Abstract
The Mandevilla fragrans leaves extract was evaluated as an environmental-friendly corrosion inhibitor for mild steel in 1 mol L−1 HCl. The inhibitive properties of the extract were tested by gravimetric measurements, polarization curves, linear polarization resistance, and electrochemical impedance spectrometry. The performed experiments showed a good correlation with each other and, using a concentration of 0.800 g L−1, an average anti-corrosion efficiency of 94.1% was reached. The mechanism of inhibition is based on the adsorption of molecules present in the extract on the steel surface generating a layer that protects the surface-to-corrosion process. The surface analysis of the material in the inhibitor presence in aggressive medium using atomic force microscopy sustains the surface protection by the inhibitor adsorption. The study of the adsorption isotherms showed that the Langmuir model is the most adequate one.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Metallic corrosion is the cause of several expenses that directly impact the economy, besides being the cause of accidents and environmental impacts [1]. Mild steel is one of the most widely used metallic materials in industries due to properties such as hardness and ductility, along with having low economic cost. However, this material does not have a good corrosion resistance and several industry operations occur in corrosive media. An example of those is the usage of hydrochloric acid in acid pickling processes [2,3,4]. In this kind of operation, there is a need for surface protection to increase the useful life of the metallic material [5].
One of the most usual protection methods for different types of alloys is the application of inorganic or organic compounds as corrosion inhibitors. These compounds can be classified according to their operation, where the most common are anodic, cathodic, and adsorption inhibitors [6, 7]. In the past, chromates were widely used as inorganic inhibitors, but these compounds are highly toxic, motivating their prohibition [8, 9].
Organic inhibitors generally act by adsorption on the metallic surface [10, 11]. Corrosion inhibitors are heteroatom-containing compounds such as N, S, and O and compounds containing π bonds. The presence of these elements increases the adsorptive properties of the compounds as the electron pairs can be donated to vacant d orbitals from atoms on the material surface [12,13,14,15,16,17]. Organic inhibitors are generally less toxic than inorganic inhibitors, however, these compounds may still be harmful to the environment [10, 18].
The improvement of environmental issues and the application of green chemistry principles induce the search for less toxic and environmentally friendly inhibitors. This type of inhibitor can be an organic compound that has been synthesized using the principles of green chemistry or obtained from the extraction of some natural products [19]. The natural extracts usually do not contain toxic elements, are biodegradable, come from a renewable source and act as a great corrosion inhibitor for metals. These extracts include products from animal [20, 21] or plant origin [22,23,24,25,26,27,28,29,30,31,32,33,34,35,36], and recently, tests with algae extract were reported [37,38,39]. The inhibitory properties were related to the presence of compounds such as alkaloids, flavonoids, or condensed tannins [40,41,42]. All common compounds found in natural extracts have characteristics such as heteroatoms presence and π electrons, as already aforementioned, important for acting as a corrosion inhibitor.
The Mandevilla fragrans belongs to Apocynaceae family which is one of the most studied families with respect to pharmacological products [43, 44]. The Mandevilla genus has several pharmacological applications as snake venom inhibition [45], anti-edematogenic activity [46], and anti-inflammatory activity [47].
Some species in this family have even been reported as possible corrosion inhibitors. The alkaloids extract of Geissospermum leave [48] was tested in HCl 1.0 mol L−1 solution. Using a concentration of 100 mg L−1, a maximum efficiency of 92% was obtained. The extract of Ervatamia coronaria [49] was evaluated in 1.0 mol L−1 HCl and H2SO4 solutions and presented a maximum efficiency of 86.8% and 89.9%, respectively, in a concentration of 50% w/v. Cryptostegia grandiflora [50] leaves methanolic extract was studied as a corrosion inhibitor in H2SO4 1.0 mol L−1 medium and obtained a 87.5% efficiency at a concentration of 500 mg L−1. The analysis of the extract showed the presence of flavonoids and phenolic compounds, and the main components were Myricetin and Rutin. Tabernaemontana divaricate [51] ethanolic extract was tested in HCl 1.0 mol L−1 solution and reached maximum efficiency of 95.2% at 0.500 g L−1.
In this work, we show a new application for Mandevilla fragrans extract. Despite its extract has not yet been reported with any pharmacological or corrosion inhibiting action, the analyses of species of same genus showed the presence of several compounds that can act as corrosion inhibitors. This is a species of easy farming and growth. The extraction method was performed as simply as possible and using an environmental-friendly solvent, in agreement with the green chemistry principles. To analyze the phenolic contents in aqueous extract, thin-layer chromatography was used and quantification of flavonoids and condensed tannins was performed with UV–Vis Spectrophotometric. To evaluate the efficiency of the aqueous extract of Mandevilla fragrans leaves as corrosion inhibitor, gravimetric measurement, potentiodynamic polarization, linear polarization resistance, and electrochemical impedance spectrometry were performed and the metallic surface was analyzed using atomic force microscopy.
2 Experimental
2.1 Materials
The corrosive medium was a HCl 1 mol L−1 solution prepared with analytical grade HCl (37%, Vetec S/A) diluted with ultrapure water.
Samples of A36 mild steel were the materials used in this study. The chemical composition of the steel is presented in Table 1.
The solvents used for the extraction and chromatography were of analytical grade from Vetec S/A. Chromatographic analyses were performed on Merck S/A silica gel 60 F254 plates. Spectrophotometric quantification analysis of tannins and flavonoids was performed on a BIOCHROM LIBRA S50 spectrophotometer.
2.2 Preparation and Characterization of the Mandevilla fragrans Leaves Extract
Mandevilla fragrans samples were collected at the Restinga of Jurubatiba, located in Carapebus, in the northern state of Rio de Janeiro in December 2010. This material was separated into leaves, stems, and flowers and dried at room temperature.
The aqueous extract was obtained from 100 g of Mandevilla fragrans leaves by turbolysis method, using a semi-industrial blender (MetVisa brand—Light Line) at room temperature for five minutes of stirring with 1000 mL of pure water. The procedure was performed twice. After freeze drying of the combined extracts, 4.77 g of a light brown-colored solid was obtained.
