Abstract
The effect of fluoride ions was investigated on the austenitic stainless steel in a typical solution of phosphoric acid that is used in industrial process, for clear understanding the damage that caused by corrosion to special alloying material Uranus B6 at temperature of 80 °C. For that the electrochemical and spectroscopy methods were used like potentiodynamic and electrochemical impedance spectroscopy, X-ray diffraction and UV–visible–IR reflection, Scanning electron microscopy and energy-dispersive spectroscopy analysis (SEM–EDX). The electrochemical methods show the drastic effect of fluoride on the passivation parameters, especially icrit, ipass and Epit caused by decreasing the alloy elements in the surface alloy, specially the molybdenum. Moreover, the spectroscopy results reflect that fluoride has two axes of interaction with the surface alloy; chemical interaction that was appeared by the dissolution mechanism, which it increases the dissolution of the base alloy atom and the alloying elements and this result was improved by the UV–visible–IR and EDX analysis. And the physical effect was demonstrated by X-ray diffraction, which is showing a deformation of the crystalline structure of surface alloy; and different pit types were detected by SEM. And the suggestion of the effect of fluoride on the nickel percent in the passive film and also on the molybdenum and copper has been proposed as a mechanism to explain these results.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Austenitic stainless steel is used commonly in the industry for its excellent mechanical and chemical properties at cryogenic and furnace temperatures, and exhibits superb properties of welding, for that is corresponding 70% of the stainless steel manufacture [1]. It has 16% to 25% chromium and nickel, and up to 6% molybdenum and minor contains nitrogen, copper, and tungsten. These mixtures of elements give anti-corrosive properties to this type of stainless steel. It displays excellent resistance to many aggressive environments causes normal corrosive and stress corrosion cracking that can attack steels like 304L and 316L alloys.
In general, most stainless steels exhibit poor performance in hydrohalic acids aqueous solution because they are unable to maintain passive chromium oxide films on the entire surface, resulting in active corrosion [2,3,4]; and for the austenitic stainless steels in some conditions of hydrofluoric liquid have good quality up to a temperature of 100 °C, exceeding 100 °C, the corrosion rates increase to undesirable levels [3]. UNS N08904 stainless steel is an austenitic stainless steel was proposed for chemical processing as phosphoric acid production, with high-quality resistance to attacks, especially to pitting corrosion in harsh corrosion conditions [5, 6]; but it present some partial resistance in the presence of free halide ions [7,8,9,10], especially in its iso-corrosion at temperature of 80 °C and 2% of hydrofluoric acid [3]. Furthermore, in our case, the system is the polluted phosphoric acid, which the parameters of 2% HF and the temperature of 80 °C may exist in the industrial production process. And there is a little published data on the behavior of this alloy in this condition, and the influence of fluoride ion on its deterioration in the industrial phosphoric acid has not been given much attention.
Hence, the main of this present investigation is to analyze and understand the influence of 2% fluoride on the corrosion of high austenitic stainless steel in contaminated phosphoric acid at 80 °C. In this regard, the electrochemical methods and spectroscopy analyses are used in this investigation.
2 Experimental Details
2.1 Materials and Test Solution
The austenitic stainless steel used in this study was Uranus B6 (UB6) called stainless steel UNS N08904; the chemical compositions of the samples are listed in Table 1. Before each test, the sample was abraded with abrasive paper and cleaned with water. The reference solution (blank solution) was prepared from 40% phosphoric acid added to the water, then the 0.42% KCl to 4% H2SO4 mixture was added, and the amount of 2% HF was included to study its influence on the corrosion resistance of austenitic stainless steels at 80 °C.
2.2 Electrochemical Measurements
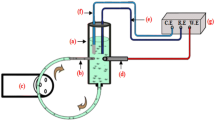
The electrochemical measurements were used in this investigation by cell composes of three electrodes; the saturated calomel electrode (SCE) and auxiliary electrode of platinum, and the working electrode connected to VoltaLab PGZ 301 Potentiostat. The polarization interval from − 0.3 to 1.2 V/SCE was applied with a scan rate of 1 mV s−1, after the steady state condition of 1800 s. Electrochemical impedance spectroscopy (EIS) was used at open circuit potential with the amplitude of 10 mV and a frequency range of 100 kHz to 100 mHz.
