Abstract
Life History Theory proposes that individuals facing uncontrollable threats to their survival—for example, uncontrollable threats of infectious disease—will pursue a “fast” sexual strategy, characterized by earlier and more promiscuous sexual behavior. Consistent with this idea, individuals with genes that equip them with poorer defenses against infectious disease could also tend to pursue a faster sexual strategy. Within a sample of 180 women, we find that women with a genetic marker of lower immunocompetence—namely, homozygosity at the Major Histocompatibility Complex (MHC) region of the genome—report more positive attitudes towards short-term sexual relationships, a younger age at sexual debut, a more promiscuous sexual history (such as more one-time sex partners), and a greater number of lifetime sexual relationships but not romantic relationships. These findings identify the MHC as a possible source of genetic variation in women’s sexual strategies and highlight the need for further research examining the complex links between genes, immunocompetence, and social behavior in humans.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Sexual attitudes and behavior vary widely, both between and within cultures. Whereas about half of college-aged women in the U.S. consider love to be a prerequisite to sex, half do not (England and Bearak 2014). Similarly, large cross-cultural investigations have revealed vast individual variation in sexual attitudes and behavior within each of dozens of nations worldwide (e.g., Schmitt 2005). But why does this individual variation exist?
Past research has identified social, psychological, and genetic factors associated with individual differences in sexual attitudes and behavior. For example, religious involvement predicts less promiscuous sexual attitudes and behavior (e.g., Thornton and Camburn 1989). In contrast, media exposure and parental divorce have been linked to more promiscuous sexual attitudes and behaviors (Hetherington 1972; Miller and Bingham 1989; Newcomer and Udry 1987). At the molecular level, other work has linked variation in promiscuous behavior to polymorphisms at specific genetic loci (Garcia et al. 2010; Zietsch et al. 2015; Zion et al. 2006).
Additionally, a large body of research testing predictions from Life History Theory has documented associations between growing up in a harsh or unpredictable environment and more promiscuous sexual attitudes and behaviors (see Belsky et al. 1991; Chisholm 1999; Griskevicius et al. 2013; Kaplan and Gangestad 2005). Here, we extend these life history predictions to empirically assess the possibility that genetic susceptibility to pathogens contributes to individual differences in sexual strategies.
Life History Theory and Sexual Strategies
Life history theory examines how organisms strategically allocate finite resources such as time and energy to necessary tasks, including growth (somatic development), maintenance (immune function and rebuilding damaged tissue), and reproduction (mating and parenting), in ways that enhance fitness (or, in humans, did so ancestrally). The finite nature of these resources entails making tradeoffs both between different tasks pursued concurrently and between tasks pursued now versus in the future. For example, one key tradeoff is between quality and quantity of offspring: an individual could produce a small number of offspring and invest heavily in each or produce a large number of offspring but invest little in each. Another key tradeoff is between current and future reproduction: an individual could spend its energy to reproduce now but have little left for future reproduction or forego reproduction now to conserve energy for future reproduction (see Del Giudice et al. 2015; Del Giudice and Belsky 2011; Roff 2002).
These tradeoffs give rise to different “life history strategies,” which fall on a slow-to-fast continuum. Fast life history strategies prioritize current over future reproduction and quantity over quality of offspring. Accordingly, they are characterized by faster somatic development, an earlier age at reproductive maturity, earlier and more promiscuous mating, a larger number of offspring but less parental investment in each one, and a shorter lifespan (Ellis et al. 2009; Figueredo et al. 2005; Griskevicius et al. 2013). Slow life history strategies prioritize future over current reproduction and quality over quantity of offspring, with corresponding slower somatic development, later age at reproductive maturity, and later and less promiscuous mating.
Life history theory further proposes that the fitness payoffs of pursuing a fast or slow life history strategy depend crucially on the environment (see Del Giudice et al. 2015). Environments high in extrinsic mortality threats—threats to an individual’s survival that it cannot avoid by changing how it allocates resources to growth, maintenance, and reproduction—are expected to favor faster life history strategies. Given little control over their own or their offspring’s chances of survival, individuals that quickly produce a large number of offspring will increase the likelihood that at least some offspring survive. In contrast, environments low in extrinsic mortality threats are expected to favor slower life history strategies. In these environments, individuals can take longer to grow and capture energy from the environment. They can later invest this energy in a small number of offspring, thereby increasing each offspring’s prospects for survival and reproductive success (e.g., Del Giudice 2009). Consistent with these Life History Theory predictions, studies have found that individuals who grow up in environments high in extrinsic mortality threats (e.g., high pathogen prevalence, intergroup violence, or resource instability) tend to adopt a faster life history strategy, including a faster sexual strategy (e.g., earlier and more promiscuous sexual behavior; see Belsky et al. 2007; Del Giudice 2009; Quinlan 2007; Chisholm 1999; Griskevicius et al. 2013; Kaplan and Gangestad 2005; Nettle 2012; Nettle and Cockerill 2010; Wilson and Daly 1997).
Disease Threat and Sexual Strategies
Infectious disease has historically been, and continues to be, one of the most common extrinsic threats to human mortality worldwide, and has likely accounted for more human deaths than all wars, natural disasters, and noninfectious diseases combined (Anderson and May 1991; Inhorn and Brown 1990; Wolfe et al. 2007). This threat has implications for calibration of life history and sexual strategies. People in ecologies characterized by higher infectious disease prevalence display less nurturant and investing parenting, consistent with the characteristics of a faster life history strategy (Quinlan 2007). Similarly, small scale societies inhabiting regions of higher pathogen prevalence are significantly more likely to favor polygynous—rather than monogamous—marriage systems (Low 1990).