Thin-layer chromatography (TLC) was performed for the following classes of metabolites: phenolic acids, flavonoids, tannins, anthraquinones, cardiotonics, coumarins, saponins, and alkaloids. The conditions for the analyses were according to Wagner and Bladt [52] for phytochemical screening. Chemical patterns and markers were used and revealing for each class of metabolite researched.
Tannin levels were determined by the vanillin method, according to Morrison [53]. A calibration curve was obtained with epicatechin at concentrations of 25—250 µg mL−1. The experiments were performed in triplicate.
The quantification of the flavonoid concentration of the sample was performed based on the Brazilian Pharmacopoeia 5th edition [54] with adaptations, by colorimetric method, based on a calibration curve constructed by diluting a standard solution of rutin in the range 1.5—30 μg mL−1. Quantification was performed in triplicate.
2.3 Gravimetric Study
Gravimetric study was performed following ASTM G1-03. The ASTM A36 mild steel samples were grounded sequentially with 400 and 600 grit emery paper followed by water, surfactant, ethanol, and acetone washing. The samples then were dried, weighed, and immersed in solutions containing 1.0 mol L−1 HCl. The samples were in contact with the corrosive medium for 5 h. The tests were repeated in the presence of 0.05, 0.100, 0.300, 0.500, and 0.800 g L−1 of the aqueous extract of Mandevilla fragrans leaves (AEMF) at room temperature. After 5 h, the samples were removed from the corrosive medium and subjected to a chemical pickling with Clark's solution (HCl p.a.; SbCl3; SnCl2), washed with water, surfactant, ethanol, and acetone and dried. Finally, the mild steel mass was measured again. With the initial and final mass values, it was possible to calculate the corrosion rate (CR) values from Eq. 1, where A is the total exposed area (cm2), Δm is the mass variation (g), t is the immersion time (h), d is the steel density (g cm−3), and 8.76 × 104 is the proportionality constant. The efficiency (ɳ) of the inhibitor was obtained from Eq. 2, where CR and CRinh are the corrosion rates in the absence and presence of inhibitor, respectively.
2.4 Electrochemical Tests
The electrochemical measurements were performed using an Autolab PGSTAT128N potentiostat/galvanostat powered by NOVA 2.1 software. An electrochemical cell with three electrodes was employed: mild steel (0.25 cm2) was used as the working electrode, platinum foil as the counter electrode, and an Ag|AgCl (3.0 mol L−1) electrode as the reference electrode. The Open Circuit Potential (OCP) was determined by the immersion of the electrodes in the HCl 3.0 mol L−1 for 3600 s. The electrochemical impedance spectroscopy was carried out in the range of 100 kHz to 10 MHz at the OCP with an amplitude of ± 10 mV. The efficiency (ɳ) of the inhibitor was calculated using Eq. 3, where Rct is the charge transfer resistance.
Linear polarization resistance (Rp) was measured after the variation of ± 10 mV at the OCP. The efficiency (ɳ) of the inhibitor was calculated using Eq. 4, where Rp is the polarization resistance.
Potentiodynamic polarization was performed with a scan rate of 1.0 mV s−1 in the range of ± 300 mV (vs. OCP).
2.5 Surface Analysis
The effect of AEMF on surface of the ASTM A36 mild steel was analyzed by Atomic Force Microscopy (AFM). The images were acquired in three different situations: after grounded with 600 grit emery paper, following an immersion in 1.0 mol L−1 HCl without inhibitor and after been immersed in 1.0 mol L−1 HCl with 0.800 g L−1 of AEMF.
3 Results and Discussion
3.1 Extract Characterization
The classes of metabolites found in the TLC phytochemical analysis of Mandevilla fragrans aqueous extract obtained by the method described in this paper were phenolic acids, flavonoids, hydrolyzable and condensed tannins, anthraquinones, cardiotonics, and saponins (Table 2).
The classes of substances identified in this study are in agreement with those found in the literature for the Apocynaceae family [55, 56].
Plant tannins have been quantified by several types of technics, such as precipitation of metals or proteins and colorimetric methods, the latter being more common. The vanillin test is widely used to quantify proanthocyanidins (condensed tannins) in vegetables, particularly grain [53]. It is based on the reaction of 1% vanillin with phenolic in strongly acidic medium, a colored compound with maximum absorption at 500 nm. Catechin and epicatechin have been widely used as standard due the satisfactory response to this reaction.
The content of condensed tannins for the aqueous extract (0.25 mg mL−1), expressed in milligrams of epicatechin per milliliter of extract, was 0.0229 (± 0.00196, R2 = 0.099026).
The flavonoid content for the aqueous extract (3.0 mg mL−1), expressed in milligram equivalent of rutin, was 0.020399 (± 0.000198, R2 = 0.999439).
It is noteworthy that the metabolite content of plant extracts depends on some factors such as the method and conditions of extraction, extraction solvents, and yield of the plant part. The aqueous extraction at room temperature was performed in order to obtain an extract that solubilizes in the various types of measurements such as the anti-corrosion tests performed in this work. On the other hand, aqueous extraction avoids the high toxicity of organic solvents and waste generated that is one of the biggest challenges of natural bioactive substances extraction.
3.2 Inhibitor Action
The electrochemical measurement known as potentiodynamic polarization (PP) allows to evaluate the drop in the cathodic and the anodic currents in the presence of the corrosion inhibitor as well as the change in the corrosion potential. Figure 1 shows AEMF concentration dependence of cathodic and anodic currents in the polarization curves.
A considerable drop in both currents (cathodic and anodic) which is more pronounced as AEMF concentration increases can be seen in the polarization plots, indicating that those reactions are suppressed by the corrosion inhibitor [57, 58]. The corrosion potential (Ecorr) varies less than ± 35 mV in the presence of AEMF related to blank solution (Table 3). Variations of the corrosion potential (Ecorr) less than ± 85 mV are associated to mixed-type corrosion inhibitors [59,60,61,62].