2.3 X-ray Diffraction (XRD) Analysis
XRD analysis was executed on the surface samples after the immersion time of 48 h in both solutions at 80 °C, by Shimadzu 6100 diffractometer equipped with a copper anticathode (λCuKα = 1.541838 Å), a thin film attachment THA-1101 and counter monochromator CM-4121. The samples were scanned at a speed of 2.0°/min between 40 and 120″ (2θ) at a tube voltage of kV and a current of 30 mA.
2.4 UV–Vis–NIR Spectroscopy
The samples and the solutions were analyzed after 48 h immersion of austenitic stainless steel at 80 °C, by UV–Vis–NIR spectrophotometer (Jasco V670 model) equipped with a 150 mm integrating sphere (ILN-925 model). The reflectance spectra and the absorbance of the samples were obtained at room temperature. To present the results the smoothing of Savitzky–Golay and the second derivative method applied to recover the band’s result, as well as to eliminate the baseline shift [4, 11].
2.5 Scanning Electron Microscopy and Energy-Dispersive Spectroscopy Analysis
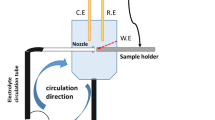
Scanning electron microscopy and energy-dispersive spectroscopy analysis was executed on the surface of austenitic stainless steel after 48 h of immersion in different conditions at 80 °C. The scanning electron microscope (SEM) JEOL JSM-IT 100 was equipped with an EDX through an accelerating voltage of 20 kV.
3 Results and Discussion
3.1 Potentiodynamic Polarization Curves
The polarization curves of the austenitic stainless steel are plotted after immersion for 30 min in polluted phosphoric acid solutions to study the effect of fluoride at 80 °C (Fig. 1). The electrochemical parameters extracted from this figure are collated in Table 2.
The graph clearly shows that fluoride affects the field anodic, which considerably modifies the parameters describing this phenomenon. In both cases, the anodic polarization shows that when the potential is made more positive, the dissolution current reaches the highest value and then decreases abruptly to a low value, as shown schematically in Fig. 1. This behavior is due to the reaction of surface metals with water to form oxides [11] (Eq. (1)).
Moreover, in the presence of fluoride, the active current density is eight times greater and the passive current density nine times higher. On the other hand, the pit nucleation resistance value (Epit − Ecorr) is considerably reduced from 1131.1 to 627.8 mV. In addition, the critical passivation potential of the oxide film (Ep) is slightly modified; instead, the critical passivation current (icrit) strongly depends on the compositions of the solution, particularly the presence of fluoride which prevents passivation. Otherwise, the critical passivation current density increases considerably, reflecting the decreasing stability of the oxide films with respect to dissolution and the difficulty of passivation. It is known that Cr and Mo are the main passivating elements forming Cr2O3 and MoO3 that enriched the surface of iron alloys during active dissolution [12]. As a result, fluoride may affect these elements, resulting in an increase in iron activity in the alloy.
Usually, the protective capacity of the film is measured by the passive current density passing through it, this current increasing considerably in the case of HF. It appears that fluoride affects the passive process, leading to the formation of a weak passive film. This means that the passage of metal ions through the oxide film is very fast, producing higher current. On the other hand, the current begins to increase considerably at the pitting potential due to localized rupture of the passive film, suggesting that fluoride induces the pitting corrosion that controls the dissolution process. Consequently, the correlation between the passivation current density and the pit nucleation resistance was observed. Moreover, the effect of fluoride on the corrosion of the austenitic stainless steel is not yet clarified in depth in the phosphoric acid solution. Many researchers have discussed the effect of chloride, including its effect on the formation of pitting corrosion [8, 9, 13,14,15,16,17].
According to [5, 18,19,20,21] the adsorption and penetration are the essential mechanisms which that halide ion effect the film/solution interface; the high electronegativity and small ion radius of fluoride led it enters in competition with adsorbed water and dissolved oxygen [22]; these process may increase the electrochemical parameters of the system, especially for anodic dissolution that leading to breakdown of passivity. However, the temperature increases the adsorption of fluoride and chloride on the passive film [23,24,25], which leads to change the passive film behavior and decrease in pitting resistance.
