Abstract
Human female preferences for male traits may be indicative of sexual selection pressures. Previous data suggest women’s preferences for masculine features appear to be highest around peak times of fertility. Preferences for sexually dimorphic features at different times of risk of conception suggest women may be shifting mate preferences or mating strategies. Two datasets presented here show women’s preferences for digitally masculinized or feminized faces after recently giving birth and caring for a young baby. Findings from an online sample (n = 211) and the Philippines (n = 155) indicate women during the first year after giving birth expressed a preference for feminized male faces. Moreover, in the same Philippines study, nulliparous women (n = 105) similarly displayed a preference for feminized male faces. These findings suggest women caring for a young infant may be utilizing contextual mating strategies in a time of low-risk of conception, while a preference for feminine male faces may also be suggestive of the wider variation in face dimorphism preferences cross-culturally.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Women undertake dramatic changes in reproductive effort in the process of conceiving, carrying a pregnancy to term, and in postpartum care. This amounts to life history shifts from mating to parenting effort (Ellison 2003; Trivers 1972; Stearns 1992). The postpartum phase is characterized by intensive parenting effort, typically at expense to mating effort. Indeed, intensive breastfeeding interacts with other energetic and health factors in contributing to lactational amenorrhea (Ellison 2003; Ellison 2009), where the maternal benefits of lactation operate at expense to ovarian cycling. These shifts in reproductive effort are reflected in steroid hormone changes. For example, low testosterone and estradiol levels, both sex steroids which are lower after birth compared to pre-pregnancy levels (see Barrett et al. 2013; Nelson 2011), may decrease women’s sexual behaviors (Meston and Frohlich 2001; Simon et al. 2005; Sarrel et al. 1998). An increase in oxytocin, particularly during breastfeeding, after giving birth may indicate a shift in care towards an infant (Brizendine 2006; Lancaster and Kaplan 2009).
A downregulation of mating effort may be seen through a decline in sexual function (Weeks-Shackelford et al. 2007; Escasa-Dorne 2015), including selectivity for mating-related traits. Postpartum mothers tend to exhibit lower rates of sexual activity compared to pre-pregnancy levels (Hicks et al. 2004; Hipp et al. 2012; Leeman and Rogers 2012; Sayasneh and Pandeva 2010; Woranitat and Taneepanichskul 2007), and rates of dyspareunia during the year after giving birth are as high as 92 % (Andrews et al. 2008).
A preference for sexually dimorphic traits provides us with information on the evolution of human mating psychology (Puts 2010, 2016). Sexually dimorphic traits, including masculine facial features, may be one cue indicating genetic potential or phenotypic quality of a possible mate (Andersson 1994; Johnstone 1995; Roberts et al. 2004; Zahavi 1991). Women’s preferences for masculinity, particularly for traits of masculinity in faces, in a potential partner appear to be contextual (see Gildersleeve et al. 2014). For example, Little et al. (2002) found preferences for feminine facial features appear higher among women who are not in a relationship and for a long-term (rather than a short-term) context among women who were not using oral contraceptives; however, face preferences differed among women who were currently using oral contraceptives. Additionally, while masculine faces may be indicative of certain beneficial traits when selecting a partner, many studies find only slight preferences for masculine features (Rhodes et al. 2003; Puts 2005; DeBruine et al. 2006; Little et al. 2008) or preferences for feminine features (Perrett et al. 1998; Penton-Voak et al. 1999a, b; Little et al. 2001; Welling et al. 2007). In one study among the Hadza of Tanzania, breastfeeding women preferred feminized male voices played over an audio recording compared to women who were not breastfeeding (Apicella and Feinberg 2009). Among 28 women in the UK, recruited during pregnancy and followed through to 12 weeks postpartum, participants had a stronger preference for masculine faces during pregnancy compared to postpartum (Cobey et al. 2015). Further, compared to a group of nulliparous women, women using hormonal contraceptives had a higher preference for masculine male faces (Cobey et al. 2015).