3.3 Weight Loss Study
In agreement with the rules from the American Society for Testing Materials (ASTM G1-03), gravimetric measurements were performed on mild steel specimens to evaluate the anticorrosive activity from the aqueous extract of Mandevilla fragrans leaves (AEMF) at 303 K. The results are displayed in Table 4.
The results revealed that the presence of the organic molecules reduces significantly the corrosion rate, even at lower concentrations (0.050 g L−1 – 64.0%), reaching a maximum anticorrosive efficiency of 94.8% at 0.800 g L−1. It is well discussed and reported that organic molecules act as corrosion inhibitor by adsorbing on the metal surface and forming a protective film that prevents the contact with the electrolyte [12,13,14]. Hence, the inhibitor efficiency (η) can be associated with the metallic surface coverage (θ). It can be observed in Table 4 that the raise in AEMF concentration increases the value of θ, meaning more molecules are adsorbed on the mild steel surface, protecting it against corrosion [63, 64].
To corroborate this hypothesis, the surface of the mild steel was analyzed by Atomic Force Microscopy (AFM) and various isotherms were simulated to understand how the organic molecules present in the AEMF interact with mild steel surface.
3.4 Atomic Force Microscopy (AFM)
Atomic Force Microscopy (AFM) was performed to evaluate the mild steel surface and the average surface roughness in three different scenarios (after polishing, after an immersion in 1.0 mol L−1 HCl without inhibitor and with 0.800 g L−1 of AEMF). Figure 2 shows the 2D and 3D images of the steel surface and Table 5 presents the average roughness of the steel surface in the three different scenarios.
In Fig. 2a, the metal surface after polishing presents the marks from the SiC paper and the lowest average roughness. Different from Fig. 2a, Fig. 2b displays a very damaged surface after the corrosive attack in the absence of the organic molecules and a large increase in average roughness was observed. When exposure to the corrosive medium occurs in the presence of the inhibitor (Fig. 2c), the surface of mild steel has a smoother appearance, clearly less attacked by the corrosive medium and with reduced average roughness, being much closer to the polished surface. This result points to AEMF components acting by forming a compact film that protects the mild steel surface from corrosion [21, 50, 65, 66].
3.5 Adsorption Isotherm
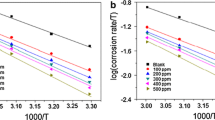
The adsorption of the AEMF organic molecules on the metal surface was fitted using ten different isotherms (Kiselev, Langmuir, Frumkin, Elovich, Hill-de Boer, Temkin, Dubinin–Radushkevich, Freundlich, Flory–Huggins, and Fowler–Guggenheim). As shown in Fig. 3, our data fitted best the Langmuir theory (mathematically defined by Eq. 5; R2 = 0.9994), so AEMF adsorbs as a monolayer and the organic molecules do not interact with neighboring molecules [67, 68].
where Kads is the adsorption constant, Cinh is the inhibitor concentration.
The inverse of the y-intercept provides the value of Kads. For the aqueous extract of Mandevilla fragrans, the adsorption constant is 31.35 L g−1, a high value for natural extracts, showing that the equilibrium is dislocated towards the adsorption of the inhibitor on the mild steel surface [69].
There are two possible mechanisms for the adsorption of the inhibitor compounds on the mild steel surface. The first one is the chemisorption and this type of adsorption occurs when a lone electron pair of heteroatoms such as N, O, and S and π electrons present in the phytochemicals of the AEMF interact with vacant d orbitals from iron atoms in the mild steel surface. The second is the physisorption. In acid media, some AEMF molecules can be protonated and interact electrostatically with chloride ions on mild steel surface. Physisorption is a weaker form of adsorption than chemisorption, thus the nature of adsorption can be analyzed with ΔGads values [19, 20].
Our system is formed by several organic molecules as seen in Table 2. Due to this complex system it is not possible to calculate the molecular mass. So, as shown in many studies, when discussing a natural extract, it is not possible to obtain thermodynamic adsorption parameters (ΔGads, ΔHads, ΔSads) because the molecular mass is unknown.[20, 70, 71]. However, precisely because the AEMF is formed of several organic molecules, is plausible admit that both mechanisms of inhibition, chemisorption and physisorption, are occurring on mild steel surface in our system, where some molecules will donate their lone electron pair and other protonated molecules will interact electrostatically with the chloride ions on the steel surface.
3.6 Linear Polarization Resistance (LPR)
Varying the potential (E) ± 10 mV (at OCP) and recording the respective response in current density (j) allow to calculate the polarization resistance (Rp), according to Eq. 6.
From the data displayed in Table 6, it can be observed a higher value of Rp as more concentration of AEMF is added to the acid medium, indicating a better protection of the metallic surface. The corrosion inhibition efficiency values corroborated the obtained via gravimetric experiments, reaching a maximum of 93.0% at 0.800 g L−1 [19, 72].
3.7 Electrochemical Impedance Spectroscopy (EIS)
To understand more about the mechanism of protection and the anticorrosive efficiency of AEMF, Electrochemical Impedance Spectroscopy was performed. Figure 4 shows the Nyquist plot of different concentrations of the organic extract.
The depressed semi-circles observed in the graph occurs due to the inhomogeneity of the mild steel surface [19, 73, 74]. It is well reported that the diameters of the semi-circles in the Nyquist plots are associated with the charge transfer resistance of the material tested [75, 76]. It can be seen in Fig. 4 that the increase in AEMF concentration leads to an enlargement of the diameters, pointing to a better protection of the metallic surface. The single loop format indicates that the corrosion process is mainly associated with charge transfer [77,78,79].
To simulate the phenomena occurring in the interface metal/solution, an equivalent electric circuit (Fig. 5) was used composed of a solution resistance (Rs) in series with a constant phase element (CPE, defined by Y0 and n) in parallel with a charge transfer resistance (Rct) [30, 80, 81]. A pure capacitor was not used due to surface roughness and inhomogeneity [81]. Table 7 displays the Impedance data.
