Abstract
Mating is a double-edged sword. It can have great adaptive benefits, but also high costs, depending on the mate. Disgust is an avoidance reaction that serves the function of discouraging costly mating decisions, for example if the risk of pathogen transmission is high. It should, however, be temporarily inhibited in order to enable potentially adaptive mating. We therefore tested the hypothesis that sexual arousal inhibits disgust if a partner is attractive, but not if he is unattractive or shows signs of disease. In an online experiment, women rated their disgust towards anticipated behaviors with men depicted on photographs. Participants did so in a sexually aroused state and in a control state. The faces varied in attractiveness and the presence of disease cues (blemishes). We found that disease cues and attractiveness, but not sexual arousal, influenced disgust. The results suggest that women feel disgust at sexual contact with unattractive or diseased men independently of their sexual arousal.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Mating and pathogen avoidance both pose fundamental adaptive challenges. They have substantially shaped the evolution of traits in non-human animals, as well as in humans (Buss and Symons 2015; Dixson 2009; Schaller 2015; Trivers 1996). However, the two are in tension (Lee et al. 2014): Mating behavior like kissing or sex necessarily involves close physical contact and exchange of bodily fluids, which poses a large risk of infection with pathogens (Fleischman et al. 2015). Mating decisions must therefore weigh the costs of pathogen transmission with the benefits of mating success (Tybur and Gangestad 2011; Tybur et al. 2013).
The potential costs and benefits of mating pivot around the mates. Mating decisions therefore incorporate information about particular mates in question. Particularly relevant are their genetic quality and health status (Tybur and Gangestad 2011). For both of these properties, there exist easily available visual cues that provide a heuristic for assessments (Sugiyama 2016). Physical attractiveness is assessed within milliseconds of seeing a new face (Willis and Todorov 2006) and is a reliable indicator of genetic quality and health (Little 2014; Re and Rule 2016). Disease often manifests itself visibly on the organisms, for example through skin blemishes (Bundy 2012; Ryan et al. 2012). Hence, visual information about a potential mate should induce avoidance and approach tendencies in line with anticipated costs or benefits of mating (Al-Shawaf, Conroy-Beam, Asao, & Buss, 2016; Tooby and Cosmides 2008).
Disgust evolved primarily to protect the organism from pathogen threats by encouraging avoidance of potential pathogen vectors (Curtis et al. 2004; Oaten et al. 2009; Tybur and Lieberman 2016). Hence, such stimuli also elicit it (Rozin et al. 2008). But the emotion also promotes adaptive mating decisions (Al-Shawaf, Lewis, & Buss, 2015; Tybur et al. 2013). This is evidenced by the fact that people experience disgust not only towards individuals who show signs of bad health, pathogens, or potentially contagious disease (Mortensen et al. 2010; Rozin et al. 2008; Ryan et al. 2012) but also towards for example mates outside of a fertile age range (Fessler and Navarrete 2003; Tybur et al. 2009). Disgust therefore discourages costly mating.
While disgust encourages avoidance, sexual arousal facilitates approach. This psycho-physiological state facilitates sexual engagement through the following means: preparing the body physiologically for intercourse (Masters and Johnson 1966), temporarily increasing sexual desire (Toates 2009), narrowing attention to sexual stimuli, increasing approach motivation towards them, and inhibiting negative emotions (Barlow 1986; Janssen and Bancroft 2007; Prause et al. 2008). Similar to disgust, it is sensitive to traits of the mate, particularly physical attractiveness (Buunk et al. 2002; Hawk et al. 2007; Stone et al. 2011). Both disgust and sexual arousal therefore partly determine mating decisions based on visual information about a mate.
Disgust and Sexual Arousal Influence Each Other
Disgust and sexual arousal motivate avoidance and approach, respectively, and are therefore oppositional. Accordingly, people tend to experience only one of them at a time (Koukounas & McCabe, 2001; Vonderheide and Mosher 1988). Because sexual intercourse inherently involves stimuli that can trigger strong aversion (e.g., genitals; Rozin et al. 1995), sexual arousal should facilitate sexual engagement by temporarily inhibiting avoidance tendencies and therefore also disgust responses (Borg and de Jong 2012). This also corresponds to predictions by clinical models of sexual response: According to the dual-control model of sexual response (Janssen and Bancroft 2007), negative emotions like disgust need to be suppressed to enable seamless sex, in that they draw attention away from sexual stimuli and therefore disrupt the sexual response cycle. More specifically, de Jong et al. (2013) propose that sexual arousal and disgust inhibit one another. They claim that in a given moment, the more prominent of the two suppresses the other and gives rise to the congruent response. That means that if disgust outweighed sexual arousal, it would pull away attention from arousing stimuli and guide it towards the disgust elicitor, and vice versa. Accordingly, this would reduce sexual arousal and therefore reduce approach, facilitating avoidance.
Research indeed indicates that disgust inhibits sexual arousal. Fleischman et al. (2015) and Andrews et al. (2015) experimentally induced disgust in a group of male and female participants and found that those groups reported less subsequent sexual arousal to erotic movies than the control groups. Thus, the existing evidence for an inhibitory effect of disgust on sexual arousal is relatively clear cut in both sexes. Three experiments also showed convincingly that men’s disgust sensitivity decreases under sexual arousal (Ariely and Loewenstein 2006; Lee et al. 2014; Stevenson et al. 2011).Footnote 1 Findings on the effect of sexual arousal on disgust in women, however, have been very mixed.
Does Sexual Arousal Inhibit Disgust in Women?
A Note on Disgust Domains
The studies we are going to review have discriminated between types/domains of disgust, and have used different classifications to do so. We will therefore briefly clarify the terms used. Tybur et al. (2013) coined the term sexual disgust to refer to disgust towards sexual acts that are evolutionarily disadvantageous, e.g., sex with a partner in an infertile age range. However, van Overveld et al. (2013) used it in the context of disgust towards sex-related stimuli more generally, interchangeably with sex-related disgust. Borg and de Jong (2012) have used the word sex-related disgust to describe disgust towards sex-related stimuli. Sex-related disgust is therefore a more general term that also encompasses both usages of sexual disgust. We will therefore refer to sexual disgust according to Tybur et al.’s (2013) definition and to sex-related disgust as elicited by sex-related stimuli. Pathogen disgust is generally used to refer to disgust elicited by stimuli of potentially infectious organisms (Tybur et al. 2013). This can also exclude sexual stimuli, but we will use it to refer to disgust to only sex-unrelated stimuli.
Four studies have directly examined whether women experience a reduction in disgust when sexually aroused. They all used a similar experimental setup, in which participants were either sexually aroused or in a control state, and rated their disgust towards various stimuli. Borg and de Jong (2012) asked sexually aroused, positively/physically aroused, and unaroused women to perform certain disgusting tasks. The tasks were either sex-related (e.g., touching a lubricated condom) or not (e.g., eating a biscuit with an insect on it). The sexually aroused group, compared to the other two groups, completed the highest number of disgusting tasks and reported finding all the tasks less disgusting. These group differences were larger for sex-related tasks than sex-unrelated tasks.
