suspended and bed sediments (ATSDR 2005; Environmental Protection Agency (EPA) 1979). Natural background of total zinc concentration is usually from 10 to 300 mg/kg dry weight in soils (Buchauer 1973). ATSDR (2005) stated that increased levels of zinc in soils can be attributed to natural occurrence of zinc enriched ores (as in the study area), anthropogenic sources including the disposal of zinc wastes from metal manufacturing industries and coal ash from electric utilities, sludge and fertilizer or even through abiotic and biotic processes. Toxicity in human may occur if zinc concentration in water approaches 400 mg/kg. This is characterized by symptoms of irritability, muscular stiffness, pain, loss of appetite and nausea. Zinc appears to have a protective effect against the toxicities of both cadmium and lead (Alloway 1995; Fergussion 1990).
Copper (Cu)
From the result, copper was observed in nearly all the locations except in Umuoze-Okoha and Ikenyi, and it was well dispersed. Copper has a mean concentration of 13.40 mg/kg and a range of 126 mg/kg for the dry season samples, while rainy season samples range from 0.16 to 115.17 mg/kg, with mean value of 15.49 mg/kg, and the standard deviation is 1.08. Copper is more concentrated at Ameka and Aghamegu (Fig. 3b). Geochemical background value was set as 55 mg/kg in Earth’s crust and 50 mg/kg in shale. The high concentrations of copper can be attributed to the mining activities and the chemical behaviour of copper. The movement of copper in soil is determined by the physical and chemical interactions of copper with soil components (Andrews and Sutherland 2004). Tyler and McBride (1982) noted that most copper deposited in soil from the environment and sludge disposal will be strongly adsorbed and remains in the upper layers of soil. In general, copper is mostly adsorbed to organic matter, carbonate minerals, clay minerals or hydrous iron and manganese oxides (Callahan et al. 1979; Fuhrer 1986). Apart from the fact that the distribution of copper in soils is less affected by pH than other metals, it binds to soil much more strongly than other divalent cations (Gerritse and Van Driel 1984). Tyler and McBride (1982), in a study of competitive adsorption and leaching of metals in soil columns of widely different characteristics, showed that copper eluted much more slowly and in much lower quantities than Zn, Cd and Ni from two mineral soils and not at all from peat soil, which contained the greatest amount of organic matter. Hermann and Neumann-Mahlkau (1985) demonstrated that copper shows a pronounced solubility in the oxidizing environment, than in the reducing environment, possibly due to the formation of sulphides.
Lead (Pb)
Lead–zinc deposits are the major source of lead in the environment. Other sources in dust and soil include lead that falls to the ground from the air and deterioration of products of lead-based paint from buildings (ATSDR 2007). Landfills in industrial areas are also potential sources, as they may contain waste from lead ore mining or other industrial activities such as battery production (Denaix et al. 2001). Deposits of lead-containing dust from the atmosphere (in industrial areas) and wastewater from industries handle raw materials that contain lead (primarily iron and steel industries and lead producers), urban run-off and mining piles (Finster et al. 2004). This analysis shows high dispersion for lead in the study area. A mean concentration of 18.49 mg/kg with the range of 0.00–74.3 mg/kg, for the dry season samples, was recorded for lead, while the result of rainy season samples ranged from 0 to 74.05 mg/kg, with mean concentrations of 19.71 mg/kg. The standard deviation is 0.37. The maximum concentration was recorded in samples around the Ameka and Mkpuma Akpatakpa mining area (Fig. 3c). Geochemical background value was set as 12.5 mg/kg in Earth’s crust and 20 mg/kg in shale. The occurrence of lead zinc ores (galena) in the study area notwithstanding the chemical behaviour of lead contributes to its higher values in the soils of the area. Studies by ATSDR (2007) showed that once lead falls onto soils, it sticks strongly to soil particles and remains in the upper layer of soil. Lead is immobilized by ion exchange with hydrous oxides or clays or by chelation with humic or fulvic acids in the soil (Olson and Skogerboe 1975). Reddy et al. (1995) have also concluded that the mobility of lead will increase in environments having low pH due to the increased solubility of lead under acidic conditions. Both studies showed that the accumulation of lead in most soils is primarily a function of the rate of deposition from the atmosphere. High concentrations of lead are retained strongly in soil, and very little is transported through run-off to surface water or leaching to groundwater except under acidic conditions EPA (1986) and NSF (1977), noted.