To calculate the double-layer capacitance (Cdl) values from CPE, the formulas listed below (Eq. 7) were used [28]. Associating these values (Table 7) to the Helmholtz equation (Eq. 8), it is possible to explain the drop on Cdl values as the concentration of AEMF increases due the adsorption of more organic molecules on the mild steel surface substituting water molecules. This fact leads to a lower value of local dielectric constant and surface exposed to the aggressive medium, at the same time that generates a higher thickness value of the protective film formed, all contributing to a lower value of double-layer capacitance [82, 83].
As shown in the aforementioned experimental results, the aqueous extract of Mandevilla fragrans leaves achieves a good corrosion inhibition efficiency at low concentration (0.050 g L−1 – 70.3%) and reaches a maximum of 94.0% at 0.800 g L−1, an excellent result.
3.8 Comparison of Techniques and with Other Extracts
In this study, many techniques were applied to analyze the corrosion inhibitory capacity of the AEMF. With the first one, Weight Loss Study, the corrosion rate was calculated, and consequently the anticorrosive efficiency, with high accuracy due to the long duration of the experiment. However, is not possible to obtain information about how the inhibitor acts [84, 85].
Different from that, in the electrochemical experiments, a lot of information from the interface mild steel/solution can be accessed as well as the mechanism of action of the corrosion inhibitor. But the electrochemical analyses are more susceptible to experimental conditions and have to be performed carefully.
Therefore, come the importance of the comparison between all those measurements to see a corroboration between them.
Figure 6 shows the correlation among all the techniques used to evaluate AEMF as a corrosion inhibitor for mild steel in acid medium. It can be observed an excellent concordance between Weight Loss Study and the electrochemical techniques, Linear Polarization Resistance and Electrochemical Impedance Spectroscopy. This result validates the entire discussion in this study on the behavior of the AEMF as a corrosion inhibitor for mild steel.
Another important comparison to be made is with other extracts already reported in the literature as corrosion inhibitor for mild steel in 1.0 mol L−1 HCl, both from the point of view of anti-corrosion efficiency and also in the form of extraction procedure (easier and environmentally friendly). Table 8 presents these data.
In this scenario, the AEMF presented an excellent efficiency with a reasonable concentration, having the characteristics necessary for a great corrosion inhibitor.
When AEMF is compared to other crude extracts from the same family, Cryptostegia grandiflora and Tabernaemontana divaricate [50, 51], a similar performance is observed regarding the anticorrosive efficiency. However, from an environmental point of view, the AEMF has the advantage to be an aqueous extract which makes it even more sustainable and environmentally friendly, hence, a better option as a green corrosion inhibitor.
4 Conclusions
The aqueous extract of Mandevilla fragrans acts as a great corrosion inhibitor for mild steel in acid medium (1.0 mol L−1 HCl). Weight Loss Study, Electrochemical Impedance Spectroscopy, and Linear Polarization Resistance show an average corrosion inhibition efficiency of 94.1% at 0.800 g L−1 of AEMF. The adsorption process obeys the Langmuir theory of a protective monolayer. Potentiodynamic Polarization reveals that the inhibitor acts as mixed-type and Atomic Force Microscopy images suggest the formation of a protective film by the organic molecules.
References
Kutz M (2005) Handbook of environmental degradation of materials, 2a edN. William Andrew. https://doi.org/10.1016/C2010-0-66227-4
Roberge PR (2012) Handbook of corrosion engineering, 2a edn. McGraw-Hill Education.
Zhang QH, Hou BS, Li YY, Zhu GY, Liu HF, Zhang GA (2020) Two novel chitosan derivatives as high efficient eco-friendly inhibitors for the corrosion of mild steel in acidic solution. Corros Sci 164:108346. https://doi.org/10.1016/j.corsci.2019.108346
Abdeli M, Ahmadi NP, Khosroshahi RA (2009) Nile Blue and Indigo Carmine organic dyes as corrosion inhibitor of mild steel in hydrochloric acid. J Solid State Electrochem 14(7):1317–1324. https://doi.org/10.1007/s10008-009-0925-z
Finšgar M, Jackson J (2014) Application of corrosion inhibitors for steels in acidic media for the oil and gas industry: a review. Corros Sci 86:17–41. https://doi.org/10.1016/j.corsci.2014.04.044
Sayin K, Karakaş D (2013) Quantum chemical studies on the some inorganic corrosion inhibitors. Corros Sci 77:37–45. https://doi.org/10.1016/j.corsci.2013.07.023
Rani BEA, Basu BBJ (2012) Green inhibitors for corrosion protection of metals and alloys: an overview. Int J Corros 2012:1–15. https://doi.org/10.1155/2012/380217
Fernandez M, Breedon M, Cole IS, Barnard AS (2016) Modeling corrosion inhibition efficacy of small organic molecules as non-toxic chromate alternatives using comparative molecular surface analysis (CoMSA). Chemosphere 160:80–88. https://doi.org/10.1016/j.chemosphere.2016.06.044
Winkler DA, Breedon M, White P, Hughes AE, Sapper ED, Cole I (2016) Using high throughput experimental data and in silico models to discover alternatives to toxic chromate corrosion inhibitors. Corros Sci 106:229–235. https://doi.org/10.1016/j.corsci.2016.02.008