Notably, however, an individual’s risk of mortality from to infectious disease is not determined solely by the magnitude of pathogenic threats in the environment (i.e. external to the individual); it is also determined by the individual’s immunocompetence and other internal factors. All else equal, individuals with a poorer ability to defend themselves from pathogens are more vulnerable to infectious disease – and therefore more susceptible to these extrinsic, unpredictable threats. Pursuing a faster life history strategy in these circumstances may historically have served to increase individuals’ chances of reproducing before succumbing to inescapable and unpredictable disease-related mortality (see Waynforth 2012; Hill et al. 2016; Hill et al. 2015). Consistent with this logic are preliminary results suggesting that people with a history of greater vulnerability to illness report a lower ability to delay gratification, more heavily discount the future, and score more towards the “fast” end of the Arizona Life History Battery (Hill et al. 2016). Specific to sexual strategies, results from several studies suggest that adolescents with chronic illness have sex earlier and may be more likely to engage in risky sexual behavior than healthy adolescents (Choquet et al. 1997; Suris et al. 2008; Suris and Parera 2005; reviewed by Valencia and Cromer 2000). Other work has found that adults who had been diagnosed in childhood with a chronic illness that reduces life expectancy (e.g., cancer, severe asthma, type I diabetes, or epilepsy) had an earlier age at first reproduction than did healthy individuals (Waynforth 2012). Indeed, lower life expectancy appears to be the largest single predictor of earlier reproductive timing in women worldwide (Low et al. 2008).
The Major Histocompatibility Complex
The major histocompatibility complex offers a prime opportunity for testing the possibility that internal factors that increase susceptibility to infectious disease will favor the adoption of faster sexual strategies. The major histocompatibility complex (MHC), also called the human leukocyte antigen system (HLA) in humans, is a group of genes encoding cell surface molecules. These cell surface molecules present antigens found within the cell or in the extracellular environment to immune system cells. The immune system cells determine whether the antigen is “self” or “non-self” (a possible pathogen) and, if needed, proceed to initiate an immune response. Therefore, the MHC plays a critically important role in pathogen recognition and immune response.
A few features of the MHC allow it to carry out this role. First, the MHC is the most polymorphic region of the human genome, with hundreds or thousands of variants (alleles) for each MHC gene. This extreme level of polymorphism on the MHC is thought to have been maintained by selection largely because heterozygotes have greater immunocompetence than homozygotes (Janeway et al. 2001). Also, MHC alleles are expressed codominantly, meaning that both maternally and paternally inherited alleles contribute to the variety of cell surface molecules on an individual’s cell membranes. The more heterozygous an individual is, the wider the variety of antigens his or her cell surface molecules can bind and present to immune systems cells, and hence, the wider the variety of pathogens his or her immune system could potentially recognize and destroy (Bhutta 2007; Penn et al. 2002). Along these lines, research indicates that MHC heterozygosity is associated with greater immunocompetence in humans and nonhuman animals (e.g. Carrington and O’Brien 2003; Hraber et al. 2007; Jeffery et al. 2000; Penn et al. 2002). For example, among people infected with HIV-1, greater MHC-heterozygosity is associated with a slower progression to AIDS (see Carrington and O’Brien 2003).
Current Research
A Life History Theory perspective suggests that individuals whose genetics equipped them with poorer defenses against pathogens—thereby making them more vulnerable to extrinsic threats of mortality from infectious disease—may pursue a faster sexual strategy. To test this idea, we examined the relationship between MHC status and sexual strategies in a sample of women participating in a larger study examining the roles of scent, genetics, and hormones in attraction. In addition to pragmatic reasons for limiting the sample to women, past research suggests that women’s sexual strategies may be more responsive than men’s to disease-related threats (e.g., Schaller and Murray 2008). The logical hypothesis warranted by Life History Theory suggests that women who are more MHC-homozygous (a genetic marker of lower immunocompetence) would exhibit attitudes and behaviors consistent with a faster sexual strategy.
Method
Participants
One hundred eighty women between the ages of 18 and 40 (mean = 22.1, SD = 4.45) participated at one of two data collection sites. This sample size was predetermined based on project-specific funding, and data collection was terminated once the target sample size was reached. The first data collection site was at a Lesbian and Gay Pride Festival held in southern California (n = 95). The second site was at UCLA (n = 85). Preliminary analyses revealed that the groups did not meaningfully differ on any of the dependent measures of interest (p’s > .05), so the two samples were combined for the analyses reported below. Of the total sample of participants, 38.9% reported being heterosexual, 34.4% reported being lesbian, 19.4% reported being bisexual, and 5.0% reported being of another sexual orientation (2.2% did not report a sexual orientation). Participants reported the ethnic ancestry of both of their parents. The sample was ethnically diverse: 28.9% reported Hispanic (including Mexican and Puerto Rican) ancestry for both parents, 22.8% reported European ancestry for both parents, 17.8% reported Asian ancestry for both parents, 5% reported other ancestry, and 25.6% reported ancestry from multiple ethnicities.