Cadmium (Cd)
Cadmium can be released to the atmosphere through metal production activities, fossil fuel combustion and waste materials incineration. Cadmium can also accumulate in soils due to atmospheric deposition and direct application methods such as the use of phosphate fertilizer and sewage sludge disposal. Geochemical analysis indicates concentrations of cadmium with a range of 8.25 mg/kg, with mean concentration of 1.08 mg/kg for the dry season samples, while rainy season samples ranged from 0.00 to 16.46 mg/kg with mean concentrations of 1.56 mg/kg. The standard deviation is 0.06. This indicates very high concentration, especially for the Ameka and Mkpuma Akpatakpa mining areas (Fig. 3d) when compared to the geochemical background value of 0.15 mg/kg on the Earth’s crust and 0.3 mg/kg in shale. ATSDR (2007) noted that high concentration of cadmium is commonly associated with the occurrences of zinc, lead and copper ores. This is the case of the study area. Garvey et al.(2013) and Harrison et al. (1981) also noted that in soils, physical and chemical factors like pH, oxidation–reduction reactions, formation of complexes and the availability of organic matter are affected by the mobility of cadmium. Low pH (acidity) increases the availability of cadmium (Elinder 1992). Generally, cadmium is more immobilized in soils. This is because it binds strongly to organic matter (Autier and White 2004). Studies by Kamau, (2001) and Elinder (1985) showed that while soluble forms may migrate in water, it is relatively nonmobile in insoluble complexes or adsorbed to sediments. Although cadmium may be released into the air as particles and vapour, the net flux to soil will be positive as cadmium will eventually deposit onto soils. Harrison et al. (1981) also observed that cadmium may precipitate as insoluble cadmium compounds or form complexes or chelates by interaction with organic matter.
Chromium (Cr)
Chromium was evenly distributed in the study area. The mean concentration of Cr is 9.00 mg/kg with a range of 0.00-28.32 mg/kg for the dry season samples, while rainy season samples range from 0.00 to 51.87 mg/kg with mean of 8.20 mg/kg. Higher concentrations were observed around the mines than other areas (Fig. 4a). Geochemical background value was set as 100 mg/kg in Earth’s crust and 73 mg/kg in shale.
Although chromium occurs naturally in rocks, animals, plants and soil, where it exists in combination with other elements to form various compounds, ATSDR (2012) indicated that anthropogenic activities like the manufacture of chromium-based products, leather tanning and the burning of natural gas, oil or coal can release chromium in the environment. (Ashley et al. (2003) noted that the movement of chromium in soil is dependent upon the speciation of chromium, which is determined by the redox potential and the pH of the soil. Researches by Barnhart (1997) and Robson (2003) have noted that in most soils, chromium will be present predominantly in the chromium (III) oxidation state. This form has very low solubility and low reactivity, resulting in low mobility in the environment. Many researchers including James et al. (1997), Balasoiu et al. (2001), Jardine et al. (1999) and Robson (2003) have studied the solubility and mobility of chromium. James et al. (1997) noted that chromium (VI) may be present in soil as CrO −24 and HCrO4 under oxidizing conditions and Sahuquillo et al. (2003) demonstrated a leachability study comparing the mobility of several metals including chromium and showed that chromium had the least mobility of all of the metals studied. Balasoiu et al. (2001), Jardine et al. (1999) and Robson (2003) have supported previous data findings that chromium is not very mobile in soil, especially in the trivalent oxidation state. Leachability investigations by Sheppard and Thibault (1991) carried out in a period of 4 years in a sandy loam also showed that chromium mobility is low while Jardine et al. (1999) emphasized that in deeper soil where anaerobic conditions exist, chromium (VI) will be reduced to chromium (III) by S−2 and Fe+2 present in soil.