Lee et al. (2014) looked at sexual and pathogen disgust. Participants sexually aroused themselves at home in their own preferred way and then filled out the Three Domains of Disgust Scale that comprises pathogen, moral, and sexual disgust (Tybur et al. 2009). Sexually aroused women were less sexual disgust sensitive than the control group, but also more pathogen disgust sensitive. Van Overveld and Borg (2015) found no difference in disgust towards neutral, pathogenic, or sexual images between a sexual arousal and a threat arousal group. Finally, Fleischman et al. (2015) found no influence of sexual arousal on women’s pathogen disgust. Arousal was measured both by self-report and with a physiological measure of genital blood flow (vaginal plethysmography). Women who were aroused did not rate highly pathogen salient images (e.g., excrement, dead animals, and injured, diseased, or dead humans) as less disgusting than women who were not aroused. The authors also found that both physiological and subjective measures of sexual arousal did not decrease subsequent ratings of disgust at these images. Taken together, these studies suggest that sexual arousal does not inhibit disgust generally. Effects have been selective on particular classes of stimuli in single studies, but with inconsistent results across studies. One limitation of all four studies is that they assessed participants’ disgust towards stimuli that are largely irrelevant for mating. But as we have argued, avoidance and approach motives should be regulated according to properties of potential mates. The interactional effect of sexual arousal and disgust should therefore depend on the sexual partner at hand. Sexual arousal may facilitate intercourse with physically attractive, healthy-looking mates, whereas disgust may prevent intercourse with unattractive or diseased mates.
Study and Hypotheses
In the present study, we tested whether sexual arousal downregulates women’s disgust towards potential mates and whether this is moderated by the mates’ physical attractiveness and disease cues (rosacea blemishes). Participants rated their disgust towards different men when they were sexually aroused and when they were not. We thereby extended previous methods by testing the effect of sexual arousal on disgust within participants. The focus was on women because past studies have found the link between sexual dysfunction and disgust only in women, not in men (Borg et al. 2010; Grauvogl et al. 2015) and because there are significant sex differences in disgust (Fleischman 2017; Skolnick 2013; Tybur et al. 2011), sexual arousal patterns (Carvalho et al. 2013; Chivers 2010), and the interaction of the two (Andrews et al. 2015; Lee et al. 2014). We hypothesized that sexual arousal will decrease disgust towards attractive, but not towards unattractive mates (hypothesis 1) and that sexual arousal will only reduce disgust towards mates without disease cues, but not towards mates with disease cues (hypothesis 2).
Method
Materials
Faces
Twenty pictures of male faces were obtained with permission from a modeling website and from the Warsaw set of emotional facial expression pictures (Olszanowski et al. 2015). We chose to show only faces because women generally pay most attention to and infer most relevant information about a mate by looking at the face (Re and Rule 2016; Wagstaff et al. 2015). They were rated for attractiveness by 121 women from the social network of the first author on a scale ranging from 0 = not at all, to 10 = extremely, with the midpoint 5 = average. We choose the six pictures with the highest and the six with the lowest attractiveness ratings. The mean rating of the attractive faces was 6.60 (SD = .60) and the mean of the unattractive ones was 2.51 (SD = .54). This difference was statistically significant with a large effect size, t = 55.73, p < .000, d = .96. A professional photo editor then first smoothed the skin of all faces, because skin quality is a cue to disease as well (Bundy 2012; Re and Rule 2016; Sugiyama 2016). He then made a version of each picture in which the face contained blemishes typical of the disease rosacea. See Fig. 1 for example pictures.
Videos
There was one video for each of the two conditions. The video in the sexual arousal condition was a 6-min women friendly erotic clip showing a Caucasian mixed-sex couple. The first minute consisted of non-genital foreplay, followed by about 2 min of cunnilingus, and another 3 min of penile-vaginal intercourse in the man-on-top and rear entry position. Forty-five women in previous studies (Hamilton and Meston 2013; Harte and Meston 2008) have reacted to it with both physiological and self-reported sexual arousal (L. D. Hamilton, personal correspondence, December 8, 2015). The video also corresponded to the recommendations for female sex research by Woodard et al. (2008). The neutral condition contained a 7-min video blog of a Caucasian, mixed-sex couple’s hiking trip (Flying The Nest 2015). The video was chosen because it was simple, physical, and pleasant and thus relatively comparable to the erotic clip. Furthermore, the couple was comparable in physical attractiveness to the pornographic actors.
Procedure
When signing up for the study, we instructed participants to participate online from home where likely most comfortable being sexually aroused. Prior studies have successfully employed related methods (Ariely and Loewenstein 2006; Lee et al. 2014). When participants opened the link to the study on Qualtrics, they were randomly assigned to one of the two conditions and received informed consent accordingly. The instructions emphasized that they should be alone and undisturbed for at least 20 min. They were then asked to provide information on demographics, sexual orientation, their menstrual cycle, relationship status, use of oral contraception, and recent illness. Parts of this information were used to control for factors that have been shown to influence women’s disgust reactions (Fessler and Navarrete 2003; Fleischman 2014), perception of potential partners’ faces (DeBruine 2014; Sacco et al. 2012), and reaction to visual sexual stimuli (Mass et al. 2009; Renfro et al. 2015; Wallen and Rupp 2010).
Participants were then shown one of the videos according to their randomly assigned condition. If they were in the sexual arousal condition, they were reminded of the explicit content and had to open the video in a separate window to view it. They were only able to continue with the survey after the respective video was over. They were then asked about their level of sexual arousal and disgust as a manipulation check (How sexually aroused/disgusted are you feeling right now?). The questions were answered on scale from 0 = Not at all to 4 = Very much.Footnote 2
Participants consequently viewed six pictures of male faces, half of which were attractive and half of which were unattractive. Three of them contained blemishes. Because the blemishes could not be evenly divided among unattractive and attractive faces, we counterbalanced across participants whether two attractive and one unattractive face contained blemished or vice versa. Furthermore, we used a Latin square and counterbalanced the order of the faces and conditions across participants in order to eliminate order and priming effects (DeBruine 2014).
Participants indicated how attractive they found the men depicted on a scale from 0 = Not at all attractive to 4 = Very attractive. They also indicated how disgusted they would feel “Talking to that person,” “Hugging that person,” “Kissing that person,” and “Having sex with that person.” We chose these four behaviors in order to cover sexual and non-sexual behaviors, for which we expected different effects based on previous studies (Borg and de Jong 2012; Stevenson et al. 2011). The items also ranged from a casual interpersonal behavior to intimate physical contact and should therefore differ greatly in the disgust they elicit (Ryan et al. 2012). Furthermore, sampling across behaviors enabled us to account for effects that a single behavior might have and avoid floor or ceiling effects. The items were answered on a scale from 0 = Not at all disgusting to 6 = Extremely disgusting.