Measures
Sexual Strategy
We measured three key components of participants’ sexuality that are characteristic of a faster life history strategy. First, we assessed participants’ attitudes toward short-term mating using the three attitudinal items from Simpson and Gangestad’s (1991) Sociosexual Orientation Inventory: “Sex without love is OK,” “I can imagine myself being comfortable with and enjoying ‘casual’ sex with different partners,” and “I would have to be closely attached to someone (both emotionally and psychologically) before I could feel comfortable and fully enjoy having sex with him or her” (reverse scored). Participants indicated their agreement with these items on a 9-point Likert scale. Participants’ responses to the three items were highly intercorrelated (r’s > .54, Cronbach’s Alpha = .79) and were therefore combined to create a single short-term mating attitudes score, with higher scores indicating more favorable attitudes toward short-term mating. Second, we created a short-term mating behavior score, with higher scores indicating a more promiscuous sexual history. This consisted of the composite of participants’ self-reported lifetime number of sex partners, number of sex partners in the past year, and number of one-time sex partners (both male and female partners for each measure). These values were highly intercorrelated, r’s > .63, Cronbach’s Alpha = .78). Preliminary tests indicated the presence of two clear outliers, who scored more than four standard deviations above the mean on each of these three behavioral measures. For these two outliers we truncated their scores to the next-highest score for each of the three measures before computing their composite score. In addition, we asked participants to report the age at which they had first had sex. Some participants reported that they had not yet had sex (n = 15), and another 13 participants reported having had sex but did not provide an age. In order to maximize statistical power for the multivariate analysis below, for the 15 participants who reported not yet having had sex we imputed their current age as the age at which they had first had sex in order to represent a crude lower bound value.Footnote 1 The thirteen participants who did not provide an age were excluded from the central analyses. Finally, as an additional measure of women’s sexual strategies, participants reported their lifetime number of “sexual” and “romantic” relationships.
Procedure
Pride Site
As part of a larger study examining the roles of scent and genetics in women’s attractions, we collected data at a booth at the Long Beach Lesbian and Gay Pride Festival for two days. Our booth was marked by large signs advertising a “UCLA Study of Women’s Attraction.” Festival attendees who approached our booth were provided with a brief description of the study and, if interested in participating, screened to determine their eligibility. Women were eligible to participate if they were between the ages of 18–40 years (inclusive) and had drunk fewer than four alcoholic beverages in the past two hours. All participants gave informed consent prior to completing any study tasks. In order to prevent contamination of saliva samples, participants were asked to refrain from eating, drinking, and chewing gum until the end of their session.
Participants completed a questionnaire, provided a saliva sample for genotyping, and completed a scent rating task unrelated to the present study. The questionnaire assessed a variety of variables unrelated to this study, as well as the variables of interest described above. Participants were supplied with a clipboard and encouraged to find a private area near the booth to complete the questionnaire. Research assistants stood by to answer participants’ questions and to prevent them from talking to other festival attendees while they worked. After the questionnaire, participants provided saliva via passive drool using an industry-standard Oragene® saliva collection kit (DNA Genotek Inc., Ottawa, ON, Canada). If a participant complained of an inability to produce sufficient saliva, she was given the option of using a small amount (just a dab on her moistened fingertip) of artificial sweetener (e.g., Equal, Sweet ‘n Low) to stimulate saliva production. Following the saliva sample, participants were debriefed and paid $10.
UCLA Site
As part of a separate larger study examining the roles of scent, genetics, and hormones in women’s attractions, we also collected data at UCLA for two days. Women who participated at UCLA were recruited at the Long Beach Pride Festival (or learned of the study through friends who had attended Pride) or via fliers posted on the UCLA campus. Women were eligible if they were between the ages of 18–40 years, smoked cigarettes fewer than three times per week, did not report an impaired sense of smell, were not pregnant, had not given birth within the last year, were not breastfeeding, were not going through menopause or post-menopausal, had not used emergency hormonal contraception (“Plan B”) in the last three months, and had not used hormonal medications in the last three months (not including hormonal contraception, which was acceptable). Several of the inclusion criteria (e.g., requiring that participants were not pregnant) reflect the fact that other portions of the study examined women’s hormone responses. All participants gave informed consent prior to completing any study tasks. As in the Pride sessions, they were asked to refrain from eating, drinking, and chewing gum until the end of their session.
Participants completed a body scent rating task unrelated to the present study. The participants also completed a questionnaire very similar to the one used at Pride, which again assessed a variety of variables including those of interest in this study. Participants completed the questionnaire in a quiet classroom, seated several feet from other participants and with cardboard carrels to ensure their privacy. They then provided a saliva sample using the same methods as at Pride. At the end of their sessions, participants were debriefed and paid $35 if they were recruited at UCLA or $100 if they were recruited at or through attendees of Pride. The latter received a larger payment in order to reimburse them for their travel (e.g., the cost of gas to drive to UCLA from Long Beach) and other expenses related to their participation.
Genotyping
In compliance with manufacturer protocols, the saliva samples were stored at room temperature until data collection was complete. The samples then underwent DNA extraction at a UCLA genetics lab before being transported to the UCLA Immunogenetics Sequencing Laboratory for standard medium-resolution HLA typing at three loci: HLA-A, HLA-B and HLA-DRB1. These are the three “classic” MHC genes most commonly typed in similar investigations. Likewise, similar investigations typically use low- or medium-resolution typing, consistent with the procedures followed here (for more details on HLA sequencing and analysis, see Shaw 2009).