Preferences for masculine traits appear to be highest around the time of ovulation, when conception risk is highest (Gildersleeve et al. 2014; Gangestad et al. 2015; Gangestad and Thornhill 1998). The ovulatory-shift hypothesis (Gangestad et al. 2005) argues that the preference for cues indicative of high genetic quality in a potential mate are highest when risk of conception is highest, compared to times of low risk of conception. For example, Penton-Voak and Perrett (2000) analyzed facial masculinity preferences of 139 women who were not using oral contraceptives. Based on self-reports of menstrual cycle status, women who were placed in a high-conception risk group (i.e., around ovulation) had higher preferences for masculinized male faces compared to women in a low-conception risk group. Other studies using similar self-report methods find similar effects of higher masculinity preference around ovulation (Johnston et al. 2001; Jones et al. 2005a, b, 2008; Penton-Voak et al. 1999a, b; Penton-Voak and Perrett 2000). Shifting preferences over the menstrual cycle may be due to an adaptive benefit of extra selectiveness of traits indicative of high genetic quality in a partner during a time of high risk of conception, an adaptive benefit of selectivity of high partnership quality during most non-conceptive times of a cycle.
Shifting preferences may also be a result of fluctuations in hormones, particularly the sex steroid hormones, across the menstrual cycle. Estradiol and progesterone shift, with the highest levels of estradiol near ovulation, whereas progesterone levels peak during the luteal phase. These shifts in sex steroid hormone levels may alter women’s facial preferences over the menstrual cycle. Within-participant changes in estradiol level predicted women’s preferences for facial masculinity (Roney and Simmons 2008; Roney et al. 2011). Among 70 women, Welling et al. (2007) found women’s preferences for masculine faces were higher on days on which testosterone levels were higher. In another study, Marcinkowska et al. (2016) analyzed risk of conception using estradiol as a marker of between-subjects cycling status of 115 Polish women and found that estradiol was not associated with masculinity preferences of faces in a short-term or long-term context, nor did the study find differences in preferences when the women were divided into groups of high or low probability of conception.
Preferences for masculine male faces appear to provide cues of a high quality partner; however, masculine features may be contextually less desirable for numerous reasons. Males with higher testosterone levels, for example, may also be more aggressive, more likely to have extra-pair relationships, and be less invested in spouses or parenting (Gray et al. 2002; Mazur and Booth 1998). Males with higher facial masculinity were rated as more likely to cheat on a partner, to get into more physical fights, to be a lower quality spouse and father, and to be less emotionally supportive in a relationship compared to males with higher facial femininity (Boothroyd et al. 2007; Kruger 2006). Moreover, cross-cultural variation appears to impact the degree of preference for masculine or feminine facial features, with arguments that the preference for masculinity may be impacted by the pathogen load in the environment (DeBruine et al. 2010a; Moore et al. 2013; Scott et al. 2014).
Despite the availability of research on women’s face preferences for males, little research focuses on post-birth shifts in female preferences for male traits. Because of the change in sex steroid hormones, and the behavioral shifts from mating to parenting effort, during the postpartum phase, an understanding of facultative face preferences among women after giving birth is warranted. Women’s post-birth preferences for male traits may be altered, also complementing research on facultative female preferences across the menstrual cycle. Women’s sexual and romantic preferences in a partner after giving birth have received little previous research focus, leaving an empirical gap to be filled. Additionally, the preferences of women who are in lactational amenorrhea may provide insight into the evolution of our behavioral reproductive milieu, as women in natural fertility groups spend a majority of their reproductive years in this phase, and unlike industrialized groups (Escasa-Dorne et al. 2013; Eaton et al. 1994; Strassmann 1997, 1999).
Here, we test preferences for facial masculinity or femininity among mothers caring for a young baby. Based on tradeoffs between mating and parenting effort, we predicted mothers from our sample would have a preference for feminine faces, and that this would be stronger for breastfeeding women. To explore potential variation in female preferences of male faces during the time after giving birth, we asked participants to complete a face preference task. Participants were recruited from two populations: an online, convenience sample of women who had given birth in the past year and a sample of women from the Philippines who had 1) given birth in the past year, or 2) had never before given birth. The Philippines sample of nulliparous women provided a comparative sample of women who were not breastfeeding nor had given birth, and would more closely resemble previous study populations where facial preferences have been studied. As the Philippines sample also provided saliva samples for steroid hormone analysis, this population was chosen to provide a pool of participants who were more likely to breastfeed extensively and less likely to use hormonal contraceptives (NSO 2009).