Furthermore, in the case without fluoride (Fig. 1), the dissolution of the passive film starts at the potential below 950 mV, That is attributed to chromate formation from Cr3+ to Cr6+, and the presence of alloys elements like Mo is responsible for stable passivity extending up to the potential of transpassive Cr dissolution [12]. In a study was released by Jabs et al. [26] using both XPS and time of flight secondary ion mass spectrometry proposed that the Molybdenum is one of the principal elements that suppressed the passive film dissolution at potentials superior to 500 mV; instead in our case, this value is decreased to less noble value about 450 mV in the presence of fluoride Table 2. This may an indication that fluoride affects this element. This suggestion finds its explication in the analysis of surface material was used after the polarization test in the solution with fluoride (Fig. 2). Furthermore, the SEM shows that the morphology of the pits is modified in the case of fluoride; different types of pits have been detected with irregular forms and the small pits (Fig. 2b). In the opposite case, without fluoride, a simple form of pits has been observed (Fig. 2a).
From these observations, many propositions are possible to explain this effect, first that localized activation of wide, shallow pits may be taken more at the edges of the pits in the presence of fluoride; indicating partial reactivation. Second, this may be due to the fact that the HF has a role on the acidity at the pit site. Third, we also observe that like a part of the alloy was rejected from the surface alloy (Fig. 2b); this may a sign of another pitting mechanism, which suggests intervener of the chemical and physical processes. However, in acidic solutions, as the HF was added the pitting potential decreased in the less noble direction indicating the destruction effect of HF.
EDX analysis showed that the concentration of Mo in the surface alloy (Fig. 3a1) is lower than in the corrosion products (Fig. 3a2–a4). The surface enrichment by this element was found especially in the corrosion product that exists at pit spot, which can be an important reason for explaining the increase of the pitting corrosion. The positive influence of the addition of Mo on the pitting resistance of iron-chromium alloys is attributed to the reduction of iron activity [12]. Guenbour et al. [7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27] conclude that the presence of Mo in the Fe–Cr–Ni alloy increases its resistance to attack by the chloride ions in the phosphoric medium, Ilevbare and Burstein have studied the role of Mo in inhibition of pitting corrosion in acidified chloride solution [28]. The particularly beneficial effect of molybdenum can be explained by its ability to form stable and protective compounds [7] and by its influence on the self-passivation kinetics of stainless steels [29].
3.2 Electrochemical Impedance Spectroscopy Measurements
EIS measurements were employed to investigate the relative stability of the passive films formed on Uranus B6 (UB6) in the contaminated phosphoric acid solution.
Figure 4 shows the Nyquist plots of the impedance of austenitic stainless steel in the polluted phosphoric acid with and without 2% of HF at 80 °C. The plots present an individual depressed capacitive loop, which characterizes the charge transfer process [8, 30,31,32]. Moreover, the diameter of the semicircle provides the polarization resistance; that is used to estimate the corrosion resistance of stainless steel toward the aggressive environment. Plus, decreasing the value of this parameter during an investigation indicates the weakness of the system. Besides, the capacity of the double layer can also be calculated by the frequency of the high imaginary impedance value.
Generally, the inverse relationship exists between these parameters. Further, the same plots in both cases, suggesting a similar mechanism that controls the corrosion of austenitic stainless steel. Moreover, the impedance data were adjusted using the equivalent circuit shown in Fig. 4, where Rs characterizes the resistance of the solution, Rp is the polarization resistance, and the constant-phase element (CPE) is used to describe the non-ideal capacitive response,
The impedance of this element is defined as [33]:
where Q is the CPE constant, ω is the angular frequency (rad/s), j2 = − 1 is the imaginary number and α is the CPE exponent. Depending on α, CPE can represent resistance (α = 0, Z0 = R), capacitance (α = 1, Z0 = C), or Warburg impedance (α = 0.5, Z0 = W). The CPE elements, Q, have been converted into a pure capacitance (C) by means of the following equation [34]:
The resistance, capacitance and α values obtained by adjusting the experimental data shown in Fig. 4 are summarized in Table 3.