One-hundred and thirty (72.2%) of the women were not homozygous at any of the three loci. Of the 50 women who were homozygous at any locus, 41 were homozygous at just one locus, seven were homozygous at two loci, and two were homozygous at all three loci. Consistent with past research (e.g. Roberts et al. 2005), we categorized women as homozygous if they were homozygous at one or more loci.
Ethnic-origin groups were statistically similar to one another on both predictor and dependent measures, with the exception that participants reporting Asian ethnicity were more likely to be MHC-homozygous (43.8% vs. 24.3%, p = .026), reported less promiscuous sexual behavior on the three-item composite (−.33 vs. .06, p = .024), and reported an older age at sexual debut (18.7 vs. 16.6 years, p < .001) compared with the other ethnic groups. Given these patterns, and given other work suggesting that MHC status may influence Asian samples differently (e.g., Saphire-Bernstein et al. 2016), we included Asian ethnicity as a dichotomous covariate (0 = no; 1 = yes) in all analyses reported below.
Also, the sample was not representative of the general population in terms of its inclusion of a large number of bisexual and lesbian women. The bisexual women in our sample reported a significantly higher frequency of sexual contact with men than with women (e.g., mean number of lifetime male sex partners =7.50, SD = 10.88, mean number of lifetime female sex partners =3.80, SD = 6.01, p = .026). Furthermore, past research has shown that lesbian women are much more similar to heterosexual women than to heterosexual or gay men in many aspects of their evolved mating psychology (Bailey et al. 1994). For these reasons, we think it is likely that the predictions outlined above will apply to women of all sexual orientations. Nonetheless, comparing the non-heterosexual women to the heterosexual women did reveal some potential differences. Namely, non-heterosexual women reported more promiscuous sexual behavior and an earlier age at sexual debut (p’s < .01). Therefore, to be conservative, we included a dichotomous sexual orientation predictor (heterosexual =1; non-heterosexual =0) in the analyses.
Finally, because variables comprising the short-term mating behavior score necessarily increase with age (e.g., total number of lifetime sexual partners) we included age as a covariate in our analyses.
Results
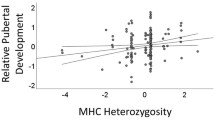
Raw mean differences between MHC groups are presented in Table 1 and in Figs. 1, 2 and 3. To test whether MHC-homozygous women exhibit more favorable attitudes towards short-term mating, a younger sexual debut, and a more promiscuous sexual history and to test whether the strength of such associations varied across dependent measures, we performed a multivariate analysis of covariance (MANCOVA). MHC homozygosity (1 = homozygous, 0 = heterozygous) was our dichotomous predictor of interest. In addition, as controls, we included ethnicity (1 = non-Asian, 0 = Asian) and sexual orientation (1 = non-heterosexual, 0 = heterosexual) as dichotomous predictor variables and participant age as a covariate. The three key dependent variables were short-term mating attitudes, age at sexual debut, and sexually promiscuous behavior.
Results from this omnibus test revealed that homozygosity significantly predicted the set of dependent variables, Wilk’s Λ = .91, F(3155) = 5.10, p = .002, partial η2 = .090. We followed up this MANCOVA with univariate tests examining each of the key dependent variables separately, with homozygosity, ethnicity, sexual orientation, and age as predictors. Homozygosity again predicted all three dependent variables: being homozygous at one or more MHC loci predicted more favorable short-term mating attitudes, (F(1171) = 11.60, p = .001, partial η2 = .064), a younger age at sexual debut (F(1158) = 6.63, p = .01, partial η2 = .040), and a more promiscuous sexual history, (F(1166) = 4.51, p = .035, partial η2 = .026).Footnote 2
Given the study’s focus on evolved reproductive strategies, we performed a subsequent MANCOVA predicting the three dependent variables that excluded participants who reported being exclusively lesbian. Results from this test were similar to those reported above: despite the loss in statistical power, the unique effect of MHC (while controlling for ethnicity and age) was significant, Wilk’s Λ = .90, F(3,99) = 3.67, p = .015, partial η2 = .100.
Although life history logic offered no predictions regarding potential interactive effects between the independent variables, we conducted exploratory analyses of the full-factorial model in which we included as predictors the four main effect variables, three two-way interactions between MHC, ethnicity, and sexual orientation, and their three-way interaction. The MANCOVA and all three ANCOVAs revealed a main effect of MHC that was similar in magnitude to our main-effect analyses (for the multivariate test, F(3151) = 3.59, p = .015, partial η2 = .067).Footnote 3 No two- or three-way interactions involving MHC approached significance, p’s > .40. Therefore, the main effects of MHC detailed above do not appear to be qualified by any interactive effects of sexual orientation or ethnicity. Although not relevant to our predictions about MHC homozygosity, a sexual orientation by ethnicity interaction emerged in the MANCOVA and two of the three ANCOVAs (p’s < .03). Because of this interaction, and because of the interest in potential differential effects of MHC in heterosexual, bisexual, and lesbian populations, we performed further analyses for each of these groups separately.
The raw means on each of the three dependent variables, split by sexual orientation and MHC status, are shown in Table 2. As can be seen from the table, the mean differences between MHC homozygous and heterozygous participants were generally similar for each of the three sexual orientation groups (the one exception to this pattern was age at sexual debut within lesbian participants, which was nearly identical between MHC groups). We also performed the same MANCOVA but for each sexual orientation group separately. Results from these analyses are shown in Table 3. These results revealed that the effect size estimates of MHC were larger in the bisexual and lesbian subgroups (partial η2’s = .148 and .149, respectively) than in the heterosexual subgroup (partial η2 = .066). However, given these small sample sizes of these subgroups, and given that no interactions involving MHC status in the preceding analyses approached significance, any inferences from these exploratory results should be made with caution.