Stimuli
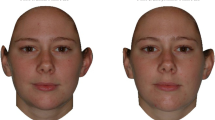
All participants were asked to view multiple sets of photographs of male faces. A total of five sets of male faces were presented, each set presented a male of a different ethnic background (composite images were obtained from male participants in South Asia, East Asia, South America, Europe, and African-Caribbean). The photograph was formed from a composite of images from each ethnicity. Each set of photographs consisted of one photo that was an unaltered photograph of a male, one photograph that was digitally altered to increase masculine features by 60 %, and one photograph that was digitally altered to increase feminine features by 60 % (see Scott et al. 2014 for more information on the methods used to digitally alter the photographs). The stimuli have been used in previous studies on multiple populations ranging from foraging groups to market economy groups (Scott et al. 2014). In random order, each set of three photographs was presented and participants were asked to choose which male was the most attractive in a short-term context and in a long-term context. Participants were also asked to select the male photo which appeared to be the “nicest”, “nastiest or meanest”, and “healthiest.” Each participant rated five sets of photographs.
Data were analyzed using SPSS v23.0 for Mac. All analyses assumed an alpha of .05 and were two-tailed. Once participants selected a photograph for each trait, the preference was entered into a database. The masculinized photograph was entered as a 60, the feminized photograph was entered as a −60, and the neutral photograph was entered as a 0 to reflect the percentage of manipulation (in either a feminized or masculinized direction) to each photograph. Each participants’ ratings were averaged across all 5 ratings (for the 5 photographs). Independent t-tests and ANOVAs were used for group comparisons, and bivariate correlation analyses were used for correlation effects. The methods for study 1 and 2 were reviewed and approved by the Institutional Review Board at the University of Nevada, Las Vegas.
Study 1
Methods
All questionnaires were completed online using SurveyMonkey.com. A link to the study was posted on various outlets (e.g., parenting blogs), and provided a convenience sample pool based primarily in the US. Women between the ages of 18–45 who had given birth in the past year were asked to complete the study. A total of 287 women started the survey. After excluding duplicate IP addresses and incomplete questionnaires, 211 participants’ data were used for analysis. After providing informed consent online, participants were directed to a questionnaire including demographic information (see Table 1). After completing the survey questions, participants were directed to the photographs to complete the stimuli task.
Study 1 Results
Of the 211 participants ratings, there was a significant preference for feminine faces (t(165) = 2.98, p = .003, d = 0.64) in rating long-term attractiveness, and a non-significant masculinity preference for a short-term context (t(165) = −.99, p = .324, d = −0.154). There was no significant difference for either long-term preferences between the women who were cycling (35 % preference for feminized faces) and women who were non-cycling (34.2 % preference for feminized faces) (t(151) = −.21, p = .836, d = −0.03), nor short-term preferences between cycling (30.2 %) and non-cycling women (27.7 %) (t(151) = −.27, p = .785, d = −0.04). There was no significant difference for either long-term preferences between the women who were breastfeeding (35 % preference for feminized faces) and women who were not breastfeeding (35.5 % preference for feminized faces) (t(164) = .15, p = .882, d = 0.02), nor short-term preferences between breastfeeding (30.9 %) and women who were not breastfeeding (24.2 %) (t(164) = 1.07, p = .287, d = 0.17).
There was no correlation between the age of the infant (M = 6.6, SD = 3.8 months) and long-term preference for faces (r(151) = −.10, p = .243) nor short-term preference for faces (r(151) = −.05, p = .539), nor age of the participant (M = 29.01, SD = 5.5 years) and long-term preference for faces (r(139) = .04, p = .625), nor short-term preference for faces (r(139) = −.13, p = .113). There was no significant difference between women who were or were not using a hormonal contraceptive and long-term preference for faces (t(164) = −.32, p = .753, d = −0.05) nor short-term preference for faces (t(164) = −.56, p = .575, d = −0.09).
Study 2
Methods
In the second study, 260 participants were recruited from neighborhood health centers in Manila, Philippines (see Table 2 for demographic data). Participants were placed into one of two groups: women who had never before given birth (n = 105) and women who were breastfeeding an infant (n = 155). For additional analyses, the breastfeeding women were grouped into ‘cycling’ or ‘non-cycling’ based on information from the questionnaire. Cycling status was determined based on the woman’s last reported menstrual cycle and time since birth. Eighty-six women had reported having at least two menstrual cycles since giving birth and were at least 12 weeks postpartum, while 48 women reported not having resumed any menstrual cycles since becoming pregnant. We were unable to determine the cycling status of 21 women, thus these women were excluded from additional analyses.