From the adjustment results, it was found that fluoride decreased the polarization resistance by about 35.5%. In contrast, the capacity of the double layer increased by about 30.4%, which means that fluoride did not have a significant impact on the corrosion resistance of austenitic stainless steel. In addition, the double layer, once formed at the interface, is created mainly from water molecules and it is known that the HF acid is a very weak acid and has a very strong hydrogen bond with the molecules of water. Therefore, in the presence of the fluoride, the small fluoride anion surrounds the protons and the water molecules. This may explain the weak effect of fluoride on the corrosion resistance at OCP, if we consider that temperature is a catalytic factor.
Furthermore, BenSalah et al. [31] were investigated the effect of temperature and the aging time of 24 h on the corrosion resistance of austenitic stainless steel in an industrial phosphoric acid solution containing impurities H2SO4, KCl and HF. They mentioned that the temperature favorites the formation of a porous polyphosphate layer that gives small protection. In our condition, the time immersion has been just 30 min, and it may be an inadequate time to see clearly the effect of fluoride. That is why we will discuss the effect of aging time of 48 h.
3.3 UV–Vis–NIR Spectroscopy
The UV–Vis–NIR spectroscopy of the austenitic stainless steel surface and the tested solutions is shown in Figs. 5 and 6, respectively. The results of spectra were used to reveal the effect of fluoride ions on the metal oxides in the surface of austenitic stainless steel and expose the existence of metal ions in the solutions after the immersion times.
The effect of fluoride on the austenite is measured as the change in the intensity or the amplitude of the peaks; as an evidence of surface degradation. The spectra of the surface of austenitic stainless steel reveal a change in the amplitude of the surface composition in the presence of fluoride ions and do not appear new peaks, instead, the spectra of the tested solutions indicate that the amplitude bands are increased in the presence of fluoride, as illustrated in Fig. 5, which mean that surface of alloy conserve its compositions and do not form new film, this a signification that surface was under the dissolution attack; as proposed by the following reaction (Eq. (4)) [35]:
Moreover, the identifications of Fe and Cr oxides [4, 36,37,38,39] on the surface are verified by this method and also the metals complex compounds in the solutions, Whereas, the results of the tested solutions showed a large absorbance at UV domain (Fig. 6), this indicates the presence of iron phosphate complex in great quantity and other peaks of iron phosphates appeared at 645, 680 nm, the chromium complex was also detected at bands of 425 nm and 580 nm [40]; and the nickel complex at 656 nm, 720 nm [41]. Consequently, the fluoride acts as a dissolution agent which reacts with the metal in this order Fe > Cr > Ni.
3.4 Chemical Composition and Surface Morphology
Figure 8 shows the EDX spectrum of the samples which were immersed for 48 h at 80 °C in the polluted phosphoric acid. In the case of a blank solution, the EDX spectrum of the substrate is illustrated in Fig. 8b; it reveals a typical spectrum of the austenitic stainless steel. And the spectrum of the sample when we added fluoride ions is shown in Fig. 8c, its spectrum is similar to the reference, and a change in the intensity of the peaks has been observed.
Furthermore, the peaks of base elements are detected on the surface, indicating no formation of a new film over the substrate; and the surface appears to constitute by a mixture of the basis oxides film from the Cr, Fe, Ni, Mo. To support the EDX analysis, the SEM images were also investigated see Fig. 7. The pitting corrosion process was examined, and it seems that the decrease of intensity was from the pits and deep gap. However, the EDX analysis investigation on the corrosion products and on the surface in different areas was released (Fig. 9), to discover the cause of this delicate effect of fluoride.
It’s appeared that in both cases, the surface material characteristics are affected by the aggressive properties of the halide anions that inhibit the formation of the film, and attack preexisting, protective passive layer on alloy, in particular, the presence of fluoride, the pitting corrosion is induced.
Moreover, EDS mapping shows a homogeneous distribution of fluoride and heterogeneous distribution of chlorides and sulfides (Fig. 10), instead, the uniform distribution of these elements was detected in the absence of fluoride. This conflict may disturb the formation of salt metals that inhibit the dissolution.