After these ratings, we again assessed levels of sexual arousal via self-report. Participants in the neutral condition then filled out the pathogen disgust scale from the Three Domains of Disgust Scale (Tybur et al. 2009). This enabled us to control for individual differences in disgust sensitivity. Furthermore, based on a trend in their data, Fleischman et al. (2015) suggested that individual differences in disgust sensitivity may predict the degree to which sexual arousal influences motivation to engage with attractive and unattractive targets with and without disease cues. Only one subscale was used, because it appears to be the most valid one and relates most strongly to disgust sensitivity (Tybur et al. 2010). Its reliability was adequate, Guttman’s L-2 = .70.
Finally, we asked respondents “How seriously did you take this survey?” and they replied once again on a scale from 0 = Not at all to 4 = Very much. After filling out this first part, participants were asked to fill out the second part the next day for which they received a reminder per email. The second questionnaire placed them in the other of the two conditions. It comprised a different set of faces, comparable in physical attractiveness ratings and blemishes, to rule out effects of familiarity (Little et al. 2014). Eventually participants were fully debriefed about the purpose of the study.
Statistical Power and Sample Size Estimation
There were two analyses of our prime interest: first, comparing overall disgust levels between the two conditions and second, our hypothesis; hence, the interaction effect of sexual arousal and attractiveness/blemishes on disgust. Effect sizes of the main effect of sexual arousal between groups in previous studies were an approximate eta2 of .08 (Lee et al. 2014; Stevenson et al. 2011). We therefore estimated that it would take 34 participants in a repeated measures design to obtain a power of .8 (Guo et al. 2013). We stopped data collection after we reached a sufficient number of participants for this analysis (54; Simmons et al. 2011).
Participants
The raw data consisted of 91 participants that had completed part one and 54 that had completed part two. Some of the responses of the two parts could not be connected due to missing information.Footnote 3 Several participants in the sexual arousal condition (11 in part one and four in part two) reported no sexual arousal at all and were hence excluded from the analysis. Two participants who identified as homosexual were also excluded.
The analyses were performed on two nested datasets. One was the fully repeated measurements sample (RM data; n = 38), in which each participant had been placed in both the sexual arousal and the neutral condition. The other dataset consisted only of those who completed part one of the study, with each participant in only one of the conditions (n = 79). There were 34 women in the sexual arousal and 45 in the neutral condition.Footnote 4 It was therefore analyzed between participants (BP data). All participants in the RM data also completed part one. Therefore, we report the demographics of the participants in the exhaustive BP dataset.
Participants were female psychology undergraduates from the participant pools of the authors’ universities. Their ages ranged from 18 to 43 years, with a mean of 21.66 (SD = 4.10). Most (N = 66; 83.5%) were Caucasian, and the others were of various other ethnicities. Three participants reported to be bisexual, the rest identified as mostly or exclusively heterosexual. Forty-four participants were single and the other 35 were in a relationship; 37 used oral contraceptives, 42 did not. Recruitment took place via internal advertisement in the universities and participation was rewarded with course credits.
Results
Manipulation Check
We performed manipulation checks on both the BP and the RM data. Table 1 shows the means of the two sexual arousal self-reports (after the video and after the faces), baseline disgust (measured after the video), and how seriously participants took the survey, per dataset. The table also depicts the outcomes of the t tests between the sexual arousal and the neutral condition. The between and within participant tests of the two datasets corresponded to each other. Participants reported significantly and substantially more sexual arousal in the sexual arousal condition than the neutral condition, both before and after seeing the faces. The sexual arousal condition also scored higher on baseline disgust. Seriousness scores were high overall—no participant had a score smaller than two—and did not differ significantly between conditions. The manipulation therefore worked as expected, with disgust levels as a possible confound.
Main Analysis
Disgust
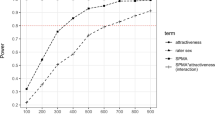
Hypothesis 1 predicted that sexual arousal would decrease disgust towards attractive, but not unattractive faces; hypothesis 2 predicted that sexual arousal would not reduce disgust towards blemished faces. Thus, we expected interaction effects between sexual arousal and attractiveness, and sexual arousal and blemishes. We computed a fully repeated measures analysis of variance (RM ANOVA) using the RM data, with attractiveness, blemishes, sexual arousal, and behavior as predictors. Behavior was added as a control variable within participants. The dependent variable was disgust towards each behavior. Table 2 shows the corresponding statistics. We found significant main effects of behavior, attractiveness, and blemishes, but not of sexual arousal. Unattractive faces (M = 3.49, SE = .12) elicited significantly more disgust than attractive faces (M = 2.19, SE = .11), and blemished faces (M = 3.11, SE = .12) elicited more disgust than unblemished faces (M = 2.57, SE = .11). Behaviors became increasingly disgusting as they became more sexual (see Fig. 2). All depicted differences in Fig. 2 reached statistical significance (see Table 3 for within participant contrasts). There was a significant behavior by attractiveness interaction. As can be seen in Fig. 2, the more sexual the behaviors became, the larger the difference in disgust between attractive and unattractive faces. The mean differences in the order of behaviors were .25 (.73) for talking, .88 (1.15) for hugging, 1.99 (1.25) for kissing, and 2.24 (1.38) for sex. There were no other significant interaction effects. Both hypotheses were not confirmed.
Individual Differences and Between Participant Analysis
We also controlled for baseline disgust after seeing the video and for the individual difference variables age, conception risk, oral contraception, recent illness, being in a relationship, and pathogen disgust sensitivity. Because these variables all varied between, not within participants, we computed a mixed design ANOVA with the BP sample of part one (n = 79), retaining our main variables sexual arousal, behavior, attractiveness, and blemishes in the analysis. Sexual arousal varied between participants in this analysis, and as can be seen in Table 4, it again did not influence disgust ratings. Overall, this analysis mirrored the results of the fully repeated measurement design.
When adding all the individual difference variables in one model, there were a few significant, yet meaningless interaction effectsFootnote 5 that most likely resulted from an inflated type I error rate. Therefore, we are reporting the tests for adding each variable separately to the mixed design ANOVA. This analysis was also more meaningful in terms of statistical power. Age and conception risk were added as covariates, oral contraception, recent illness, and relationship status as between participant dummy variables. We computed conception risk using the estimation for irregularly menstruating women by Wilcox et al. (2000) and excluded participants that used oral contraception from the computation (missing data). For pathogen disgust sensitivity, we performed a median split on the data similarly to Fleischman et al. (2015) and therefore treated it as a between participant dummy variable as well.Footnote 6 The test statistics are listed in Table 4. None of the variables reached statistical significance.