After the researchers explained the purpose of the study in Tagalog, and obtained informed consent, each participant provided a saliva sample via passive drool to be analyzed for testosterone and estradiol levels. Saliva samples were stored in a cooler with ice immediately after collection and placed in a freezer at the end of each collection day. Samples were shipped to the UNLV Evolution and Behavior Lab via overnight delivery on dry ice, and were stored in a −20 °C freezer until analysis. Saliva samples were assayed in batches, and run in duplicate, for testosterone and estradiol at the UNLV Evolution and Human Behavior Lab using Salimetrics kits. Inter-assay coefficients of variation were 3.3 % and 3.0 % for testosterone and 7.8 % and 4.3 % for estradiol. The intra-assay coefficients of variation were 4.2 % and 7.3 %, for testosterone and estradiol respectively.
The same demographic questionnaires and photo stimuli as Study 1 were used in Study 2. All questionnaires were translated into Tagalog, and participants were given the option to complete the study in English or Tagalog. Although Tagalog is spoken by most individuals as either a first or second language in the Philippines, Tagalog (or Filipino) and English are the national languages of the Philippines. Participants completed all methods with the researcher in a semi-private location of the health centers. After the participants completed the questionnaire, the researchers placed the photographs in front of the participant and asked the participant to choose the photo which represented her choice for the same traits listed in Study 1 (most attractive in a short-term context, most attractive in a long-term context, nicest, nastiest, and healthiest).
Study 2 Results
Of the 260 participant’s ratings, there was a statistically significant preference for feminine faces in a long-term (t(238) = 6.58, p < .001, d = 0.85) and short-term (t(238) = 4.02, p < .001, d = 0.52) context. There was no significant difference for either long-term preferences between the women who had never given birth (56.2 % preference for feminized faces) and women who were breastfeeding (51.6 % preference for feminized faces) (t(237) = .58, p = .561, d = 0.08), nor short-term preferences between women who had never given birth (42.9 %) and women who were breastfeeding (48.4 %) (t(237) = −0.97, p = .335, d = −0.13) (see Table 3). There was no significant difference between cycling status and long-term preferences (F(2, 219) = .25, p = .779, ηp2 = 0.002) nor short-term preferences (F(2, 219) = .35, p = .703, ηp2 = 0.003).
There was no correlation between the age of the infant (M = 8.5, SD = 5.8 months) and long-term preference for faces (r(140) = −.14, p = .089) nor short-term preference for faces (r(140) = .01, p = .918). There was a significant correlation between age and long-term preference for faces (r(203) = −.16, p = .026), but not for short-term preference for faces (r(203) = −.03, p = .636). After entering age as a covariate, overall preference for feminized faces was still significant (F(1, 204) = 5.06, p = .026). There was no significant difference in face preferences between relationship status and long-term preference for faces (t(208) = −.85, p = .399, d = −0.12) nor short-term preference for faces (t(208) = −1.48, p = .141, d = −0.21).
There was no correlation between estradiol levels (M = 1.46 pg/mL, SD = .97) and long-term preference (r(176) = −.01, p = .934) nor short-term preference (r(176) = −.03, p = .671), nor between testosterone levels (M = 58.79 pg/mL, SD = 24.25) and long-term preferences (r(173) = .02, p = .833) nor short-term preferences (r(173) = −.01, p = .671).
Discussion
This study was designed to determine male facial masculinity preferences among women caring for a young baby. Data from both samples indicate no masculinity facial preferences among postpartum women. Women in both studies had a preference for feminine faces; however, there was no difference between the breastfeeding and nulliparous women in the Philippines sample. Previous studies find support for masculine face preferences, albeit with small to modest effects (Gildersleeve et al. 2014; Rhodes et al. 2003; DeBruine et al. 2006), while other studies suggest a feminine face preference or no preference (see DeBruine et al. 2006). The variation in face preferences may be due to the variation in methods utilized by each study, low perception of variation in morphology of stimuli, or could suggest the wide variation in preferences across ecological contexts (Germine et al. 2015).