Umoren SA, Eduok UM (2016) Application of carbohydrate polymers as corrosion inhibitors for metal substrates in different media: a review. Carbohydr Polym 140:314–341. https://doi.org/10.1016/j.carbpol.2015.12.038
Hu K, Zhuang J, Zheng C, Ma Z, Yan L, Gu H, Zeng X, Ding J (2016) Effect of novel cytosine-l-alanine derivative based corrosion inhibitor on steel surface in acidic solution. J Mol Liq 222:109–117. https://doi.org/10.1016/j.molliq.2016.07.008
Vengatesh G, Sundaravadivelu M (2019) Non-toxic bisacodyl as an effective corrosion inhibitor for mild steel in 1 M HCl: thermodynamic, electrochemical, SEM, EDX, AFM, FT-IR, DFT and molecular dynamics simulation studies. J Mol Liq 287:110906. https://doi.org/10.1016/j.molliq.2019.110906
Obot IB, Onyeachu IB, Wazzan N, Al-Amri AH (2019) Theoretical and experimental investigation of two alkyl carboxylates as corrosion inhibitors for steel in acidic medium. J Mol Liq 279:190–207. https://doi.org/10.1016/j.molliq.2019.01.116
Gadow HS, Farghaly TA, Eldesoky AM (2019) Experimental and theoretical investigations for some spiropyrazoles derivatives as corrosion inhibitors for copper in 2 M HNO3 solutions. J Mol Liq. https://doi.org/10.1016/j.molliq.2019.111614
Babić-Samardžija K, Hackerman N (2005) Triazole, benzotriazole and substituted benzotriazoles as corrosion inhibitors of iron in aerated acidic media. J Solid State Electrochem 9(7):483–497. https://doi.org/10.1007/s10008-004-0584-z
Ahamad I, Prasad R, Quraishi MA (2010) Experimental and theoretical investigations of adsorption of fexofenadine at mild steel/hydrochloric acid interface as corrosion inhibitor. J Solid State Electrochem 14(11):2095–2105. https://doi.org/10.1007/s10008-010-1041-9
Abdel Hameed RS, Al-Bagawi AH, Shehata HA, Shamroukh AH, Abdallah M (2020) Corrosion inhibition and adsorption properties of some heterocyclic derivatives on C-steel surface in HCl. J Bio Tribo Corros. https://doi.org/10.1007/s40735-020-00345-y
Loto RT, Loto CA (2019) Data on the corrosion inhibition effect of non-toxic organic derivatives on high carbon steel in dilute acid media. Chem Data Collect. https://doi.org/10.1016/j.cdc.2019.100214
Fernandes CM, Alvarez LX, dos Santos NE, Maldonado Barrios AC, Ponzio EA (2019) Green synthesis of 1-benzyl-4-phenyl-1H-1,2,3-triazole, its application as corrosion inhibitor for mild steel in acidic medium and new approach of classical electrochemical analyses. Corros Sci 149:185–194. https://doi.org/10.1016/j.corsci.2019.01.019
Fernandes CM, Ferreira Fagundes TdS, Escarpini dos Santos N, de Shewry M, Rocha T, Garrett R, Borges RM, Muricy G, Valverde AL, Ponzio EA (2019) Ircinia strobilina crude extract as corrosion inhibitor for mild steel in acid medium. Electrochim Acta 312:137–148. https://doi.org/10.1016/j.electacta.2019.04.148
Bidi MA, Azadi M, Rassouli M (2020) A new green inhibitor for lowering the corrosion rate of carbon steel in 1 M HCl solution: Hyalomma tick extract. Mater Today Commun. https://doi.org/10.1016/j.mtcomm.2020.100996
Wang Q, Tan B, Bao H, Xie Y, Mou Y, Li P, Chen D, Shi Y, Li X, Yang W (2019) Evaluation of Ficus tikoua leaves extract as an eco-friendly corrosion inhibitor for carbon steel in HCl media. Bioelectrochemistry 128:49–55. https://doi.org/10.1016/j.bioelechem.2019.03.001
Singh A, Ahamad I, Singh VK, Quraishi MA (2010) Inhibition effect of environmentally benign Karanj (Pongamia pinnata) seed extract on corrosion of mild steel in hydrochloric acid solution. J Solid State Electrochem 15(6):1087–1097. https://doi.org/10.1007/s10008-010-1172-z
Sanaei Z, Ramezanzadeh M, Bahlakeh G, Ramezanzadeh B (2019) Use of Rosa canina fruit extract as a green corrosion inhibitor for mild steel in 1 M HCl solution: a complementary experimental, molecular dynamics and quantum mechanics investigation. J Ind Eng Chem 69:18–31. https://doi.org/10.1016/j.jiec.2018.09.013
He T, Emori W, Zhang RH, Okafor PC, Yang M, Cheng CR (2019) Detailed characterization of Phellodendron chinense Schneid and its application in the corrosion inhibition of carbon steel in acidic media. Bioelectrochemistry 130:107332. https://doi.org/10.1016/j.bioelechem.2019.107332
Dehghani A, Bahlakeh G, Ramezanzadeh B (2019) Green Eucalyptus leaf extract: a potent source of bio-active corrosion inhibitors for mild steel. Bioelectrochemistry 130:107339. https://doi.org/10.1016/j.bioelechem.2019.107339
Srivastava M, Tiwari P, Srivastava SK, Kumar A, Ji G, Prakash R (2018) Low cost aqueous extract of Pisum sativum peels for inhibition of mild steel corrosion. J Mol Liq 254:357–368. https://doi.org/10.1016/j.molliq.2018.01.137
Alvarez PE, Fiori-Bimbi MV, Neske A, Brandán SA, Gervasi CA (2018) Rollinia occidentalis extract as green corrosion inhibitor for carbon steel in HCl solution. J Ind Eng Chem 58:92–99. https://doi.org/10.1016/j.jiec.2017.09.012
Asadi N, Ramezanzadeh M, Bahlakeh G, Ramezanzadeh B (2019) Utilizing Lemon Balm extract as an effective green corrosion inhibitor for mild steel in 1M HCl solution: a detailed experimental, molecular dynamics, Monte Carlo and quantum mechanics study. J Taiwan Inst Chem Eng 95:252–272. https://doi.org/10.1016/j.jtice.2018.07.011
Mourya P, Banerjee S, Singh MM (2014) Corrosion inhibition of mild steel in acidic solution by Tagetes erecta (Marigold flower) extract as a green inhibitor. Corros Sci 85:352–363. https://doi.org/10.1016/j.corsci.2014.04.036