Our data also allowed us to perform a discriminant test of whether homozygosity predicts women’s lifetime number of sexual relationships specifically (consistent with life history predictions) or predicts their lifetime number of romantic relationships more generally. To do this we analyzed two variables: participants’ self-reported lifetime number of “sexual” relationships and their lifetime number of “romantic” relationships. Means and standard deviations for these variables are reported at the bottom of Table 1. To examine associations between homozygosity and these two types of relationship experience, we conducted two univariate ANCOVA’s with the same predictors included in the analyses above. Convergent with the results reported above, homozygosity significantly predicted women’s lifetime number of sexual relationships, F(1169) = 7.04, p = .009, partial η2 = .040. In contrast, whereas age and sexual orientation significantly predicted women’s number of romantic relationships (partial η2’s = .043 and .023, respectively), homozygosity did not, F(1170) = 0.01, p = .90, partial η2 = .001. These results suggest that women’s MHC-homozygosity might influence their strategies towards sexual relationships specifically, rather than their orientation to romantic relationships more generally.
Discussion
A large body of empirical and theoretical work indicates that individuals tend to pursue faster life history strategies—often manifesting as earlier and more promiscuous sexual behavior—when they are more vulnerable to uncontrollable mortality threats. Given that MHC-homozygosity is an uncontrollable internal factor that reduces immunocompetence, it follows that MHC-homozygous individuals will also adopt a faster life history strategy, manifesting in more promiscuous sexual attitudes and behaviors and earlier sexual debut. Supporting this logic, as compared with MHC-heterozygous women, MHC-homozygous women reported more favorable attitudes toward short-term sex, an earlier sexual debut, a larger lifetime number of sex partners, and a larger lifetime number of sexual—but not romantic—relationships. To our knowledge, this is the first study to document an association between MHC homozygosity and individuals’ own sexual attitudes and behaviors, thus highlighting potentially fruitful directions for future research.
A few limitations deserve note. First, relative to the general population, recruiting methods dictated that the women who participated in this study were diverse in terms of their sexual orientation. Although the majority of the sample identified as heterosexual (38.9%) or bisexual (19.4%) and reported sexual experience with men, another 34.4% identified as lesbian. Importantly, controlling for both the main effects and interactive effects of sexual orientation in the analyses did not meaningfully alter the magnitude of the associations between MHC homozygosity and the dependent measures. Further, analyses that excluded lesbian women yielded an estimated effect size for MHC that was nearly identical to the effect size based on the full sample.
Comparing estimated effect sizes between women who identify as heterosexual versus those who identify as bisexual or lesbian suggested that MHC more strongly predicted fast sexual strategies in non-heterosexual women. However, no interactions between MHC status and sexual orientation approached significance. Nonetheless, it remains important to be cautious about making generalizations based on this unique sample, and we encourage replications in other samples.
Second, the current sample was highly ethnically diverse. While ethnically diverse samples are often lauded in psychological research, such samples can pose problems for drawing inferences from gene-based studies. Indeed, for this reason, researchers investigating MHC-related questions often restrict their samples to one ethnic group in order to reduce confounds and increase statistical power (e.g. Roberts et al. 2005). However, the groups in our sample were similar on our variables of interest--only participants of Asian ancestry differed in any statistically meaningful way, which is consistent with other recent research (Saphire-Bernstein et al. 2016). It is also noteworthy that controlling for ethnicity in the present sample increased the magnitude of the association between MHC homozygosity and sexual attitudes and behavior. Therefore, although analyses revealed significant associations between ethnicity and some of the outcome variables, these effects appear to have obscured—rather than artificially enhanced—associations between MHC homozygosity and sexual attitudes and behavior.
Third, although this study is firmly theoretically couched within a life history framework, the sexual-based outcome variables measured here reflect only one facet of life history strategy and do not fully capture variation in life history strategy per se. This study did not assess other variables that are thought to be indicative of a faster life history strategy, such as higher impulsivity, greater temporal discounting, and lower ability to delay gratification. However, recent preliminary evidence suggests that these variables are indeed associated with vulnerability to infection (Hill et al. 2016), and we expect that future work will further link these variables to MHC homozygosity as well.
Fourth, although this logical framework specifically places MHC heterozygosity as the cause of variation in sexual strategies, the present data cannot distinguish between this and other possible causal explanations. For example, it is possible that MHC heterozygosity is associated with greater overall genetic diversity (not assessed in this study). Overall genetic diversity is often associated with better general condition, which in turn could lead to calibration towards slower life history strategies. Finally, it is possible that attractiveness (not assessed in this study), rather than immunocompetence, mediates the association between MHC homozygosity and sexual strategies. However, we think this is unlikely. Previous work has found that MHC heterozygosity is associated with greater physical attractiveness (e.g., Lie et al. 2008). In turn, research has found that attractiveness is generally associated with a more promiscuous sociosexual orientation and an earlier sexual debut (e.g., Weeden and Sabini 2007). Therefore, one might have predicted that MHC heterozygosity would be associated with faster sexual strategies by way of heterozygosity’s positive effects on attractiveness. This is opposite of the pattern we predicted and observed in this study. Nonetheless, it will be important for further research to place attractiveness and overall genetic diversity into increasingly comprehensive explanatory models of sexual attitudes and behavior as this work continues to grow.