The preference for feminine faces has been interpreted as a potential mating strategy, in which females select traits which may be indicative of pair-bonding quality (Little et al. 2002), or avoidance of characteristics detrimental to pair-bonding (Boothroyd et al. 2007; Gray et al. 2002; Kruger 2006; Mazur and Booth 1998). While the interpretations of mate preference have occurred primarily among women who are regularly cycling, the preference of male faces may be of particular importance during the postpartum phase, where women are negotiating trade-offs between mating and parenting effort, and undergoing birth spacing strategies (Escasa-Dorne et al. 2013). Also consistent with this view, one study reported lower preferences for masculine faces during pregnancy, compared to women who were not pregnant, with high preferences for feminine faces during the third trimester (Limoncin et al. 2015). Moreover, a longitudinal study entailing two assessments of women’s preferences for faces showed decreases in preferred facial masculinity but no differences in female facial masculinity postpartum compared to pregnancy (Cobey et al. 2015). As also noted in the study by Cobey et al. (2015), changes in preferences for masculinity or femininity of male faces may be a feature of physiological trade-offs in parenting status, rather than primarily attributed to changes in the physiology of the post-partum phase.
Previous data have suggested a preference for masculine traits or features as they may indicate a tolerance to disease in an environment of higher pathogen load (risk) (DeBruine et al. 2010a, b, c) However, the preference for feminine faces overall in the Philippines study may be consistent with cross-cultural data which find a higher preference for feminine faces among women in higher pathogen load populations (Scott et al. 2014). The lack of differences in Filipina women’s preferences depending on motherhood status (mothers vs. childless) for male faces suggests some broader cultural or contextual factor (rather than reproductive condition) may underlie face preferences. Yet the similar patterns in women’s face preferences, perhaps with potential differences in pathogen loads, between the U.S.-based Internet sample and Philippines samples also argues against any clear or direct connection to pathogen loads.
There were no differences in facial masculinity preference between breastfeeding status or cycling status. The authors analyzed cycling status based on self-reported data and did not measure any surges in luteinizing hormone (LH) to determine cycling status. Testosterone and estradiol were both lower among the non-cycling group compared to the cycling and nulliparous groups (F(2, 172) = 12.3, p < .001, ηp2 = 0.13 and F(2, 175) = 23.1, p < .001, ηp2 = 0.21 respectively), consistent with previous studies (see Nelson 2011) which find lower sex steroid hormone production among women in lactational amenorrhea. Thus, these data confirm expected differences in hormone levels according to cycle status, even as cycling status was unrelated to women’s preferences for male faces. Future studies may benefit from employing a longitudinal design among women, following them from pregnancy through lactational amenorrhea to the resumption of ovulatory cycles based on LH surges to determine cycling status.
To summarize, the current two studies tested preferences for facial masculinity among mothers caring for a young baby. Both populations of mothers preferred feminine faces; however, there were no significant differences between breastfeeding status or cycling status. The preferences of women during the time after giving birth may help provide contextual information for the reproductive strategies of women, and may be useful to a broader understanding of tradeoffs between mating and parenting effort, birth spacing strategies, differential investment, and parenting-mating interactions over successive offspring. Research on women’s mate preferences after birth calls for further analysis, and the current study provides a glimpse into mate preferences throughout life history transitions.
References
Andersson, M. (1994). Sexual selection. Princeton: Princeton University Press.
Andrews, V., Thakar, R., Sultan, A. H., & Jones, P. W. (2008). Evolution of postpartum perineal pain and dyspareunia – a prospective study. European Journal of Obstetrics & Gynecology and Reproductive Behavior, 137, 152–156.
Apicella, C. L., & Feinberg, D. R. (2009). Voice pitch alters mate-choice-relevant perception in hunter–gatherers. Proceedings of the Royal Society of London B: Biological Sciences, 276(1659), 1077–1082.
Barrett, E. S., Tran, V., Thurston, S., Jasienska, G., Furberg, A. S., Ellison, P. T., & Thune, I. (2013). Marriage and motherhood are associated with lower testosterone concentrations in women. Hormones and Behavior, 63(1), 72–79.
Boothroyd, L. G., Jones, B. C., Burt, D. M., & Perrett, D. I. (2007). Partner characteristics associated with masculinity, health and maturity in male faces. Personality and Individual Differences, 43, 1161–1173.
Brizendine, L. (2006). The female brain. Broadway Books
Cobey, K. D., Little, A. C., & Roberts, S. C. (2015). Hormonal effects on women's facial masculinity preferences: the influence of pregnancy, post-partum, and hormonal contraceptive use. Biological Psychology, 104, 35–40.