On the other hand, the residual element content of the stainless steel, in particular, the copper content has appeared in corrosion product. However, it is reduced significantly from the surface, which can stimulate corrosion. Further, the various papers [42,43,44], have been studying the role of molybdenum in the corrosion resistance of austenitic stainless steel, such as the high Mo–Mo bond energy of molybdenum, which prevents the active locations, and formation of insolubility molybdenum chlorides, that avoid the dissolution of the alloy. For that, the alloys contain this element was used in the aggressive environment includes chlorides.
In addition, EDS mapping shows a uniform distribution of molybdenum in the absence of fluoride (Fig. 10), but rather less and an increase in intensity in some areas in the presence of fluoride, as well as molybdenum, is identified in the EDS corrosion product; this can be a significant influence of fluoride on molybdenum in the matrix of the alloy. Moreover, the SEM displays that the dissolution mechanism is distributed horizontally in the surface, and no pit was observed in the case of the blank solution (Fig. 7b).
Generally, the EDS spectrum and mapping display clear evidence of the decreasing density of the chemical elements on the surface alloy in the presence of fluoride. Consequently, this effect may result from the reaction of fluoride ions with the base metals of alloy and especially with molybdenum and copper.
3.5 X-ray Diffraction (XRD) Analysis
Figure 11 shows the X-ray diffraction patterns produced from three austenitic stainless steel samples after immersion for 48 h at a temperature of 80 °C: the first sample is the reference sample analyzed before the test, the second sample was in the blank solution, and the third sample was in the blank solution with fluoride. In the reference sample, five peaks of diffraction can be observed, peaks (110) c, (200) c, (220) c, (311) c and (400) c of austenitic under Cu radiation Ka reflecting the crystalline structure of austenitic stainless steel. This material has a single-phase structure with centered cubic faces (FCC), as shown in Fig. 11.
In addition, in the case of the solution with fluoride, the XRD pattern does not save the reflection peak at 44.44°, it may be a sign that plan (1 1 0) in FCC has a low-energy value under the effect of fluoride. Moreover, the fluoride may influence the stacking fault energy of the crystalline structure, which the base metal and the alloying elements control this parameter [33, 34, 45, 46]. Therefore, the effect of fluoride on the alloying elements may lead to the development of the strains or the internal stresses that accompany its absorption in the surface alloy, which might provide a significant driving force for austenite decomposition and deformation. As a result, the γ-phase stability is decreased. This suggestion may proofed by a peak shift was noted at the plan (2 2 0) that may came from the shear plane of (1 1 0).
Furthermore, there is another suggestion. It is quite common that the main chemical compositions of austenitic stainless steel are iron and chromium, nickel, molybdenum. In the presence of fluoride, the peak (1 1 0) containing Fe, Cr, Ni, Mo disappears, but the diffraction pattern of the intensity (200) containing Fe, Cr, Ni is increased (Fig. 5), which led us to propose that the alloy was under local corrosion. A study was published on the white cast iron [47], it revealed a decrease and the disappearance of some ferrite peaks, whose authors found that the alloy was subjected to selective corrosion.
And SEM images of the alloy can confirm this suggestion (Fig. 7). Moreover, the results of the absorption spectra of the solutions (Fig. 6) showed an increase in iron ions and chromium and nickel in the solution. And the effect on molybdenum was observed at the mass fraction of molybdenum in the corrosion product, and at the bottom of the pit where it was increased (Fig. 9a, b). In addition, an increase in peak (200) containing Fe, Cr, Ni can be interpreted as the result of increasing the mass fraction of nickel in the unaffected area (Fig. 9d). In the recent research, Guobo Zou et al. [30] studied the effect of the HF solution on the corrosion behavior of austenitic stainless steel. They discovered that a deposited layer of NiF2, Fe2O3, and Cr2O3 was responsible for the isolation of the base metal, while NiF2 inhibited passivation of the alloy in HF. In our case, the basic solution was phosphoric acid polluted with sulfate and chloride ions and the fluoride concentration was 2% HF at a temperature of 80 °C. These parameters can lead to the formation of insoluble oxide layer reach by the metal elements in this order Fe > Ni > Cr > Mo does not cover the entire surface. That can direct to the appearance of the active zones resulting from the attack by the halide ions.