Mercury (Hg)
Low concentration of mercury was observed in the study area. Only few samples around Abakaliki area showed the presence of Hg while the rest showed low concentrations (Fig. 4b). The mean concentration of mercury is 0.37 mg/kg with the range of 0.00–6.03 mg/kg for the dry season samples, while rainy season samples ranged from 0.00 to 8.1 mg/kg. Its maximum concentrations were recorded at the Amanchara and the Ameka mining areas. The accumulation of mercury in the environment is due to the weathering of minerals in rocks and soil from exposure to climatic forces and from volcanic activity, and ATSDR (2012) noted that human activities such as mining and burning of fossil fuels have resulted in its additional release to the environment. In soils and surface waters, mercury can exist in two states: mercuric (Hg+2) and mercurous (Hg+1) (Meili 2013). Temperature controls the vaporization of mercury from soils, with emissions from contaminated soils being greater in warmer weather when soil microbial reduction of Hg+2 to the more volatile elemental mercury is greatest (Lindberg et al. 1991). Sato and Sada (1992) and WHO (1990, 1991) noted that atmospheric deposition of mercury from both natural and anthropogenic sources has been identified as an indirect source of mercury to soil and sediments. This is correct as mercury is released to cultivated soils through the direct application of inorganic and organic fertilizers (e.g. sewage sludge and compost), lime and fungicides containing mercury (Andersson 1979). In their view, Glass et al. (1991) suggested that the concentration of mercury in the atmosphere is due to its long resident time in the atmosphere. They estimated the residence time in the atmosphere to range from 60 days to 2 years. Mercury concentration can also increase by the release of volatile forms in water or soil, but most mercury is adsorbed to soil and sediment (EPA 1984; Meili 2013). Mercuric mercury usually forms various complexes with chloride and hydroxide ions in soils (Boske et al. 2004; Andersson 1979).
Silver (Ag)
From the result obtained, silver was not evenly distributed compared to the heavy metals in the study area. Many samples showed the absence of silver for the two seasons. Maximum concentration of 30.29 mg/kg and 7.7 mg/kg and mean concentration of 3.14 mg/kg and 0.52 mk/kg were recorded for the dry and rainy seasons, respectively. The mining regions of Mkpuma Akpatakpa and Enyigba showed higher concentrations (Fig. 4c). Silver has a standard deviation of 1.72.
Mining processes and the weathering of silver-bearing rocks and ores like chalcopyrite, galena and siderite are the major sources of silver in soils and the environment. ATSDR (1990) also indicated that rain can also release large amounts of silver into the environment. Apart from the weathering of ores which deposits silver in the study area, other factors which affect the mobility of silver in soils are drainage (silver tends to be removed from well-drained soils); oxidation–reduction potential and pH conditions (which determine the reactivity of iron and manganese complexes which tend to immobilize silver); and the presence of organic matter (which complexes with silver and reduces its mobility) (Boyle 1968). Although the environmental fate of silver are not well studied and characterized, silver and its compounds are transported in the air, water and soil and are partitioned between these media (ATSDR 1990; Dissanayake et al. 1983). The transport and partitioning of silver in surface waters and soils are influenced by the particular form of the compound. However, according to Lindsay and Sadiq (1979), under oxidizing conditions the primary silver compounds would be bromides, chlorides and iodides, while under reducing conditions the free metal and silver sulphide would predominate.
Arsenic (As)
The result of geochemical analysis showed that arsenic was dispersed in the study area (Fig. 4d). Many of the samples locations show high concentrations of arsenic. It has a maximum concentration of 56 mg/kg with mean concentration of 11.19 mg/kg for the dry season samples, while rainy season samples showed very low concentration values of 0.00 mg/kg (in most places)—19.0 mg/kg with mean concentration of 1.21 mg/kg. The standard deviation is 23.91. Geochemical background value was set as 1.8 mg/kg in Earth’s crust and 10 mg/kg in shale. This result indicates very high contamination for the area, especially in the mining fields of Ameka and Enyigba.