It is also worth noting that intuitive logic may arrive at the opposite prediction—that higher vulnerability to disease may lead to a less promiscuous and sexual strategy as a behaviorally protective measure against human-transmitted infections, and delayed reproductive timing as a result of increased metabolic allocation to somatic maintenance. Indeed, at the psychological level, some research suggests that trait-like aversion to germs and disease vectors is associated with less promiscuous sexual attitudes and behaviors (Duncan et al. 2009; Murray et al. 2013). This alternative relationship is logically implausible on at least two accounts. First, within a life history framework, this would entail that this threat be a controllable internal factor in order for delayed reproduction (in favor of somatic development) to be an adaptive strategy. Homozygosity at the MHC confers a threat that is not controllable, as it is associated with a decreased ability to detect novel, un-encountered antigens (Bhutta 2007; Penn et al. 2002). This is consistent with other work linking uncontrollable immunity-related threats to earlier reproductive timing and higher sexual risk-taking (e.g. Choquet et al. 1997; Suris et al. 2008; Waynforth 2012). Second, the research linking aversion to disease cues to more restrictive sexual strategies is exclusive to affective, psychological reactions to potential disease transmission and, perhaps surprisingly, the relationship between these affective reactions and actual vulnerability to disease are weak (e.g. Duncan et al. 2009; Hill et al. 2015; Murray et al. 2013). Investigations are only beginning to elucidate the relationship between actual immunological vulnerability and affective reactions to disease threats. In one notable example, a set of five experiments showed that increasing women’s perceptions of their vulnerability to infection (but importantly, not their level of disgust or germ aversion) increased their desire for sexual variety and desire for novel sex partners (Hill et al. 2015).
Despite these limitations, a few strengths deserve note. First, we largely employed a community sample, and within this sample women varied widely in sexual attitudes and behavior. Second, this study is one of the first to look at MHC as a source of individual differences in social behavior. MHC in humans has been studied primarily as a predictor of attractiveness or attraction between romantic dyads (for review see Havlicek and Roberts 2009); the current study suggests that it can be used as a marker of individual differences in immunocompetence, which may have further implications for the diverse facets of our disease avoidance psychology. Third, this study conceptualizes an internal existential threat as an uncontrollable factor which, with limited exceptions (e.g., Hill et al. 2016) is to date one of the few studies to do so. Such conceptualization consequently suggests that such internal factors have implications that are similar to uncontrollable external threats. This distinction will provide fruitful avenues for research.
Future research will require examining whether these effects generalize to men. Some findings suggest that women’s sexuality is more responsive than men’s to context (e.g., Schaller and Murray 2008; Schmitt 2005). Therefore, although it is plausible that MHC-homozygous men will also exhibit a faster sexual strategy than MHC-heterozygous men, this association may be weaker for men than for women.
Future research may also examine whether other genetic markers of reduced immunocompetence are associated with faster sexual strategies in humans. Some recent work has identified specific genetic polymorphisms that are associated both with poorer immunocompetence (such as the C allele of the ACP1 gene) and with more socially cautious personality traits (Napolioni et al. 2014; see also MacMurray et al. 2014). However, the links between specific polymorphisms putatively associated with immunocompetence and their implications for sexual strategies remain to be elucidated.
The associations documented here are provocative and suggest many promising paths for future research, with potentially important implications for links between the genetics of immunocompetence, life history strategies, and human sexuality and behavior.
Notes
Excluding these 15 participants did not change the inferential nature of the results; excluding these participants from our central main effects analysis produced an estimated effect size of MHC within .002 of the estimate reported here.
Analyses which included ethnicity and sexual orientation (or both) as non-dichotomous variables (all six levels for ethnicity, all four levels for sexual orientation) produced p-value estimates for the main effect of MHC that were actually slightly smaller than those reported here. We report the dichotomous-based analyses throughout for the sake of interpretability and maximizing degrees of freedom.
Follow-up analyses also included data collection site as a control variable. Results from these analyses revealed near-identical estimated effect sizes of MHC to those reported above (e.g., in the main-effect MANCOVA, partial η2 = .088, p = .003).
References
Anderson, R. M., & May, R. M. (1991). Infectious diseases of humans (Vol. 1). Oxford: Oxford University Press.
Bailey, J. M., Gaulin, S., Agyei, Y., & Gladue, B. A. (1994). Effects of gender and sexual orientation on evolutionarily relevant aspects of human mating psychology. Journal of Personality and Social Psychology, 66, 1081–1093.
Belsky, J., Steinberg, L., & Draper, P. (1991). Childhood experience, interpersonal development, and reproductive strategy: an evolutionary theory of socialization. Child Development, 62, 647–670.
Belsky, J., Steinberg, L. D., Houts, R. M., Friedman, S. L., DeHart, G., Cauffman, E., et al. (2007). Family rearing antecedents of pubertal timing. Child Development, 78, 1302–1321.
Bhutta, M. F. (2007). Sex and the nose: human pheromonal responses. Journal of the Royal Society of Medicine, 100, 268–274.
Carrington, M., & O’Brien, S. J. (2003). The influence of HLA genotype on AIDS*. Annual Review of Medicine, 54, 535–551.
Chisholm, J. S. (1999). Death, hope and sex: steps to an evolutionary ecology of mind and morality. UK: Cambridge University Press.