The vertical and horizontal dissolution mechanisms are observed in the presence of fluoride, and pitting corrosion occurs in many forms, changing from simple pits to irregular areas and shapes (Figs. 7c, 8, 9, 10, 11, 12). This phenomenon is probably due to the instability of the oxide film in some regions, leading to local activation of the alloy.
4 Conclusions
In summary, the effect of fluoride on Uranus B6 at 80 °C in polluted phosphoric acid was studied using electrochemical methods and spectroscopy analyses. The test was applied by polarization and EIS to the potential of the open circuit, showed that fluoride did not affect the polarization of resistance much, but the passivation parameters were very sensitive to the presence of fluorine. In particular, the pitting corrosion process has been observed at a lower potential, which means that fluoride improves pitting. This effect is explained by the influence of fluoride on molybdenum. To better understand the effect of fluoride on the alloy at OCP, immersion times of 48 h were achieved under the same conditions. The drastic effect of fluoride ions was noted, and the spectroscopic analyses highlighted the presence of two interaction axes. The dissolution mechanism was proofed by UV–Vis–IR reflection, which the fluoride reacts with the metal in this order Fe > Cr > Ni. And EDS has proven that fluoride also affects molybdenum and copper. DRX reflect a decrease in the stability of the γ phase by strain effect.
The possible mechanism of this process is that the effect of fluoride results in partial, localized and discontinuous destruction by deformation of the crystal structure and dissolution mechanism, therefore, facilitating the initiation of pitting corrosion.
References
Oshima T, Habara Y, Kuroda K (2007) Efforts to save nickel in austenitic stainless steels. ISIJ Int 47:359–364
Kirchner RW (1971) An evaluation of nickel-rich alloys in wet process phosphoric acid. Mater Corros Korrosion 22:581–592
Richardson JA (2010) Corrosion in hydrogen halides and hydrohalic acids. In: Shreir’s corrosion. American Society for Metals, Metals Park, pp 1207–1225
Kerroum Y, Skal S, Guenbour A, Bellaouchou A, Tabyaoui M, Zarrouk A, Garcia Anton J (2018) Effect of fluoride on corrosion behavior of UNS N08904 stainless steel in polluted phosphoric acid. J Mol Liq 265:390–397
Pillai SR (2002) High temperature corrosion of austenitic stainless steels. In: Corrosion of austenitic stainless steels. Elsevier, Amsterdam, pp 265–286
Schorr M, Valdez B (2016) The phosphoric acid industry: equipment, materials, and corrosion. Corros Rev 34:85–102
Guenbour A, Faucheu J, Ben Bachir A (1988) on the mechanism for improved passivation by addition of molybdenum to austenitic stainless steels in o-phosphoric acid. Corrosion 44:214–221
Iken H, Basseguy R, Guenbour A, Ben BA (2007) Classic and local analysis of corrosion behaviour of graphite and stainless steels in polluted phosphoric acid. Electrochim Acta 52:2580–2587
Boudalia M, Guenbour A, Bellaouchou A, Fernandez-Domene RM, Garcia-Anton J (2013) Corrosion behaviour of a highly alloyed austenitic alloy UB6 in contaminated phosphoric acid. Int J Corros 2013:1–9
Bellaouchou A, Guenbour A, Benbachir A (1993) Corrosion behavior of stainless steel in phosphoric acid polluted by sulfide ions. Corrosion 49:656–662
Skal S, Kerroum Y, Guenbour A, Bellaouchou A, Tabyaoui H, Idrissi H, Zarrouk A, García-Antón J (2017) Effect of abrasive particles on electrochemical behaviour of passive film formed on Alloy 59 in contaminated phosphoric acid. J Mater Environ Sci 8:3234–3246
Goetz R, Laurent J (1985) The influence of minoralloying elements on the passivation behaviour of iron-chromium alloys in HCl. Corrosion 25:1115–1126