Chellouli M, Chebabe D, Dermaj A, Erramli H, Bettach N, Hajjaji N, Casaletto MP, Cirrincione C, Privitera A, Srhiri A (2016) Corrosion inhibition of iron in acidic solution by a green formulation derived from Nigella sativa L. Electrochim Acta 204:50–59. https://doi.org/10.1016/j.electacta.2016.04.015
Qiang Y, Zhang S, Tan B, Chen S (2018) Evaluation of Ginkgo leaf extract as an eco-friendly corrosion inhibitor of X70 steel in HCl solution. Corros Sci 133:6–16. https://doi.org/10.1016/j.corsci.2018.01.008
de Oliveira RR, Battistin A, Gonçalves RS (2011) Alcoholic Mentha extracts as inhibitors of low-carbon steel corrosion in aqueous medium. J Solid State Electrochem 16(2):747–752. https://doi.org/10.1007/s10008-011-1422-8
Ayoola AA, Fayomi OSI, Akande IG, Ayeni OA, Agboola O, Obanla OR, Abatan OG, Chukwuka CJ (2020) Inhibitive corrosion performance of the eco-friendly aloe vera in acidic media of mild and stainless steels. J Bio Tribo Corros. https://doi.org/10.1007/s40735-020-00361-y
Ragul R, Ravichandran J, Murugesh A (2020) Adsorption and inhibitive action of Mitracarpus hirtus extract towards the corrosion of mild steel in H2SO4 medium. J Bio Tribo Corros. https://doi.org/10.1007/s40735-020-00360-z
Saxena A, Kumar J (2020) Phytochemical screening, metal-binding studies and applications of floral extract of Sonchus oleraceus as a corrosion inhibitor. J Bio Tribo Corros. https://doi.org/10.1007/s40735-020-00349-8
Abboud Y, Abourriche A, Ainane T, Charrouf M, Bennamara A, Tanane O, Hammouti B (2009) Corrosion inhibition of carbon steel in acidic media Bybifurcaria Bifurcataextract. Chem Eng Commun 196(7):788–800. https://doi.org/10.1080/00986440802589875
Verma DK, Khan F (2016) Green approach to corrosion inhibition of mild steel in hydrochloric acid medium using extract of spirogyra algae. Green Chem Lett Rev 9(1):52–60. https://doi.org/10.1080/17518253.2015.1137976
de Souza GPG, de Sampaio TGM, Furtado BA, Buzzetti HMP, Ramos JBC, Teixeira LV, Velasco AC, Damasceno NR, Ponzio AE (2019) Study of the efficiency of the algae Prasiola crispa extract as a corrosion inhibitor in HCl 1 mol L-1. Rev Virtual Química 11(5):1521–1539. https://doi.org/10.21577/1984-6835.20190106
Rahim AA, Rocca E, Steinmetz J, Kassim MJ, Adnan R, Sani Ibrahim M (2007) Mangrove tannins and their flavanoid monomers as alternative steel corrosion inhibitors in acidic medium. Corros Sci 49(2):402–417. https://doi.org/10.1016/j.corsci.2006.04.013
Lebrini M, Robert F, Lecante A, Roos C (2011) Corrosion inhibition of C38 steel in 1M hydrochloric acid medium by alkaloids extract from Oxandra asbeckii plant. Corros Sci 53(2):687–695. https://doi.org/10.1016/j.corsci.2010.10.006
M’hiri N, Veys-Renaux D, Rocca E, Ioannou I, Boudhrioua NM, Ghoul M (2016) Corrosion inhibition of carbon steel in acidic medium by orange peel extract and its main antioxidant compounds. Corros Sci 102:55–62. https://doi.org/10.1016/j.corsci.2015.09.017
Wen S, Chen Y, Lu Y, Wang Y, Ding L, Jiang M (2016) Cardenolides from the Apocynaceae family and their anticancer activity. Fitoterapia 112:74–84. https://doi.org/10.1016/j.fitote.2016.04.023
Chan EWC, Wong SK, Chan HT (2016) Apocynaceae species with antiproliferative and/or antiplasmodial properties: a review of ten genera. J Integr Med 14(4):269–284. https://doi.org/10.1016/s2095-4964(16)60261-3
Biondo R, Pereira AMS, Marcussi S, Pereira PS, França SC, Soares AM (2003) Inhibition of enzymatic and pharmacological activities of some snake venoms and toxins by Mandevilla velutina (Apocynaceae) aqueous extract. Biochimie 85(10):1017–1025. https://doi.org/10.1016/s0300-9084(03)00138-x
Mattos WM, Campos MM, Fernandes ES, Richetti GP, Niero R, Yunes RA, Calixto JB (2006) Anti-edematogenic effects of velutinol A isolated from Mandevilla velutina: evidence for a selective inhibition of kinin B1 receptor-mediated responses. Regul Pept 136(1–3):98–104. https://doi.org/10.1016/j.regpep.2006.04.011
de Almeida DA, Rosa SI, da Cruz TC, Pavan E, Sabino Damazo A, Soares IM, Ascencio SD, Macho A, Martins DT (2017) Mandevilla longiflora (Desf.) Pichon improves airway inflammation in a murine model of allergic asthma. J Ethnopharmacol 200:51–59. https://doi.org/10.1016/j.jep.2017.02.015
Faustin M, Maciuk A, Salvin P, Roos C, Lebrini M (2015) Corrosion inhibition of C38 steel by alkaloids extract of Geissospermum laeve in 1M hydrochloric acid: electrochemical and phytochemical studies. Corros Sci 92:287–300. https://doi.org/10.1016/j.corsci.2014.12.005
Sethuraman MG, Aishwarya V, Kamal C, Jebakumar Immanuel Edison T (2017) Studies on Ervatinine—the anticorrosive phytoconstituent of Ervatamia coronaria. Arab J Chem 10:S522–S530. https://doi.org/10.1016/j.arabjc.2012.10.013
Prabakaran M, Kim S-H, Hemapriya V, Chung I-M (2016) Evaluation of polyphenol composition and anti-corrosion properties of Cryptostegia grandiflora plant extract on mild steel in acidic medium. J Ind Eng Chem 37:47–56. https://doi.org/10.1016/j.jiec.2016.03.006
Rose K, Kim B-S, Rajagopal K, Arumugam S, Devarayan K (2016) Surface protection of steel in acid medium by Tabernaemontana divaricata extract: physicochemical evidence for adsorption of inhibitor. J Mol Liq 214:111–116. https://doi.org/10.1016/j.molliq.2015.12.008
Wagner H, Bladt S (1996) Plant drug analysis. A thing layer chromatography atlas, 2nd edn. Springer, New York
Morrison I (1995) Determination of lignin and tannin contents of cowpea seed coats. Ann Bot 76(3):287–290. https://doi.org/10.1006/anbo.1995.1097
Brasil. Farmacopeia Brasileira, volume 1 / Agência Nacional de Vigilância Sanitária. Brasília: Anvisa, 2010.548p., 1v/il, 5th edition.