Attractiveness
We also examined how ratings of attractiveness of the faces were influenced by attractiveness level,Footnote 7 blemishes, and sexual arousal of the participants. We ran a fully RM ANOVA with attractiveness ratings as the DV (see Table 5). Attractiveness ratings were influenced by attractiveness level and blemishes, but not by sexual arousal. This also validated our attractiveness manipulation. Furthermore, there was an interaction effect between attractiveness level and blemishes. For unattractive faces, there was no difference in attractiveness ratings between blemished (M = 1.62, SD = .76) and unblemished (M = 1.68, SD = .67) faces, t(90) = .65, p = .516, d = .14 . But in attractive faces, there was a large difference between blemished (M = 3.21, SD = .91) and unblemished (M = 4.00, SD = .86) faces, t(90) = 6.50, p < .001, d = 1.37. Hence, blemishes only reduced the attractiveness ratings of attractive faces, and attractive unblemished faces were rated as particularly attractive. Individual difference variables also did not affect attractiveness ratings, but for conciseness, the tests are not reported here.
Discussion
The main goal of this study was to investigate the effect of sexual arousal on women’s disgust towards potential mates, moderated by the mates’ attractiveness and disease cues. We hypothesized that sexual arousal would decrease disgust towards attractive, but not towards unattractive or blemished men. Both hypotheses were not confirmed. Disgust, as well as attractiveness ratings, were influenced by attractiveness and blemishes, but not by sexual arousal. Sexual arousal also did not interact with any of the variables. Individual difference variables did not have any effects either.
Attractiveness and Blemishes
As expected, attractiveness decreased women’s disgust towards behaviors with the men depicted. This is in line with the findings of Principe and Langlois (2011) that attractive faces elicit less disgust than unattractive faces and with Mehrabian and Blum (1997) who showed that attractive faces elicited more positive emotions in viewers. These findings highlight the desirability of physical attractiveness, particularly as mates (Eastwick et al. 2014; Zsok et al. 2017). Attractiveness seems to reduce disgust and therefore also avoidance tendencies—probably because it signals good health and small risk of pathogen transmission (Gangestad 1993; Sugiyama 2016; Tybur and Gangestad 2011).
We also found that the more physically intimate the behaviors were, the less disgust elicited attractive as opposed to unattractive men. This also seems like an adaptive choice considering potential mating costs. The behaviors we assessed (talking to, hugging, kissing, sex) differed in the extent that they require physical contact and therefore also in the potential adaptive costs associated with pathogen transmission (Rozin et al. 1995). Sex, the behavior that elicited the greatest disgust, and the greatest difference between attractive and unattractive faces, additionally involves costs/benefits associated with potentially producing offspring. The more potentially costly a behavior is on average, the more important it is to choose a partner that optimizes the cost-benefit ratio. Accordingly, our study shows that anticipated disgust seems to be a particularly useful indicator for potential costs when they are high. Overall, women’s behavioral tendencies reflected our initial premise that attractiveness and health both signal potentially beneficial mating and that disgust is indicative of potential costs with suboptimal mates.
In line with Ryan et al. (2012), our study shows that blemished faces, independently of their attractiveness, elicit more disgust than unblemished faces This might explain why people with face blemishes seem to experience more negative social interactions (Kent 2002; Schachter et al. 1971; Wright et al. 1970). Blemishes reduced the attractiveness of attractive faces, but not of unattractive faces. This could be because there are many attributes that determine the attractiveness of a face (Little 2014; Re and Rule 2016; Sugiyama 2016). Unattractive faces contain other cues that might show detrimental traits, so the blemishes we added might not have made large difference. Similarly, the effect cosmetic make-up has on women’s faces is rather small compared to the inherent properties of the face (Jones and Kramer 2015). Another explanation might be that the blemishes made the threat of infection salient, and participants therefore became more critical in evaluating the attractiveness of the faces (Little, DeBruine, & Jones, 2011; Young et al. 2011).
Sexual Arousal and Disgust
Sexual arousal did not influence disgust ratings, even when we controlled for the specific behaviors, individual differences, and baseline disgust. Contrary to Istvan et al. (1983), there was also no effect of sexual arousal on attractiveness ratings. Studies in men, however, consistently show that sexual arousal decreases their disgust sensitivity (Ariely and Loewenstein 2006; Lee et al. 2014; Stevenson et al. 2011).
de Jong et al.’s (2013) model provides a possible explanation for the null results of this and previous studies (Fleischman et al. 2015; van Overveld & Borg, 2015): Sexual arousal might have not exceeded the level of disgust to the extent that it would occupy attentional resources and therefore inhibit disgust. Women on average have a higher disgust sensitivity and propensity than men (Andrews et al. 2015; Borg et al. 2015; Fleischman 2017; Grauvogl et al. 2015; Rohrmann et al. 2008; Tybur et al. 2011). This also implies that they require relatively more sexual arousal to outweigh disgust and elicit a sexually functioning feedback loop (de Jong et al. 2013). In other words, sexual arousal is less likely to outweigh disgust in women. This is also thought to be the underlying problem in sexual dysfunctions involving disgust (Borg et al. 2010). The expected inhibitory effect of sexual arousal on disgust might therefore be observable in experiments using a strong sexual arousal manipulation (Lee et al. 2014) and/or less disgusting stimuli. Our stimuli were not very disgusting per se, but the men depicted were unfamiliar to participants, and strangers tend to elicit more disgust than familiar others (Borg et al. 2015; Peng et al. 2013; Rozin et al. 1995). The increased baseline disgust level in the sexual arousal condition might have contributed to an imbalance between sexual arousal and disgust in favor of the latter.
The mixed findings in women could also be explained by the sex-specific challenges in evolutionary history (Buss and Schmitt 1993). Women face greater potential costs from sexual intercourse, because of parental investment, as well as greater susceptibility to disease (Fleischman 2014; Madkan et al. 2006). Hence, they favor a sexual strategy that involves mating inside long-term committed relationships, with partners whose traits they have carefully examined (Schmitt 2015). The women in our study had never seen the men they were presented before. The study therefore presented them with a short-term mating scenario. Selection pressures might have not been strong on evolving a mechanism that suppresses disgust in such scenarios, because it yields little evolutionary benefit. It might even impose costs due to suboptimal mating decisions. In sum, while sexual arousal clearly decreases disgust in men, this effect seems to be weak, and/or more dependent on context in women.
Individual Difference Variables
We did not find that any individual difference variables (age, pathogen disgust sensitivity, recent illness, relationship status, oral contraception, conception risk) influenced disgust or attractiveness, although previous research has (Curtis et al. 2004; DeBruine 2014; Fessler and Navarrete 2003; Fleischman 2014; Gruijters et al. 2016; Park et al. 2012). The fact that we did not find a link between recent illness and disgust might be because we employed only explicit measures, while other studies have found this link only on implicit measures (Lund and Boggero 2014; Miller and Maner 2011). The null effect of being in a relationship is in line with the findings of Wang et al. (2016). Failure to replicate the effects of the other variables probably stems from the fact that they have been rather small and that our study lacked the statistical power to detect them. Furthermore, some variables, for example facial masculinity, have been precisely manipulated in other studies (DeBruine et al. 2010), while our manipulations pertained to attractiveness globally. So overall, our data does not directly refute these effects, but merely confirms that they are small.