DeBruine, L. M., Jones, B. C., Little, A. C., Boothroyd, L. G., Perrett, D. I., Penton-Voak, I. S., Cooper, P. A., Penke, L., Feinberg, D. R., & Tiddeman, B. P. (2006). Correlated preferences for facial masculinity and ideal or actual partner's masculinity. Proceedings of the Royal Society of London B: Biological Sciences, 273(1592), 1355–1360.
DeBruine, L. M., Jones, B. C., Little, A. C., Crawford, J. R., & Welling, L. L. (2010a). Further evidence for regional variation in women's masculinity preferences. Proceedings of the Royal Society of London B: Biological Sciences, rspb20102200.
DeBruine, L. M., Jones, B. C., Tybur, J. M., Lieberman, D., & Griskevicius, V. (2010a). Women's preferences for masculinity in male faces are predicted by pathogen disgust, but not moral or sexual disgust. Evolution and Human Behavior, 31, 69–74.
DeBruine, L. M., Jones, B. C., Crawford, J. R., Welling, L. L., & Little, A. C. (2010b). The health of a nation predicts their mate preferences: cross-cultural variation in women's preferences for masculinized male faces. Proceedings of the Royal Society of London B: Biological Sciences, 277(1692), 2405–2410.
Eaton, S. B., Pike, M. C., Short, R. V., Lee, N. C., Trussell, J., Hatcher, R. A.,… & Hill, K. R. (1994). Women's reproductive cancers in evolutionary context. Quarterly Review of Biology, 353–367
Ellison, P. T. (2003). Energetics and reproductive effort. American Journal of Human Biology, 15(3), 342–351.
Ellison, P. T. (2009). On fertile ground: A natural history of human reproduction. Harvard University Press.
Escasa-Dorne, M. J. (2015). Sexual functioning and commitment to their current relationship among breastfeeding and regularly cycling women in Manila, Philippines. Human Nature, 26(1), 89–101.
Escasa-Dorne, M., Young, S. M., & Gray, P. B. (2013). Now or later. Evolutions Empress: Darwinian Perspectives on the Nature of Women, 260.
Gangestad, S. W., & Thornhill, R. (1998). Menstrual cycle variation in women’s preferences for the scent of symmetrical men. Proceedings of the Royal Society B: Biological Sciences, 265, 927–933.
Gangestad, S. W., Thornhill, R., & Garver-Apgar, C. E. (2005). Adaptations to ovulation: implications for sexual and social behavior. Current Directions in Psychological Science, 14, 312–316.
Gangestad, S. W., Thornhill, R., & Garver‐Apgar, C. E. (2015). Women's sexual interests across the ovulatory cycle. The Handbook of Evolutionary Psychology.
Germine, L., Russell, R., Bronstad, P. M., Blokland, G. A., Smoller, J. W., Kwok, H., & Wilmer, J. B. (2015). Individual aesthetic preferences for faces are shaped mostly by environments, not genes. Current Biology, 25(20), 2684–2689.
Gildersleeve, K., Haselton, M. G., & Fales, M. R. (2014). Do women’s mate preferences change across the ovulatory cycle? A meta-analytic review. Psychological Bulletin, 140(5), 1205.
Gray, P. B., Kahlenberg, S. M., Barrett, E. S., Lipson, S. F., & Ellison, P. T. (2002). Marriage and fatherhood are associated with lower testosterone in males. Evolution and Human Behavior, 23, 193–202.
Hicks, T. L., Goodall, S. F., Quattrone, E. M., & Ludon-Rochelle, M. T. (2004). Postpartum sexual functioning and method of delivery: summary of the evidence. Journal of Midwifery and Women's Health, 49, 430–436.
Hipp, L. E., Kane Low, L., & van Anders, S. M. (2012). Exploring women's postpartum sexuality: social, psychological, relational, and birth‐related contextual factors. The Journal of Sexual Medicine, 9(9), 2330–2341.
Johnston, V. S., Hagel, R., Franklin, M., Fink, B., & Grammer, K. (2001). Male facial attractiveness: evidence for hormone-mediated adaptive design. Evolution and Human Behavior, 22, 251–267.
Johnstone, R. A. (1995). Sexual selection, honest advertisement and the handicap principle: reviewing the evidence. Biological Reviews, 70, 1–65.