Major sources of arsenic in the environment are exploitation and processing of minerals and ores that contain copper or lead. Environmental and physical forces including leaching may transport arsenic into subsurface soil (Moore et al. 1988). However, EPA (1982), Pantsar-Kallio and Manninen (1997) and Welch et al. (1988) noted that under oxidizing conditions, leaching does not transport arsenic to great depth in the soil profile. Sanok et al. (1995) also affirmed that arsenic is largely immobile in agricultural soils; therefore, it tends to concentrate and remain in upper soil layers indefinitely. Natural or anthropogenic particles of arsenic form insoluble complexes with iron, aluminium and magnesium oxides in soil surfaces, and in this form, arsenic is relatively immobile (Merwin et al. 1994).
Conclusion
The geochemical assessment of soils and stream sediments in the mining areas of Abakaliki, Southeastern Nigeria, was carried out in all communities where active and abandoned mines are located: Enyigba, Mkpuma Akpatakpa, Ameka, Amorie, Amanchara, Agbaja and Alibaruhu. One hundred and sixteen soil/stream sediments were collected and subjected to geochemical analysis using an atomic absorption spectrophotometric method. The study showed high concentration and percentage contamination of Cd > Pb > As > Zn > Cu > Ag > Hg > Cr. This implies that arable soils around the mining areas (about 5 km radius) are not suitable for food crop production as biomagnification can occur in the food chain. Hence, agricultural activities should be concentrated in safe areas to minimize the risk of potential health hazards. This work showed that the discharge of mine wastes and gangues in mining areas has effects on the available arable soils in mining areas. This can also serve as pointers for mineral exploration in mining areas. This assessment shows higher distribution of metals in the Mkpuma Akpatakpa and Ameka areas than the Enyigba and Amanchara areas. Solid minerals with high composition of Cd, Pb, As and Zn can be harnessed in the area.
References
Agency for Toxic Substances and Disease Registry (ATSDR) (1990) Toxicological profile for Copper U.S. Department of Health and Human Services, Public Health Service, Division of Toxicology 1600, Atlanta, GA 30333
Agency for Toxic Substances and Disease Registry (ATSDR) (2005) Toxicological profile for Nickel U.S. Department of Health and Human Services, Public Health Service, Division of Toxicology 1600, Atlanta, GA 30333
Agency for Toxic Substances and Disease Registry (ATSDR) (2007) U.S. Department of Health and Human Services, Public Health Service, Division of Toxicology 1600, Atlanta, GA 30333
Agency for Toxic Substances and Disease Registry (ATSDR) (2012) Toxicological profile for Chromium U.S. Department of Health and Human Services, Public Health Service, Division of Toxicology 1600, Atlanta, GA 30333
Ailsa A, Michael A (1999) A dictionary of Earth Science. Oxford University Press, London
Akoto O, Ephraim JH, Darko G (2008) Heavy metal pollution in surface soils in the vicinity of abundant railway servicing workshop in Kumasi, Ghana. Int J Environ Res 2(4):359–364
Alexakis D (2016) Human health risk assessment associated with Co, Cr, Mn, Ni and V contents in agricultural soils from a Mediterranean site. Arch Agron Soil Sci 62(3):359–373
Alloway JB (1995) Soil pollution and land contamination. In: Harrison RM (ed) Pollution: causes, effects and control. R Soc Chem, Cambridge
Al-Momani IF (2009) Assessment of trace metal distribution and contamination in surface soils of Amman, Jordan. J Chem 4(1):77–87