Limitations
Our results should be interpreted in light of methodological constraints, for example regarding the experimental manipulation. We cannot know for sure whether participants fully complied with the instructions. Although we used a validated erotic video, participants might not have found it pleasant, which contributes to women’s sexual arousal in response to erotica (Carvalho et al. 2013). Our self-report measure of sexual arousal also did not guarantee that women were physically aroused (Chivers et al. 2010). Research to date has not yet addressed whether disgust inhibition, or attentional processes more broadly, operates differently under self-reported vs. physiologically measured sexual arousal. Participants agreed to watch pornographic material for the study, which poses a volunteer bias typical for sex research (Bogaert 1996; Hock 2015; Wiederman 1999); also, participants might be accustomed to pornography, and less sensitive to it (Kelley and Musialowski 1986; Koukounas and Over 2000). Future studies on sexual arousal might avoid these limitations by allowing participants to arouse themselves in their preferred manner (Lee et al. 2014), or self-select erotica (Goldey and van Anders 2016; Ioannou et al. 2016). Other than that, this study might have profited from measuring additional control variables, for example sociosexuality (Murray, Jones, & Schaller, 2013; Sacco et al. 2012), or baseline mood (ter Kuile et al. 2010). Also, we might have trapped participants into labeling any positive or negative feelings as attraction and disgust, respectively, and therefore we might have left out other feelings that could play a role.Footnote 8
Conclusion
This study suggests that approach and avoidance motivation in women towards unfamiliar potential mates are unaffected by their sexual arousal state, but are affected by the mates’ visible traits. Specifically, attractiveness and a lack of disease cues increase approach (attraction) and decrease avoidance motivation (disgust) towards engagement with mates.
Notes
van Overveld and Borg (2015) did not find this effect.
We used five-point and seven-point Likert scales with at least the endpoints labeled throughout the study, as Weijters, Cabooter, and Schillewart (2010) recommend.
Participants received an individual ID code in part one, but some did not report it in part two.
The assumption of homogeneity of variance was met despite the difference cell sizes.
Some interaction effects were significant, because one of the cell differences was significant and the other was not. However, these differences never reflected trends in line with the literature. Furthermore, for interaction effects, they were partly very small groups, hence low power and hence a higher type II error rate.
This variable was only available from participants who completed the neutral condition (n = 44).
As a reminder, the attractiveness level refers to the attractiveness manipulation, hence to the attractive and unattractive faces we chose based on pilot testing. Attractiveness ratings refer to how attractive participants of this study rated the faces.
We would like to thank two anonymous reviewers for pointing out some of these limitations.
References
Al-Shawaf, L., Lewis, D. M. G., & Buss, D. M. (2015). Disgust and mating strategy. Evolution and Human Behavior, 36(3), 199–205. doi:10.1016/j.evolhumbehav.2014.11.003.
Al-Shawaf, L., Conroy-Beam, D., Asao, K., & Buss, D. M. (2016). Human emotions: An evolutionary psychological perspective. Emotion Review, 8(2), 173–186. doi:10.1177/1754073914565518.
Andrews, A. R. I., Crone, T., Cholka, C. B., Cooper, T. V., & Bridges, A. J. (2015). Correlational and experimental analyses of the relation between disgust and sexual arousal. Motivation and Emotion, 39(5), 766–779.
Ariely, D., & Loewenstein, G. (2006). The heat of the moment: the effect of sexual arousal on sexual decision making. Journal of Behavioral Decision Making, 19(2), 87–98.
Barlow, D. H. (1986). Causes of sexual dysfunction: the role of anxiety and cognitive interference. Journal of Consulting and Clinical Psychology, 54(2), 140–148.
Bogaert, A. F. (1996). Volunteer bias in human sexuality research: evidence for both sexuality and personality differences in males. Archives of Sexual Behavior, 25(2), 125–140. doi:10.1007/BF02437932.
Borg, C., & de Jong, P. J. (2012). Feelings of disgust and disgust-induced avoidance weaken following induced sexual arousal in women. PloS One, 7(9), e44111.
Borg, C., de Jong, P. J., & Weijmar Schultz, W. (2010). Vaginismus and dyspareunia: automatic vs. deliberate disgust responsivity. The Journal of Sexual Medicine, 7(6), 2149–2157. doi:10.1111/j.1743-6109.2010.01800.x.
Borg, C., Heitmann, J., & de Jong, P. (2015). “Yuck, they are kissing!” Understanding the transition from disgust to desire and its implications for sexual dysfunction. Paper presented at Emotions 2015. Tilburg: 6th International conference on emotions, well-being and health.
Bundy, C. (2012). Visible difference associated with disease: skin conditions. In N. Rumsey & D. Harcourt (Eds.), The Oxford handbook of the psychology of appearance (pp. 398–413). New York, NY: Oxford University Press.
Buss, D. M., & Schmitt, D. P. (1993). Sexual strategies theory: an evolutionary perspective on human mating. Psychological Review, 100(2), 204–232. doi:10.1037/0033-295X.100.2.204.
Buss, D. M., & Symons, D. (2015). Part III: mating. In The handbook of evolutionary psychology. Somerset: John Wiley & Sons, Inc.
Buunk, B. P., Dijkstra, P., Fetchenhauer, D., & Kenrick, D. T. (2002). Age and gender differences in mate selection criteria for various involvement levels. Personal Relationships, 9(3), 271–278. doi:10.1111/1475-6811.00018.
Carvalho, J., Gomes, A. Q., Laja, P., Oliveira, C., Vilarinho, S., Janssen, E., & Nobre, P. (2013). Gender differences in sexual arousal and affective responses to erotica: the effects of type of film and fantasy instructions. Archives of Sexual Behavior, 42(6), 1011–1019. doi:10.1007/s10508-013-0076-2.
Chivers, M. L. (2010). A brief update on the specificity of sexual arousal. Sexual and Relationship Therapy, 25(4), 407–414. doi:10.1080/14681994.2010.495979.
Chivers, M. L., Seto, M. C., Lalumière, M. L., Laan, E., & Grimbos, T. (2010). Agreement of self-reported and genital measures of sexual arousal in men and women: a meta-analysis. Archives of Sexual Behavior, 39(1), 5–56. doi:10.1007/s10508-009-9556-9.
Curtis, V., Aunger, R., & Rabie, T. (2004). Evidence that disgust evolved to protect from risk of disease. Proceedings of the Royal Society B: Biological Sciences, 271(Suppl_4), S131–S133. doi:10.1098/rsbl.2003.0144.