Choquet, M., Fediaevsky, L. D. P., & Manfredi, R. (1997). Sexual behavior among adolescents reporting chronic conditions: a French national survey. Journal of Adolescent Health, 20, 62–67.
Del Giudice, M. (2009). Sex, attachment, and the development of reproductive strategies. Behavioral and Brain Sciences, 32, 1–21.
Del Giudice, M., & Belsky, J. (2011). The development of life history strategies: toward a multi-stage theory. In D. M. Buss & P. H. Hawley (Eds.), The evolution of personality and individual differences (pp. 154–176). New York: Oxford University Press.
Del Giudice, M., Gangestad, S. W., & Kaplan, H. (2015). Life history theory and evolutionary psychology. In D. M. Buss (Ed.), The handbook of evolutionary psychology. USA: Wiley.
Duncan, L. A., Schaller, M., & Park, J. H. (2009). Perceived vulnerability to disease: development and validation of a 15-item self-report instrument. Personality and Individual Differences, 47, 541–546.
Ellis, B. J., Figueredo, A. J., Brumbach, B. H., & Schlomer, G. L. (2009). Fundamental dimensions of environmental risk: the impact of harsh versus unpredictable environments on the evolution and development of life history strategies. Human Nature, 20, 204–268.
England, P., & Bearak, J. (2014). The sexual double standard and gender differences in attitudes toward casual sex among US university students. Demographic Research, 30, 1327–1338.
Figueredo, A. J., Vásquez, G., Brumbach, B. H., Sefcek, J. A., Kirsner, B. R., & Jacobs, W. J. (2005). The K-factor: individual differences in life history strategy. Personality and Individual Differences, 39, 1349–1360.
Garcia, J. R., MacKillop, J., Aller, E. L., Merriwether, A. M., Wilson, D. S., & Lum, J. K. (2010). Associations between dopamine D4 receptor gene variation with both infidelity and sexual promiscuity. PloS One, 5, e14162. doi:10.1371/journal.pone.0014162.
Griskevicius, V., Ackerman, J. M., Cantú, S. M., Delton, A. W., Robertson, T. E., Simpson, J. A., Thompson, M. E., & Tybur, J. M. (2013). When the economy falters, do people spend or save? Responses to resource scarcity depend on childhood environments. Psychological Science, 24, 97–105.
Havlicek, J., & Roberts, S. C. (2009). MHC-correlated mate choice in humans: a review. Psychoneuroendocrinology, 4, 497–512.
Hetherington, E. M. (1972). Effects of father-absence on personality development in adolescent daughters. Developmental Psychology, 7, 313–326.
Hill, S. E., Prokosch, M. L., & DelPriore, D. J. (2015). The impact of perceived disease threat on women’s desire for novel dating and sexual partners: is variety the best medicine? Journal of Personality and Social Psychology, 109, 244–261.
Hill, S. E., Boehm, G. W., & Prokosch, M. L. (2016). Vulnerability to disease as a predictor of faster life history strategies. Adaptive Human Behavior and Physiology, 2, 116–133.
Hraber, P., Kuiken, C., & Yusim, K. (2007). Evidence for human leukocyte antigen heterozygote advantage against hepatitis C virus infection. Hepatology, 46, 1713–1721.
Inhorn, M. C., & Brown, P. J. (1990). The anthropology of infectious disease. Annual Review of Anthropology, 19, 89–117.
Janeway, C. A., Travers, P., Walport, M. J., & Shlomchik, M. J. (2001). Immunobiology: the immune system in health and disease (Vol. 2). New York: Garland Publishing.
Jeffery, K. J., Siddiqui, A. A., Bunce, M., Lloyd, A. L., Vine, A. M., Witkover, A. D., et al. (2000). The influence of HLA class I alleles and heterozygosity on the outcome of human T cell lymphotropic virus type I infection. Journal of Immunology, 165, 7278–7284.
Kaplan, H. S., & Gangestad, S. W. (2005). Life history theory and evolutionary psychology. In D. M. Buss (Ed.), The handbook of evolutionary psychology (pp. 68–95). Hoboken: John Wiley & Sons.
Lie, H., Simmons, L., & Rhodes, G. (2008). Genetic diversity revealed in human faces. Evolution, 62, 2473–2486.
Low, B. S. (1990). Marriage systems and pathogen stress in human societies. American Zoologist, 30, 325–340.
Low, B. S., Hazel, A., Parker, N., & Welch, K. B. (2008). Influences on women’s reproductive lives: unexpected ecological underpinnings. Cross-Cultural Research, 42, 201–219.
MacMurray, J., Comings, D. E., & Napolioni, V. (2014). The gene-immune-behavioral pathway: gamma-interferon (IFN-γ) simultaneously coordinates susceptibility to infectious disease and harm avoidance behaviors. Brain, Behavior, and Immunity, 35, 169–175.
Miller, B. C., & Bingham, C. R. (1989). Family configuration in relation to the sexual behavior of female adolescents. Journal of Marriage and the Family, 51, 499–506.
Murray, D. R., Jones, D. N., & Schaller, M. (2013). Perceived threat of infectious disease and its implications for sexual attitudes. Personality and Individual Differences, 54, 103–108.
Napolioni, V., Murray, D. R., Comings, D. E., Peters, W. R., Gade-Andavolu, R., & MacMurray, J. (2014). Interaction between infectious diseases and personality traits: ACP1*C as a potential mediator. Infection, Genetics and Evolution, 26, 267–273.