American Public Health Association (APHA) (2005) Standard methods for the examination of water and wastewater. American Water Works Association-World Environmental Foundation, Washington, DC, 1000–3500
Andersson A (1979) Mercury in soils. In: Nriagu JO (ed) The biogeochemistry of mercury in the environment. Elsevier/North Holland Biomedical Press, New York, pp 79–112
Andrews S, Sutherland RA (2004) Cu, Pb and Zn contamination in Nuuanu watershed, Oahu, Hawaii. Sci Total Environ 324(1–3):173–182
Asaah VA, Abimbola AF, Suh CE (2006) Heavy metal concentration and distribution in surface soils of the Bassa industrial zone1, Douala, Cameroon. Arab J Sci Eng 31(2A):147–158
Ashley K, Howe AM, Demange M (2003) Sampling and analysis considerations for the determination of hexavalent chromium in workplace air. J Environ Monit 5(5):707–716
Australasian Institute of Mining and Metallurgy,(AusIMM) (2011) Field Geologists’ Manual, Fifth Edition, Monograph 9, Carlton Victoria. 480 pp Chapter 4, Section 4.7. General Notes for Geochemical Sampling
Autier V, White D (2004) Examination of cadmium sorption characteristics for aboreal soil near Fairbanks, Alaska. J Hazard Mater 106B:149–155
Balasoiu CF, Zagury GJ, Deschenes L (2001) Partitioning and speciation of chromium, copper, and arsenic in CCA-contaminated soils: influence of soil composition. Sci Total Environ 280(1–3):239–255
Barnhart J (1997) Chromium chemistry and implications for environmental fate and toxicity. J Soil Contam 6(6):561–568
Boske L, Sobczynski O, Kowalski A (2004) Distribution of mercury and other heavy metals in bottom sediments of the middle Odra River (Germany/Poland). Polish J Environ Stud 13(5):495–502
Boyle RW (1968) Geochemistry of silver and its deposit notes on geochemicalprospecting for the element. Geological Survey of Canada. Ottawa, Ont: Canada, Department of Energy, Mines and Resources. 160, l–96
Buchauer MJ (1973) Contamination of soil and vegetation near a zinc smelter by zinc, cadmium, copper, and lead. Environ Sci Technol 7:131–135
Callahan MA, Slimak HW, Gabel NW (1979) Water-relatedenvironmental fate of 129 priority pollutants. Washington, DC: Office of Water Planning and Standards, U.S. Environmental Protection Agency, ll-lll-19. EPA 440/4-79-029a
Casper ST, Mehra A, Farago ME, Gill RA (2004) Contamination of surface soils, river water and sediments by trace metals from copper processing industry in the Churnet River Valley, Staffordshire, UK. Environ Geochem Health 26:59
Chibuike GU, Obiora SC (2014) Heavy metal polluted soils: effects on plants and bioremediation methods, applied and environmental soil science, 2014: Article ID 752708, p 12, https://doi.org/10.1155/2014/752708
Denaix L, Semlali RM, Douay F (2001) Dissolved and colloidal transport of Cd, Pb, and Zn in a silt loam soil affected by atmospheric industrial deposition. Environ Pollut 113:29–38
Dikinya O, Areola O (2010) Comparative analysis of heavy metal concentration in secondary treated waste irrigated soil cultivated by different crops. Int J Environ Sci Technol 7(2):337–346
Dissanayake CB, Tobschall HJ, Palme H (1983) The abundance of somemajor and trace elements in highly polluted sediments from the Rhine river near Mainz, West Germany. Sci Total Environ 29:243–260
Elinder CG (1985) Cadmium: uses, occurrence and intake. In: Friberg L, Elinder CG, Kjellström T (eds) Cadmium and health: a toxicological and epidemiological appraisal, vol. I. Exposure, dose, and metabolism.Effects and response. CRC Press, Boca Raton, pp 23–64
Elinder CG (1992) Cadmium as an environmental hazard. IARC Sci Publ 118:123–132