Vieira IJ, Medeiros WL, Monnerat CS, Souza JJ, Mathias L, Braz-Filho R, Pinto AC, Sousa PM, Rezende CM, Epifanio Rde A (2008) Two fast screening methods (GC-MS and TLC-ChEI assay) for rapid evaluation of potential anticholinesterasic indole alkaloids in complex mixtures. An Acad Bras Cienc 80(3):419–426. https://doi.org/10.1590/s0001-37652008000300003
Moreno Rodríguez A, Robles Camargo J, Bello García FJ (2008) Actividad in vitro de la mezcla de alcaloides de Ervatamia coronaria (Jacq) Staff. Apocynaceae sobre amastigotes de Leishmania braziliensis. Rev Bras Farmacog 18:350–355. https://doi.org/10.1590/S0102-695X2008000300007
Meng Y, Ning W, Xu B, Yang W, Zhang K, Chen Y, Li L, Liu X, Zheng J, Zhang Y (2017) Inhibition of mild steel corrosion in hydrochloric acid using two novel pyridine Schiff base derivatives: a comparative study of experimental and theoretical results. RSC Adv 7(68):43014–43029. https://doi.org/10.1039/c7ra08170g
Li X, Deng S, Xie X (2017) Inhibition effect of tetradecylpyridinium bromide on the corrosion of cold rolled steel in 7.0 M H3PO4. Arab J Chem 10:S3715–S3724. https://doi.org/10.1016/j.arabjc.2014.05.004
Zhang Z, Tian N, Zhang L, Wu L (2015) Inhibition of the corrosion of carbon steel in HCl solution by methionine and its derivatives. Corros Sci 98:438–449. https://doi.org/10.1016/j.corsci.2015.05.048
Ansari KR, Quraishi MA, Singh A (2014) Schiff’s base of pyridyl substituted triazoles as new and effective corrosion inhibitors for mild steel in hydrochloric acid solution. Corros Sci 79:5–15. https://doi.org/10.1016/j.corsci.2013.10.009
Zhang K, Xu B, Yang W, Yin X, Liu Y, Chen Y (2015) Halogen-substituted imidazoline derivatives as corrosion inhibitors for mild steel in hydrochloric acid solution. Corros Sci 90:284–295. https://doi.org/10.1016/j.corsci.2014.10.032
Liao LL, Mo S, Lei JL, Luo HQ, Li NB (2016) Application of a cosmetic additive as an eco-friendly inhibitor for mild steel corrosion in HCl solution. J Colloid Interface Sci 474:68–77. https://doi.org/10.1016/j.jcis.2016.04.015
Boudjellal F, Ouici HB, Guendouzi A, Benali O, Sehmi A (2020) Experimental and theoretical approach to the corrosion inhibition of mild steel in acid medium by a newly synthesized pyrazole carbothioamide heterocycle. J Mol Struct. https://doi.org/10.1016/j.molstruc.2019.127051
Luo X, Ci C, Li J, Lin K, Du S, Zhang H, Li X, Cheng YF, Zang J, Liu Y (2019) 4-aminoazobenzene modified natural glucomannan as a green eco-friendly inhibitor for the mild steel in 0.5 M HCl solution. Corros Sci 151:132–142. https://doi.org/10.1016/j.corsci.2019.02.027
Umoren SA, Solomon MM, Ali SA, Dafalla HDM (2019) Synthesis, characterization, and utilization of a diallylmethylamine-based cyclopolymer for corrosion mitigation in simulated acidizing environment. Mater Sci Eng, C 100:897–914. https://doi.org/10.1016/j.msec.2019.03.057
Tan B, Zhang S, Liu H, Guo Y, Qiang Y, Li W, Guo L, Xu C, Chen S (2019) Corrosion inhibition of X65 steel in sulfuric acid by two food flavorants 2-isobutylthiazole and 1-(1,3-Thiazol-2-yl) ethanone as the green environmental corrosion inhibitors: combination of experimental and theoretical researches. J Colloid Interface Sci 538:519–529. https://doi.org/10.1016/j.jcis.2018.12.020
Tan B, Zhang S, Li W, Zuo X, Qiang Y, Xu L, Hao J, Chen S (2019) Experimental and theoretical studies on inhibition performance of Cu corrosion in 0.5 M H2SO4 by three disulfide derivatives. J Ind Eng Chem 77:449–460. https://doi.org/10.1016/j.jiec.2019.05.011
Fernandes CM, de Mello MVP, dos Santos NE, de Souza AMT, Lanznaster M, Ponzio EA (2020) Theoretical and experimental studies of a new aniline derivative corrosion inhibitor for mild steel in acid medium. Mater Corros 71:280–291. https://doi.org/10.1002/maco.201911065
Macedo R, Marques NDN, Tonholo J, Balaban RC (2019) Water-soluble carboxymethylchitosan used as corrosion inhibitor for carbon steel in saline medium. Carbohydr Polym 205:371–376. https://doi.org/10.1016/j.carbpol.2018.10.081
Liao LL, Mo S, Luo HQ, Li NB (2017) Longan seed and peel as environmentally friendly corrosion inhibitor for mild steel in acid solution: experimental and theoretical studies. J Colloid Interface Sci 499:110–119. https://doi.org/10.1016/j.jcis.2017.03.091
Oguzie EE (2008) Evaluation of the inhibitive effect of some plant extracts on the acid corrosion of mild steel. Corros Sci 50(11):2993–2998. https://doi.org/10.1016/j.corsci.2008.08.004