Jones, B. C., Little, A. C., Boothroyd, L., DeBruine, L. M., Feinberg, D. R., Law Smith, M. J., & Perrett, D. I. (2005a). Commitment to relationships and preferences for femininity and apparent health in faces are strongest on days of the ovulatory cycle when progesterone level is high. Hormones and Behavior, 48, 283–290.
Jones, B. C., Perrett, D. I., Little, A. C., Boothroyd, L., Cornwell, R. E., Feinberg, D. R., & Moore, F. R. (2005b). Menstrual cycle, pregnancy and oral contraceptive use alter attraction to apparent health in faces. Proceedings of the Royal Society B: Biological Sciences, 272, 347–354.
Jones, B. C., DeBruine, L. M., Perrett, D. I., Little, A. C., Feinberg, D. R., & Law Smith, M. J. (2008). Effects of menstrual cycle phase on face preferences. Archives of Sexual Behavior, 37, 78–84.
Kruger, D. J. (2006). Male facial masculinity influences attributions of personality and reproductive strategy. Personal Relationships, 13, 451–463.
Lancaster, J. B., & Kaplan, H. S. (2009). The endocrinology of the human adaptive complex. Endocrinology of Social Relationships, 95–119.
Leeman, L. M., & Rogers, R. G. (2012). Sex after childbirth: postpartum sexual function. Obstetrics & Gynecology, 119(3), 647–655.
Limoncin, E., Ciocca, G., Gravina, G. L., Carosa, E., Mollaioli, D., Cellerino, A., Mennucci, A., Di Sante, S., Lenzi, A., & Jannini, E. A. (2015). Pregnant women's preferences for men's faces differ significantly from nonpregnant women. The Journal of Sexual Medicine, 12(5), 1142–1151.
Little, A. C., Burt, D. M., Penton-Voak, I. S., & Perrett, D. I. (2001). Self-perceived attractiveness influences human female preferences for sexual dimorphism and symmetry in male faces. Proceedings of the Royal Society of London B, 268, 39–44.
Little, A. C., Jones, B. C., Penton-Voak, I. S., Burt, D. M., & Perrett, D. I. (2002). Partnership status and the temporal context of relationships influence human female preferences for sexual dimorphism in male face shape. Proceedings of the Royal Society of London B, 269, 1095–1103.
Little, A. C., Jones, B. C., Waitt, C., Tiddeman, B. P., Feinberg, D. R., Perrett, D. I., Apicella, C. L., & Marlowe, F. W. (2008). Symmetry is related to sexual dimorphism in faces: data across culture and species. PLoS ONE, 3, e2106.
Marcinkowska, U. M., Ellison, P. T., Galbarczyk, A., Milkowska, K., Pawlowski, B., Thune, I., & Jasienska, G. (2016). Lack of support for relation between woman's masculinity preference, estradiol level and mating context. Hormones and Behavior, 78, 1–7.
Mazur, A., & Booth, A. (1998). Testosterone and dominance in men. Behavioral and Brain Sciences, 21(03), 353–363.
Meston, C. M., & Frohlich, P. F. (2001). Update on female sexual function. Current Opinion in Urology, 11, 603–609.
Moore, F. R., Coetzee, V., Contreras-Garduño, J., Debruine, L. M., Kleisner, K., Krams, I., & Schaum, N. (2013). Cross-cultural variation in women's preferences for cues to sex-and stress-hormones in the male face. Biology Letters, 9(3), 20130050.
National Statistics Office (NSO), Department of Health (DOH) [Philippines] and Macro International Inc. (MI). (2009). National Demographic and Health Survey – Preliminary results for 2008. Manila: NSO and MI.
Nelson, R. J. (2011). An introduction to behavioral endocrinology (4th ed.). Sunderland: Sinauer.
Penton-Voak, I. S., & Perrett, D. I. (2000). Female preference for male faces change cyclically: further evidence. Evolution and Human Behavior, 21, 39–48.
Penton-Voak, I. S., Perrett, D. I., Castles, D. L., Kobayashi, T., Burt, D. M., Murray, L. K., & Minamisawa, R. (1999). Menstrual cycle alters face preference. Nature, 399, 741–742.
Perrett, D. I., Lee, K., Penton-Voak, I. S., Rowland, D., Yoshikawa, S., Burt, D. M., Henzi, S. P., Castles, D. L., & Akamatsu, S. (1998). Effects of sexual dimorphism on facial attractiveness. Nature, 394, 884–887.