Environmental Protection Agency (EPA) (1979) Water-related environmental fate of 129 priority pollutants. Washington, DC: U.S. Environmental Protection Agency, Office of Water Planning and Standards. (EPA) 440479029a
Environmental Protection Agency (EPA) (1982) Exposure and risk assessment for arsenic. Washington, DC: U.S. Environmental Protection Agency, Office of Water Regulations and Standards. PB85221711.(EPA)440485005. 1.14.68
Environmental Protection Agency (EPA) (1984) Health assessment document for manganese. Final draft. Cincinnati, OH: U.S. Environmental Protection Agency, Office of Research and Development. (EPA) 600883013F
Environmental Protection Agency (EPA) (1986) Air quality criteria for lead. Research Triangle Park, NC: U.S. Environmental Protection Agency, Office of Research and Development, Office of Health and Environmental Assessment, Environmental Criteria and Assessment Office. (EPA)600883028F
Ezeh HN, Anike OL, Egboka BCE (2007) The distribution of some heavy metals in soil in areas around the Derelict Enyigba mines and its environmental implication. J Min Geol 2(2):99–106
Fergussion IE (1990) The heavy elements chemistry, environmental impact and health effects. Pergamon Press, New York
Finster ME, Gray KA, Binns HJ (2004) Lead levels of edibles grown in contaminated residential soils: a field survey. Sci Total Environ 320:245–257
Fuhrer GJ (1986) Extractable cadmium, mercury, copper, lead, and zinc in the Lower Columbia Rover estuary, Oregon and Washington. In: U.S. Geological Survey water-resources investigations report. Portland
Garvey GJ, Hahn G, Lee RV (2013) Heavy metal hazards of Asian traditional remedies. Int J Environ Health Res 11(1):63–71
Gerritse RG, Van Driel W (1984) The relationship between adsorption of trace metals, organic matter, and pH in temperate soils. J Environ Qual 13:197–204
Glass GE, Sorenson JA, Schmidt KW (1991) Mercury deposition and sources for the upper Great-Lakes region. Water Air Soil Pollut 56:235–249
Gundersen P, Steinnes E (2003) Influence of pH and TOC concentration on Cu, Zn, Cd, and Al speciation in rivers. Water Res 37:307–318
Harikumar PS, Jisha TS (2010) Distribution pattern of trace metal pollutants in the sediments of an urban wetland in the southwest coast of India. Int. J. Eng. Sci. Tech. 2(5):540–850
Harrison RM, Laxen DPH, Wilson SJ (1981) Chemical association of lead, cadmium, copper and zinc in street dust and roadside soils. Environ Sci Technol 15:1378–1383
Hermann R, Neumann-Mahlkau P (1985) The mobility of zinc, cadmium, copper, lead, iron and arsenic in groundwater as a function of redoxpotential and pH. Sci Total Environ 43:1–12
James BR, Petura JC, Vitale RJ (1997) Oxidation-reduction chemistry of chromium: relevance to the regulation and remediation of chromate-contaminated soils. J Soil Contam 6(6):569–580
Jardine PM, Fendorf SE, Mayes MA (1999) Fate and transport of hexavalent chromium in undisturbed heterogeneous soil. Environ Sci Technol 33(17):2939–2944
Kamau JN (2001) Heavy metals distribution in sediments along the Killindini and Makupa creeks, Kenya. Hydrobiologia 458:235–240
Kishe MA, Machwa JF (2003) Distribution of heavy metals in sediments of Mwanza Gulf of LakeVictoria, Tanzania. Environ Int 28:619–625
Kogbe, C. A.(1976), Palegeographic history of Nigeria from Albian times. In: Kogbe CA (ed) Geology of Nig. Elizabeth Publishers Lagos, pp 237–252
Lech M, de Caritat P, McPherson A (2007) National Geochemical Survey of Australia: Field Manual. Geoscience Australia, Canberra. 53 pp Appendix 1. Soil sampling procedures for NGSA