DeBruine, L. M. (2014). Women’s preferences for male facial features. In V. A. Weekes-Shackelford & T. K. Shackelford (Eds.), Evolutionary perspectives on human sexual psychology and behavior (pp. 261–275). New York, NY: Springer Science + Business Media.
DeBruine, L. M., Jones, B. C., Tybur, J. M., Lieberman, D., & Griskevicius, V. (2010). Women’s preferences for masculinity in male faces are predicted by pathogen disgust, but not by moral or sexual disgust. Evolution and Human Behavior, 31(1), 69–74. doi:10.1016/j.evolhumbehav.2009.09.003.
Dixson, A. F. (2009). Sexual selection and the origins of human mating systems. Oxford, GBR: Oxford University Press.
Eastwick, P. W., Luchies, L. B., Finkel, E. J., & Hunt, L. L. (2014). The predictive validity of ideal partner preferences: a review and meta-analysis. Psychological Bulletin, 140(3), 623–665. doi:10.1037/a0032432.
Fessler, D. M. T., & Navarrete, C. D. (2003). Domain-specific variation in disgust sensitivity across the menstrual cycle. Evolution and Human Behavior, 24(6), 406–417. doi:10.1016/S1090-5138(03)00054-0.
Fleischman, D. S. (2014). Women’s disgust adaptations. In V. A. Weekes-Shackelford & T. K. Shackelford (Eds.), Evolutionary perspectives on human sexual psychology and behavior (pp. 277–296). New York, NY: Springer Science + Business Media.
Fleischman, D. S. (2017). Sex-differences in disease avoidance. In T. K. Shackelford & V. A. Weekes-Shackelford (Eds.), Encyclopedia of evolutionary psychological science. New York, NY: Springer Science + Business Media.
Fleischman, D. S., Hamilton, L. D., Fessler, D. M. T., & Meston, C. M. (2015). Disgust versus lust: exploring the interactions of disgust and fear with sexual arousal in women. PloS One, 10(6), e0118151. doi:10.1371/journal.pone.0118151.
Flying the Nest (2015, March 1st). Hiking the Hollywood sign [Video file]. Retrieved from https://www.youtube.com/watch?v=s1UofPVuSZo.
Gangestad, S. W. (1993). Sexual selection and physical attractiveness. Human Nature, 4(3), 205–235. doi:10.1007/BF02692200.
Goldey, K. L., & van Anders, S. M. (2016). Identification with stimuli moderates women’s affective and testosterone responses to self-chosen erotica. Archives of Sexual Behavior, 45(8), 2155–2171. doi:10.1007/s10508-015-0612-3.
Grauvogl, A., de Jong, P., Peters, M., Evers, S., van Overveld, M., & van Lankveld, J. (2015). Disgust and sexual arousal in young adult men and women. Archives of Sexual Behavior, 44(6), 1515–1525. doi:10.1007/s10508-014-0349-4.
Gruijters, S. L. K., Tybur, J. M., Ruiter, R. A. C., & Massar, K. (2016). Sex, germs, and health: pathogen-avoidance motives and health-protective behavior. Psychology & Health, 1–17. doi:10.1080/08870446.2016.1161194.
Guo, Y., Logan, H. L., Glueck, D. H., & Muller, K. E. (2013). Selecting a sample size for studies with repeated measures. BMC Medical Research Methodology, 13(1), 100.
Hamilton, L. D., & Meston, C. M. (2013). Chronic stress and sexual function in women. The Journal of Sexual Medicine, 10(10), 2443–2454. doi:10.1111/jsm.12249.
Harte, C. B., & Meston, C. M. (2008). The inhibitory effects of nicotine on physiological sexual arousal in nonsmoking women: results from a randomized, double-blind, placebo-controlled, cross-over trial. The Journal of Sexual Medicine, 5(5), 1184–1197. doi:10.1111/j.1743-6109.2008.00778.x.
Hawk, S. T., Tolman, R., & Mueller, C. W. (2007). The effects of target attractiveness on men’s sexual arousal in response to erotic auditory stimuli. Journal of Sex Research, 44(1), 96–103. doi:10.1207/s15598519jsr4401_10.
Hock, R. (2015). Human sexuality (4th ed.). Boston: Pearson.
Ioannou, S., Morris, P., Terry, S., Baker, M., Gallese, V., & Reddy, V. (2016). Sympathy crying: insights from infrared thermal imaging on a female sample. PloS One, 11(10), e0162749. doi:10.1371/journal.pone.0162749.
Istvan, J., Griffitt, W., & Weidner, G. (1983). Sexual arousal and the polarization of perceived sexual attractiveness. Basic and Applied Social Psychology, 4(4), 307–318.
Janssen, E., & Bancroft, J. (2007). The dual-control model: the role of sexual inhibition and excitation in sexual arousal and behavior. The Psychophysiology of Sex, 15, 197–222.
Jones, A. L., & Kramer, R. S. S. (2015). Facial cosmetics have little effect on attractiveness judgments compared with identity. Perception, 44(1), 79–86.
de Jong, P. J., van Overveld, M., & Borg, C. (2013). Giving in to arousal or staying stuck in disgust? Disgust-based mechanisms in sex and sexual dysfunction. Journal of Sex Research, 50(3–4), 247–262. doi:10.1080/00224499.2012.746280.
Kelley, K., & Musialowski, D. (1986). Repeated exposure to sexually explicit stimuli: novelty, sex, and sexual attitudes. Archives of Sexual Behavior, 15(6), 487–498. doi:10.1007/BF01542313.
Kent, G. (2002). Testing a model of disfigurement: effects of a skin camouflage service on well-being and appearance anxiety. Psychology & Health, 17(3), 377–386.
Koukounas, E., & Over, R. (2000). Changes in the magnitude of the eyeblink startle response during habituation of sexual arousal. Behaviour Research and Therapy, 38(6), 573–584. doi:10.1016/S0005-7967(99)00075-3.
Koukounas, E., & McCabe, M. P. (2001). Sexual and emotional variables influencing sexual response to erotica: A psychophysiological investigation. Archives of Sexual Behavior, 30(4), 393–408. doi:10.1023/A:1010261315767.
ter Kuile, M. M., Both, S., & van Uden, J. (2010). The effects of experimentally-induced sad and happy mood on sexual arousal in sexually healthy women. Journal of Sexual Medicine, 7(3), 1177–1184. doi:10.1111/j.1743-6109.2009.01632.x.
Lee, E. M., Ambler, J. K., & Sagarin, B. J. (2014). Effects of subjective sexual arousal on sexual, pathogen, and moral disgust sensitivity in women and men. Archives of Sexual Behavior, 43(6), 1115–1121. doi:10.1007/s10508-014-0271-9.