Nettle, D. (2012). Behaviour of parents and children in two contrasting urban neighbourhoods: an observational study. Journal of Ethology, 30, 109–116.
Nettle, D., & Cockerill, M. (2010). Development of social variation in reproductive schedules: a study from an English urban area. PloS One, 5, e12690.
Newcomer, S., & Udry, J. R. (1987). Parental marital status effects on adolescent sexual behavior. Journal of Marriage and Family, 49, 235–240.
Penn, D. J., Damjanovich, K., & Potts, W. K. (2002). MHC heterozygosity confers a selective advantage against multiple-strain infections. Proceedings of the National Academy of Sciences of the United States of America, 99, 11260–11264.
Quinlan, R. J. (2007). Human parental effort and environmental risk. Proceedings of the Royal Society B: Biological Sciences, 274, 121–125.
Roberts, S. C., Little, A. C., Gosling, L. M., Jones, B. C., Perrett, D. I., Carter, V., & Petrie, M. (2005). MHC-assortative facial preferences in humans. Biology Letters, 1, 400–403.
Roff, D. A. (2002). Life history evolution. Sunderland: Sinauer.
Saphire-Bernstein, S., Larson, C. M., Gildersleeve, K. A., Fales, M. R., Pillsworth, E. G., & Haselton, M. G. (2016). Genetic compatibility in long-term intimate relationships: partner similarity at major histocompatibility complex (MHC) genes may reduce in-pair attraction. Evolution and Human Behavior. doi:10.1016/j.evolhumbehav.2016.09.003.
Schaller, M., & Murray, D. R. (2008). Pathogens, personality, and culture: disease prevalence predicts worldwide variability in sociosexuality, extraversion, and openness to experience. Journal of Personality and Social Psychology, 95, 212–221.
Schmitt, D. P. (2005). Sociosexuality from Argentina to Zimbabwe: a 48-nation study of sex, culture, and strategies of human mating. Behavioral and Brain Sciences, 28, 247–311.
Shaw, B.E. (2009). Human leukocyte antigen matching, compatibility testing and donor selection. In J. Treleaven & A. J. Barrett (Eds.), Hematopoietic stem cell transplantation in clinical practice (pp. 239–247). London: Churchill Livingstone.
Simpson, J. A., & Gangestad, S. W. (1991). Individual differences in sociosexuality: evidence for convergent and discriminant validity. Journal of Personality and Social Psychology, 60, 870–883.
Suris, J. C., & Parera, N. (2005). Sex, drugs and chronic illness: health behaviours among chronically ill youth. The European Journal of Public Health, 15, 484–488.
Suris, J. C., Michaud, P. A., Akre, C., & Sawyer, S. M. (2008). Health risk behaviors in adolescents with chronic conditions. Pediatrics, 122, e1113–e1118.
Thornton, A., & Camburn, D. (1989). Religious participation and adolescent sexual behaviour and attitudes. Journal of Marriage and Family, 51, 641–653.
Valencia, L. S., & Cromer, B. A. (2000). Sexual activity and other high-risk behaviors in adolescents with chronic illness: a review. Journal of Pediatric and Adolescent Gynecology, 13, 53–64.
Waynforth, D. (2012). Life-history theory, chronic childhood illness and the timing of first reproduction in a British birth cohort. Proceedings of the Royal Society of London B: Biological Sciences. doi:10.1098/rspb.2012.0220.
Weeden, J., & Sabini, J. (2007). Subjective and objective measures of attractiveness and their relation to sexual behavior and sexual attitudes in university students. Archives of Sexual Behavior, 36, 79–88.
Wilson, M., & Daly, M. (1997). Life expectancy, economic inequality, homicide, and reproductive timing in Chicago neighbourhoods. British Medical Journal, 314, 1271–1274.
Wolfe, N. D., Dunavan, C. P., & Diamond, J. (2007). Origins of major human infectious diseases. Nature, 447, 279–283.
Zietsch, B. P., Westberg, L., Santtila, P., & Jern, P. (2015). Genetic analysis of human extrapair mating: heritability, between-sex correlation, and receptor genes for vasopressin and oxytocin. Evolution and Human Behavior, 36(2), 130–136.
Zion, Z. B., Tessler, R., Cohen, L., Lerer, E., Raz, Y., Bachner-Melman, R., et al. (2006). Polymorphisms in the dopamine D4 receptor gene (DRD4) contribute to individual differences in human sexual behaviour: desire, arousal, and sexual function. Molecular Psychiatry, 11, 782–786.
Acknowledgements
We thank the American Institute for Bisexuality (AIB) for funding this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing Interests
We have no competing interests.
Declaration of Ethical Standards
This research was carried out under the approval of the local Institutional Review Board responsible for the oversight of social/behavioral research. All participants provided their informed consent for the de-identified use of both their self-report data and genomic data.
Funding
This study and preparation of this manuscript was funded a grant from the American Institute for Bisexuality (awarded to MGH).
Additional information
Damian R. Murray and Kelly A. Gildersleeve contributed equally to this work.
Rights and permissions
About this article
Cite this article
Murray, D.R., Gildersleeve, K.A., Fales, M.R. et al. MHC Homozygosity Is Associated with Fast Sexual Strategies in Women. Adaptive Human Behavior and Physiology 3, 101–117 (2017). https://doi.org/10.1007/s40750-016-0057-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40750-016-0057-5