Levinson AA (1974) Introduction to exploration geochemistry applied sciences. Publishing Ltd. Maywood Illionois, p 614
Li Z, Zongwei M, Tsering JV, Zengwei Y, Lei H (2014) A review of soil heavy metal pollution from mines in China: pollution and Health risk assessment. Sci Total Environ 468(469):843–853
Lindberg SE, Turner RR, Meyers TP (1991) Atmospheric concentrations and deposition of mercury to a deciduous forest at Walker Branch Watershed, Tennessee, USA. Water Air Soil Pollut 56:577–594
Lindsay WL, Sadiq M (1979) Theoretical solubility relationships of silver in soils. In: Klein DA (ed) Environmental impacts of artificial ice nucleating agents. Dowden, Hutchinson, and Ross, Inc
Loska K, Wiechula D (2003) Application of principal component analysis of source of heavy metal contamination in surface sediments from the Rybnik Reservoir. Chemosphere 57:723–733
Lucassen E, Smolders AJP, Roelofs JGM (2002) Potential sensitivity of mines to drought, acidification and mobilization of heavy metals: the sediment S/Ca +Mg) ratio as diagnostic tool. Environ Pollut 120:635–646
Mcqueen KG (2008) A guide for mineral exploration through the regolith in the Cobar Region, Lachlan Orogen, New South Wales. CRC LEME, Perth. 110 pp Appendix. Recommended procedure for geochemical sampling and analysis
Meili M (2013) The coupling of mercury and organic matter in the biogeochemical cycle: towards a mechanistic model for the boreal forest zone. Water Air Soil Pollut 56:333–347
Mendoza CA, Cortes G, Munoz D (1996) Heavy metal pollution in soils and sediments of ruraldeveloping district 063, Mexico. Environ Toxicol Water Qual 11:327–333
Merwin I, Pruyne PT, Ebel JG (1994) Persistence, phytotoxicity, and management of arsenic, lead and mercury residues in old orchard soils of New York State. Chemosphere 29(6):1361–1367
Moore JN, Ficklin WH, Johns C (1988) Partitioning of arsenic and metals in reducing sulfidic sediments. Environ Sci Technol 22:432–437
Nriagu JO, Pacyna JM (1988) Quantitative assessment of worldwide contamination of air, water and soils by trace metals. Nature 333:134–139
NSF (1977) Transport and distribution in a watershed ecosystem. In: Boggess WR (ed) Lead in the environment. Washington, DC: National Science Foundation. Report No. NSFRA770214, 105-133
Nwajide CS (2013) Geology of Nigeria’s sedimentary basins. CSS Bookshops Limited, Lagos
Nwachukwu SO (1975) Temperature of formation of vein, minerals in the south portion of the Benue Trough, Nigeria. J Mining Geol 11(1–2):44–55
Obage GN (2009) Geology and mineral resources of Nigeria. Springer, London
Obasi PN (2017) Hydrogeological and geochemical assessment of the lead – zinc mining areas of abakaliki ebonyi state, Southeastern Nigeria. Unpublished Ph. D thesis. Nnamdi Azikiwe University, Awka, Nigeria
Obasi PN, Akudinobi BEB (2015) Geochemical assessment of heavy metal distribution and pollution status in soil/stream sediment in the Ameka Minning Area of Ebonyi State, Nigeria. Afr J Geo-Sci Res 3(4):01–07
Obasi PN, Akudinobi BEB (2019a) Pollution status of arable soil and stream sediment in the mining areas of Abakaliki, lower benue trough. Springer Int J Environ Sci Technol. doi:0.1007/s13762-019-02337-z
Obasi PN, Akudinobi BEB (2019b) Heavy metals occurrence, assessment and distribution in water resources in the Lead-Zinc mining areas of Abakaliki, Southeastern Nigeria. Springer Int J Environ Sci Technol. https://doi.org/10.1007/s13762-019-02489-y
Obasi PN, Obini N, Ani CC, Okolo CM (2018a) Evaluation of hydrochemical attributes of the Amachara Mining area, lower benue trough. Int J Sci Eng Sci 2(1):60–65