Little, A. C. (2014). Facial attractiveness. Wiley Interdisciplinary Reviews: Cognitive Science, 5(6), 621–634. doi:10.1002/wcs.1316.
Little, A. C., DeBruine, L. M., & Jones, B. C. (2011). Exposure to visual cues of pathogen contagion changes preferences for masculinity and symmetry in opposite-sex faces. Proceedings of the Royal Society of London B: Biological Sciences, 278(1714), 2032–2039. doi:10.1098/rspb.2010.1925.
Little, A. C., DeBruine, L. M., & Jones, B. C. (2014). Sex differences in attraction to familiar and unfamiliar opposite-sex faces: men prefer novelty and women prefer familiarity. Archives of Sexual Behavior, 43(5), 973–981.
Lund, E. M., & Boggero, I. A. (2014). Sick in the head? Pathogen concerns bias implicit perceptions of mental illness. Evolutionary Psychology, 12(4), 706–718. doi:10.1177/147470491401200403.
Madkan, V. K., Giancola, A. A., Sra, K. K., & Tyring, S. K. (2006). Sex differences in the transmission, prevention, and disease manifestations of sexually transmitted diseases. Archives of Dermatology, 142(3), 365–370.
Mass, R., Hölldorfer, M., Moll, B., Bauer, R., & Wolf, K. (2009). Why we haven’t died out yet: changes in women’s mimic reactions to visual erotic stimuli during their menstrual cycles. Hormones and Behavior, 55(2), 267–271. doi:10.1016/j.yhbeh.2008.06.007.
Masters, W. H., & Johnson, V. E. (1966). Human sexual response. Oxford: Little, Brown.
Mehrabian, A., & Blum, J. S. (1997). Physical appearance, attractiveness, and the mediating role of emotions. Current Psychology: a Journal for Diverse Perspectives on Diverse Psychological Issues, 16(1), 20–42. doi:10.1007/s12144-997-1013-0.
Miller, S. L., & Maner, J. K. (2011). Sick body, vigilant mind: the biological immune system activates the behavioral immune system. Psychological Science, 22(12), 1467–1471. doi:10.1177/0956797611420166.
Mortensen, C. R., Becker, D. V., Ackerman, J. M., Neuberg, S. L., & Kenrick, D. T. (2010). Infection breeds reticence: the effects of disease salience on self-perceptions of personality and behavioral avoidance tendencies. Psychological Science, 21(3), 440–447. doi:10.1177/0956797610361706.
Murray, D. R., Jones, D. N., & Schaller, M. (2013). Perceived threat of infectious disease and its implications for sexual attitudes. Personality and Individual Differences, 54(1), 103–108. doi:10.1016/j.paid.2012.08.021.
Oaten, M., Stevenson, R. J., & Case, T. I. (2009). Disgust as a disease-avoidance mechanism. Psychological Bulletin, 135(2), 303–321. doi:10.1037/a0014823.
Olszanowski, M., Pochwatko, G., Kuklinski, K., Scibor-Rylski, M., Lewinski, P., & Ohme, R. K. (2015). Warsaw set of emotional facial expression pictures: a validation study of facial display photographs. Frontiers in Psychology, 5, 1516. doi:10.3389/fpsyg.2014.01516.
van Overveld, M., & Borg, C. (2015). Brief emotion regulation training facilitates arousal control during sexual stimuli. The Journal of Sex Research, 52(9), 996–1005. doi:10.1080/00224499.2014.948111.
van Overveld, M., de Jong, P. J., Peters, M. L., van Lankveld, J., Melles, R., & ter Kuile, M. M. (2013). The sexual disgust questionnaire; a psychometric study and a first exploration in patients with sexual dysfunctions. The Journal of Sexual Medicine, 10(2), 396–407. doi:10.1111/j.1743-6109.2012.02979.x.
Park, J. H., van Leeuwen, F., & Stephen, I. D. (2012). Homeliness is in the disgust sensitivity of the beholder: relatively unattractive faces appear especially unattractive to individuals higher in pathogen disgust. Evolution and Human Behavior, 33(5), 569–577. doi:10.1016/j.evolhumbehav.2012.02.005.
Peng, M., Chang, L., & Zhou, R. (2013). Physiological and behavioral responses to strangers compared to friends as a source of disgust. Evolution and Human Behavior, 34(2), 94–98. doi:10.1016/j.evolhumbehav.2012.10.002.
Prause, N., Janssen, E., & Hetrick, W. P. (2008). Attention and emotional responses to sexual stimuli and their relationship to sexual desire. Archives of Sexual Behavior, 37(6), 934–949. doi:10.1007/s10508-007-9236-6.
Principe, C. P., & Langlois, J. H. (2011). Faces differing in attractiveness elicit corresponding affective responses. Cognition and Emotion, 25(1), 140–148.
Re, D. E., & Rule, N. O. (2016). Appearance and physiognomy. In D. Matsumoto, H. C. Hwang, M. G. Frank, & D. (Eds.), APA handbook of nonverbal communication (pp. 221–256). Washington, DC: American Psychological Association.
Renfro, K. J., Rupp, H., & Wallen, K. (2015). Duration of oral contraceptive use predicts women’s initial and subsequent subjective responses to sexual stimuli. Hormones and Behavior, 75, 33–40. doi:10.1016/j.yhbeh.2015.07.013.
Rohrmann, S., Hopp, H., & Quirin, M. (2008). Gender differences in psychophysiological responses to disgust. Journal of Psychophysiology, 22(2), 65–75.
Rozin, P., Nemeroff, C., Horowitz, M., Gordon, B., & Voet, W. (1995). The borders of the self: contamination sensitivity and potency of the body apertures and other body parts. Journal of Research in Personality, 29(3), 318–340. doi:10.1006/jrpe.1995.1019.
Rozin, P., Haidt, J., & Mcauley, C. R. (2008). Disgust. In M. Lewis, J. M. Haviland-Jones, & L. F. Barrett (Eds.), Handbook of emotions (3rd ed., pp. 757–776). New York, NY: Guilford Press.
Ryan, S., Oaten, M., Stevenson, R. J., & Case, T. I. (2012). Facial disfigurement is treated like an infectious disease. Evolution and Human Behavior, 33(6), 639–646.
Sacco, D. F., Jones, B. C., DeBruine, L. M., & Hugenberg, K. (2012). The roles of sociosexual orientation and relationship status in women’s face preferences. Personality and Individual Differences, 53(8), 1044–1047. doi:10.1016/j.paid.2012.07.023.
Schachter, R. J., Pantel, E. S., Glassman, G. M., & Zweibelson, I. (1971). Acne vulgaris and psychologic impact on high school students. New York State Journal of Medicine, 71(24), 2886–2890.
Schaller, M. (2015). The Behavioral Immune System. In The Handbook of Evolutionary Psychology (2nd ed.pp. 206–224). John Wiley & Sons, Inc.. doi:10.1002/9781119125563.evpsych107.