Puts, D. A. (2005). Mating context and menstrual phase affect women's preferences for male voice pitch. Evolution and Human Behavior, 26, 388–397.
Puts, D. A. (2010). Beauty and the beast: mechanisms of sexual selection in humans. Evolution and Human Behavior, 31(3), 157–175.
Puts, D. (2016). Human sexual selection. Current Opinion in Psychology, 7, 28–32.
Rhodes, G., Chan, J., Zebrowitz, L. A., & Simmons, L. W. (2003). Does sexual dimorphism in human faces signal health? Proceedings of the Royal Society of London B, 270, S93–S95.
Roberts, M. L., Buchanan, K. L., & Evans, M. R. (2004). Testing the immunocompetence handicap hypothesis: a review of the evidence. Animal Behaviour, 68(2), 227–239.
Roney, J. R., & Simmons, Z. L. (2008). Women's estradiol predicts preference for facial cues of men's testosterone. Hormones and Behavior, 53, 14–19.
Roney, J. R., Simmons, Z. L., & Gray, P. B. (2011). Changes in estradiol predict within-women shifts in attraction to facial cues of men's testosterone. Psychoneuroendocrinology, 36(5), 742–749.
Sarrel, P., Dobay, B., & Wilta, B. (1998). Estrogen and estrogen-androgen replacement in postmenopausal women dissatisfied with estrogen-only therapy. Sexual behavior and neuroendocrine responses. Journal of Reproductive Medicine, 43, 847–856.
Sayasneh, A., & Pandeva, I. (2010). Postpartum sexual dysfunction: a literature review of risk factors and role of mode of delivery. British Journal of Medical Practitioners, 3(2), 316.
Scott, I. M., Clark, A. P., Josephson, S. C., Boyette, A. H., Cuthill, I. C., Fried, R. L., & Honey, P. L. (2014). Human preferences for sexually dimorphic faces may be evolutionarily novel. Proceedings of the National Academy of Sciences, 111(40), 14388–14393.
Simon, J., Braunstein, G., Nachtigall, L., Utian, W., Katz, M., Miller, S., & Davis, S. (2005). Testosterone patch increases sexual activity and desire in surgically menopausal women with hypoactive sexual desire disorder. Journal of Clinical Endocrinology and Metabolism, 90, 5226–5233.
Stearns, S. C. (1992). The evolution of life histories. New York: Oxford University Press.
Strassmann, B. I. (1997). The biology of menstruation in Homo sapiens: total lifetime menses, fecundity, and nonsynchrony in a natural-fertility population. Current Anthropology, 38(1), 123–129.
Strassmann, B. I. (1999). Menstrual cycling and breast cancer: an evolutionary perspective. Journal of Women's Health, 8(2), 193–202.
Trivers, R. (1972). Parental investment and sexual selection. Sexual Selection & the Descent of Man, Aldine de Gruyter, New York, pp 136–179.
Weeks-Shackelford, V. A., Easton, J. A., & Stone, E. A. (2007). How having children affects mating psychology. In G. Geher & G. F. Miller (Eds.), Mating intelligence: Sex, relationships and the mind’s reproductive system (pp. 159–170). Mahwah: Lawrence Erlbaum.
Welling, L. L., Jones, B. C., DeBruine, L. M., Conway, C. A., Smith, M. L., Little, A. C., & Al-Dujaili, E. A. (2007). Raised salivary testosterone in women is associated with increased attraction to masculine faces. Hormones and Behavior, 52(2), 156–161.
Woranitat, W., & Taneepanichskul, S. (2007). Sexual function during the postpartum period. Journal of the Medical Association of Thailand, 90, 1744–1748.
Zahavi, A. (1991). On the definition of sexual selection, Fisher's model, and the evolution of waste and of signals in general. Animal Behaviour, 42(3), 501–503.
Acknowledgments
The Wenner-Gren Foundation and UNLV International Programs provided support for the Philippines project. We thank Fred Hadi for help with hormone assays and Elizabeth Brogdon, Carol Franco, Kristen Herlosky and Shelly Volsche for feedback on a previous draft of the manuscript. We also thank two anonymous reviewers for helpful feedback.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Escasa-Dorne, M.J., Manlove, H. & Gray, P.B. Women Express a Preference for Feminized Male Faces after Giving Birth. Adaptive Human Behavior and Physiology 3, 30–42 (2017). https://doi.org/10.1007/s40750-016-0048-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40750-016-0048-6