Obasi PN, Esom NE, Okolo CM, Edene EN (2018b) Assessment of water pollution status in the mining area of Ameka, South Eastern Nigeria using metal pollution index. Int J Sci Eng Sci 2(1):66–73
Obiorah SC, Chukwu A, Davies TC (2018) Heavy metals and health risk assessment of arable soils and food crops around Pb–Zn mining localities in Enyigba, Southeastern Nigeria. J Afr Earth Sci 116:182–189
Obiorah SC, Chukwu A, Toteu SF, Davies TC (2016) Assessment of heavy metals contamination in soils around Pb–Zn mining areas in Enyigba, Southeastern Nigeria. J Geol Soc India 87:453–462
Olson KW, Skogerboe RK (1975) Identification of soil lead compounds from automotive sources. Environ Sci Technol 9:227–230
Oti WJO, Nwabue FI (2013) Heavy metals effect due to contamination of vegetables from Enyigba Lead Mine in Ebonyi State, Nigeria. Environ Pollut 2(1):19-26
Pantsar-Kallio M, Manninen PKG (1997) Speciation of mobile arsenic in soil samples as a function of pH. Sci Total Environ 204(2):193–200
Reddy KJ, Wang L, Gloss SP (1995) Solubility and mobility of copper, zinc and lead in acidic environments. Plant Soil 171:53–58
Robson M (2003) Methodologies for assessing exposures to metals: human host factors. Ecotoxicol Environ Saf 56:104–109
Sahuquillo A, Rigol A, Rauret G (2003) Overview of the use of leaching/extraction tests for risk assessment of trace metals in contaminated soils and sediments. TrAC Trends Anal Chem 22(3):152–159
Sanok WJ, Ebel JG, Manzell KL (1995) Residues of arsenic and lead in potato soils on Long Island. Chemosphere 30(4):803–806
Sato K, Sada K (1992) Effects of emissions from a coal-fired power plant on surface soil trace element concentrations. Atmos Environ Pt 26A:325–331
Sheppard MI, Thibault DH (1991) A four-year mobility study of selected trace elements and heavy metals. J Environ Qual 20:101–114
Tyler LD, McBride MB (1982) Mobility and extractability of cadmium, copper, nickel, and zinc in organic and mineral soil columns. Soil Sci 134:198–205
Ukpong EE (1991) Total and partial extractable metals in anomalous and Background stream sediments samples from lead-zinc Belt of Benue Trough, Nigeria: a comparative analysis of extraction techniques. J Min Geol 18(1):209–215
Vanek A, Boruvka L, Drabek O, Mihaljevic M, Komarek M (2005) Mobility of lead, zinc abd cadmium in alluvial soils heavily polluted by smelting industry. Plant Soil Environ 51(7):316–321
Welch AH, Lico MS, Hughes JL (1988) Arsenic in groundwater of the western United States. Ground Water 26(3):333–347
World Health Organization (WHO) (1990) Guidelines for drinking water quality, Geneva
World Health Organization (WHO) (1991) Guidelines for drinking water quality, Geneva
Yousefi M, Saleh HN, Mohammadi AA, Mahvi AH, Ghadrpoori M, Suleimani H (2017) Data on water quality index for the groundwater in rural area Neyshabur County, Razavi province, Iran. Data Brief 15(2017):901–907
Zaborski PM (1998) A review of the Cretaceous System in Nigeria. Afr Geosci Rev 5(4):385–483
Zhiyuan L, Zongwei M, Tsering JV, Zengwei Y, Lei H (2014) A review of soil heavy metal pollution from mines in China: Pollution and health risk assessment. Sci Total Environ 468(469):843–853
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
I state clearly, that I have no conflict to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Obasi, P.N. Occurrence and distribution of heavy metal in arable soils around lead–zinc mining sites of Abakaliki, Southeast Nigeria. Model. Earth Syst. Environ. 6, 1887–1899 (2020). https://doi.org/10.1007/s40808-020-00800-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40808-020-00800-2