Schmitt, D. P. (2015). Fundamentals of human mating strategies. In The Handbook of Evolutionary Psychology (2nd ed.pp. 294–316). John Wiley & Sons, Inc.. doi:10.1002/9781119125563.evpsych111.
Simmons, J. P., Nelson, L. D., & Simonsohn, U. (2011). False-positive psychology: undisclosed flexibility in data collection and analysis allows presenting anything as significant. Psychological Science, 22(11), 1359–1366. doi:10.1177/0956797611417632.
Skolnick, A. J. (2013). Gender differences when touching something gross: unpleasant? No. Disgusting? Yes! Journal of General Psychology, 140(2), 144–157. doi:10.1080/00221309.2013.781989.
Stevenson, R. J., Case, T. I., & Oaten, M. J. (2011). Effect of self-reported sexual arousal on responses to sex-related and non-sex-related disgust cues. Archives of Sexual Behavior, 40(1), 79–85. doi:10.1007/s10508-009-9529-z.
Stone, E. A., Shackelford, T. K., & Goetz, A. T. (2011). Sexual arousal and the pursuit of attractive mating opportunities. Personality and Individual Differences, 51(5), 575–578. doi:10.1016/j.paid.2011.05.021.
Sugiyama, L. S. (2016). Physical attractiveness in adaptationist perspective. In D. M. Buss (Ed.), The handbook of evolutionary psychology (2nd ed., pp. 292–343). Hoboken, NJ: John Wiley & Sons Inc..
Toates, F. (2009). An integrative theoretical framework for understanding sexual motivation, arousal, and behavior. Journal of Sex Research, 46(2–3), 168–193.
Tooby, J., & Cosmides, L. (2008). The evolutionary psychology of the emotions and their relationship to internal regulatory variables. In M. Lewis, J. M. Haviland-Jones, & L. F. Barrett (Eds.), Handbook of emotions (3rd ed., pp. 114–137). New York, NY: Guilford Press.
Trivers, R. L. (1996). Parental investment and sexual selection. In L. D. Houck & L. C. Drickamer (Eds.), Foundations of animal behavior: Classic papers with commentaries (pp. 795–838). Chicago, IL: University of Chicago Press.
Tybur, J. M., & Gangestad, S. W. (2011). Mate preferences and infectious disease: theoretical considerations and evidence in humans. Philosophical Transactions of the Royal Society B: Biological Sciences, 366(1583), 3375–3388.
Tybur, J. M., & Lieberman, D. (2016). Human pathogen avoidance adaptations. Current Opinion in Psychology, 7, 6–11. doi:10.1016/j.copsyc.2015.06.005.
Tybur, J. M., Lieberman, D., & Griskevicius, V. (2009). Microbes, mating, and morality: Individual differences in three functional domains of disgust. Journal of Personality and Social Psychology, 97(1), 103–122. doi:10.1037/a0015474.
Tybur, J. M., Merriman, L. A., Hooper, A. E. C., McDonald, M. M., & Navarrete, C. D. (2010). Extending the behavioral immune system to political psychology: are political conservatism and disgust sensitivity really related? Evolutionary Psychology, 8(4), 599–616.
Tybur, J. M., Bryan, A. D., Lieberman, D., Caldwell Hooper, A. E., & Merriman, L. A. (2011). Sex differences and sex similarities in disgust sensitivity. Personality and Individual Differences, 51(3), 343–348. doi:10.1016/j.paid.2011.04.003.
Tybur, J. M., Lieberman, D., Kurzban, R., & DeScioli, P. (2013). Disgust: Evolved function and structure. Psychological Review, 120(1), 65–84. doi:10.1037/a0030778.
Vonderheide, S. G., & Mosher, D. L. (1988). Should I put in my diaphragm? Sex-guilt and turn-offs. Journal of Psychology & Human Sexuality, 1(1), 97–111.
Wagstaff, D. L., Sulikowski, D., & Burke, D. (2015). Sex-differences in preference for looking at the face or body in short-term and long-term mating contexts. Evolution, Mind and Behavior, 13(1), 1–17.
Wallen, K., & Rupp, H. A. (2010). Women’s interest in visual sexual stimuli varies with menstrual cycle phase at first exposure and predicts later interest. Hormones and Behavior, 57(2), 263–268. doi:10.1016/j.yhbeh.2009.12.005.
Wang, H., Hahn, A. C., DeBruine, L. M., & Jones, B. C. (2016). Do partnered women discriminate men’s faces less along the attractiveness dimension? Personality and Individual Differences, 98, 153–156. doi:10.1016/j.paid.2016.04.024.
Weijters, B., Cabooter, E., & Schillewaert, N. (2010). The effect of rating scale format on response styles: The number of response categories and response category labels. International Journal of Research in Marketing, 27(3), 236–247. doi:10.1016/j.ijresmar.2010.02.004.
Wiederman, M. W. (1999). Volunteer bias in sexuality research using college student participants. Journal of Sex Research, 36(1), 59–66.
Wilcox, A. J., Dunson, D., & Baird, D. D. (2000). The timing of the “fertile window” in the menstrual cycle: day specific estimates from a prospective study. BMJ, 321(7271), 1259–1262. doi:10.1136/bmj.321.7271.1259.
Willis, J., & Todorov, A. (2006). First impressions: making up your mind after a 100-ms exposure to a face. Psychological Science, 17(7), 592–598. doi:10.1111/j.1467-9280.2006.01750.x.
Woodard, T. L., Collins, K., Perez, M., Balon, R., Tancer, M. E., Kruger, M., et al. (2008). What kind of erotic film clips should we use in female sex research? An exploratory study. The Journal of Sexual Medicine, 5(1), 146–154. doi:10.1111/j.1743-6109.2007.00641.x.
Wright, E. T., Martin, R., Flynn, C., & Gunter, R. (1970). Some psychological effects of cosmetics. Perceptual and Motor Skills, 30(1), 12–14.
Young, S. G., Sacco, D. F., & Hugenberg, K. (2011). Vulnerability to disease is associated with a domain-specific preference for symmetrical faces relative to symmetrical non-face stimuli. European Journal of Social Psychology, 41(5), 558–563. doi:10.1002/ejsp.800.
Zsok, F., Haucke, M., de Wit, C. Y., & Barelds, D. P. H. (2017). What kind of love is love at first sight? An empirical investigation. Personal Relationships.
Acknowledgements
We would like to thank Andrea Schlump for her helpful comments on the study design and Aaron Kreidel for the editing of the stimuli. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zsok, F., Fleischman, D.S., Borg, C. et al. Disgust Trumps Lust: Women’s Disgust and Attraction Towards Men Is Unaffected by Sexual Arousal. Evolutionary Psychological Science 3, 353–363 (2017). https://doi.org/10.1007/s40806-017-0106-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40806-017-0106-8