Carlos MFLP, Valbon A, Neves MA, Santos MRL, Echevarria A (2018) Enaminoesters as novel corrosion inhibitors for carbon steel in acidic medium. J Braz Chem Soc 29:2542–2553
El-Lateef HMA, Abdallah ZA, Ahmed MSM (2019) Solvent-free synthesis and corrosion inhibition performance of Ethyl 2-(1,2,3,6-tetrahydro-6-oxo-2-thioxopyrimidin-4-yl)ethanoate on carbon steel in pickling acids: experimental, quantum chemical and Monte Carlo simulation studies. J Mol Liq 296:111800. https://doi.org/10.1016/j.molliq.2019.111800
Özcan M (2008) AC impedance measurement of cystine adsorption at mild steel/sulfuric acid interface as corrosion inhibitor. J Solid State Electrochem 12(12):1653–1661. https://doi.org/10.1007/s10008-008-0551-1
El-Hajjaji F, Messali M, Martinez de Yuso MV, Rodriguez-Castellon E, Almutairi S, Bandosz TJ, Algarra M (2019) Effect of 1-(3-phenoxypropyl) pyridazin-1-ium bromide on steel corrosion inhibition in acidic medium. J Colloid Interface Sci 541:418–424. https://doi.org/10.1016/j.jcis.2019.01.113
El Defrawy AM, Abdallah M, Al-Fahemi JH (2019) Electrochemical and theoretical investigation for some pyrazolone derivatives as inhibitors for the corrosion of C-steel in 0.5 M hydrochloric acid. J Mol Liq. https://doi.org/10.1016/j.molliq.2019.110994
El Faydy M, Galai M, Touhami ME, Obot IB, Lakhrissi B, Zarrouk A (2017) Anticorrosion potential of some 5-amino-8-hydroxyquinolines derivatives on carbon steel in hydrochloric acid solution: gravimetric, electrochemical, surface morphological, UV–visible, DFT and Monte Carlo simulations. J Mol Liq 248:1014–1027. https://doi.org/10.1016/j.molliq.2017.10.125
Xavier JR, Nallaiyan R (2011) Corrosion inhibitive properties and electrochemical adsorption behaviour of some piperidine derivatives on brass in natural sea water. J Solid State Electrochem 16(1):391–402. https://doi.org/10.1007/s10008-011-1330-y
Fernandes CM, Pina VGSS, Alvarez LX, de Albuquerque ACF, dos Santos Júnior FM, Barrios AM, Velasco JAC, Ponzio EA (2020) Use of a theoretical prediction method and quantum chemical calculations for the design, synthesis and experimental evaluation of three green corrosion inhibitors for mild steel. Colloids Surf A 599:124857–124871. https://doi.org/10.1016/j.colsurfa.2020.124857
Sadeghi Erami R, Amirnasr M, Meghdadi S, Talebian M, Farrokhpour H, Raeissi K (2019) Carboxamide derivatives as new corrosion inhibitors for mild steel protection in hydrochloric acid solution. Corros Sci 151:190–197. https://doi.org/10.1016/j.corsci.2019.02.019
Ma Q, Qi S, He X, Tang Y, Lu G (2017) 1,2,3-Triazole derivatives as corrosion inhibitors for mild steel in acidic medium: experimental and computational chemistry studies. Corros Sci 129:91–101. https://doi.org/10.1016/j.corsci.2017.09.025
Jiang L, Qiang Y, Lei Z, Wang J, Qin Z, Xiang B (2018) Excellent corrosion inhibition performance of novel quinoline derivatives on mild steel in HCl media: experimental and computational investigations. J Mol Liq 255:53–63. https://doi.org/10.1016/j.molliq.2018.01.133
Hassannejad H, Nouri A (2018) Sunflower seed hull extract as a novel green corrosion inhibitor for mild steel in HCl solution. J Mol Liq 254:377–382. https://doi.org/10.1016/j.molliq.2018.01.142
Poorqasemi E, Haqdar F, Abootalebi O (2009) A study on the accuracy of the Tafel extrapolation method in HCl. ECS Trans 19:13. https://doi.org/10.1149/1.3259809
Danaee I, RameshKumar S, RashvandAvei M, Vijayan M (2020) Electrochemical and quantum chemical studies on corrosion inhibition performance of 2,2’-(2-hydroxyethylimino)bis[N-(alphaalpha-dimethylphenethyl)-N-methylacetamide] on mild steel corrosion in 1M HCl solution. Mater Res. https://doi.org/10.1590/1980-5373-mr-2018-0610
Acknowledgements
Marcelo Tadeu Gomes de Sampaio and Caio Machado Fernandes thank Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for the scholarship received and Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ, E-26/102.971/2012, E-26/111.407/2013, and 26/202.790/2015) for all their financial support. The authors acknowledge the Brazilian research agencies (CAPES, FAPERJ, and CNPq) for financial support. This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
de Sampaio, M.T.G., Fernandes, C.M., de Souza, G.G.P. et al. Evaluation of Aqueous Extract of Mandevilla fragrans Leaves as Environmental-Friendly Corrosion Inhibitor for Mild Steel in Acid Medium. J Bio Tribo Corros 7, 14 (2021). https://doi.org/10.1007/s40735-020-00445-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40735-020-00445-9