Guenbour A, Hajji M-A, Jallouli EM, Ben Bachir A (2006) Study of corrosion–erosion behaviour of stainless alloys in industrial phosphoric acid medium. Appl Surf Sci 253:2362–2366
Iversen A, Leffler B (2010) Aqueous corrosion of stainless steels. In: Shreir’s corrosion. Elsevier, Amsterdam, pp 1802–1878
Burstein GT (2010) Passivity and localized corrosion. In: Shreir’s corrosion, vol 1. Elsevier, Amsterdam, pp 731–752
Meng G, Li Y, Shao Y, Zhang T, Wang Y, Wang F (2014) Effect of Cl− on the properties of the passive films formed on 316L stainless steel in acidic solution. J Mater Sci Technol 30:253–258
Luo H, Su H, Dong C, Li X (2017) Passivation and electrochemical behavior of 316L stainless steel in chlorinated simulated concrete pore solution. Appl Surf Sci 400:38–48
Heakal FET, Awad KA (2011) Electrochemical corrosion and passivation behavior of titanium and its Ti-6AL-4V alloy in low and highly concentrated HBr solutions. Int J Electrochem Sci 6:6483–6502
Soltis J (2015) Passivity breakdown, pit initiation and propagation of pits in metallic materials–Review. Corros Sci 90:5–22
Di Quarto F, Santamaria M (2004) Semiconductor electrochemistry approach to passivity and passivity breakdown of metals and metallic alloys. Corros Eng, Sci Technol 39:71–81
Kong D-S (2008) The influence of fluoride on the physicochemical properties of anodic oxide films formed on titanium surfaces. Langmuir 24:5324–5331
Govindan K, Murugesan S, Maruthamuthu P (2013) Photocatalytic degradation of pentachlorophenol in aqueous solution by visible light sensitive NF-codoped TiO2 photocatalyst. Mater Res Bull 48:1913–1919
Gossenberger F, Roman T, Groß A (2016) Hydrogen and halide co-adsorption on Pt(111) in an electrochemical environment: a computational perspective. Electrochim Acta 216:152–159
Habuda-Stanić M, Ravančić M, Flanagan A (2014) A review on adsorption of fluoride from aqueous solution. Materials (Basel) 7:6317–6366
Escrivà-Cerdán C, Blasco-Tamarit E, García-García DM, García-Antóna J, Akid R, Walton J (2013) Effect of temperature on passive film formation of UNS N08031 Cr–Ni alloy in phosphoric acid contaminated with different aggressive anions. Electrochim Acta 111:552–561
Jabs T, Borthen P, Strehblow H-H (1997) X-ray photoelectron spectroscopic examinations of electrochemically formed passive layers on Ni–Cr alloys. J Electrochem Soc 144:1231–1243
Guenbour A, Faucheu J, Ben Bachir A (1988) Electrochemical study of corrosion-abrasion of stainless steels in phosphoric acids. Corrosion 23:234–238
Becker P (1989) Phosphates and phosphoric acid. Raw materials, technology, and economics of the wet process. Marcel Dekker, New York
Ogura K, Ohama T (1984) Pit formation in the cathodic polarization of passive iron IV. Repair mechanism by molybdate, chromate and tungstate. Corrosion 40:47–51
Zou G, Shi W, Xiang S (2018) Corrosion behavior of 904L austenitic stainless steel in hydrofluoric acid. RSC Adv 8:2811–2817
BenSalah M, Sabot R, Triki E, Dhouibi L, Refait Ph, Jeannin M (2014) Passivity of Sanicro28 (UNS N-08028) stainless steel in polluted phosphoric acid at different temperatures studied by electrochemical impedance spectroscopy and Mott–Schottky analysis. Corros Sci 86:61–70
Galván JC, Larrea MT, Braceras I, Multigner M, González-Carrasco JL (2016) In vitro corrosion behaviour of surgical 316LVM stainless steel modified by Si+ ion implantation—an electrochemical impedance spectroscopy study. J Alloys Compd 676:414–427
Mosecker L, Saeed-Akbari A (2013) Nitrogen in chromium-manganese stainless steels: a review on the evaluation of stacking fault energy by computational thermodynamics. Sci Technol Adv Mater 14:033001
Reick W, Pohl M, Padilha AF (1996) Determination of stacking fault energy of austenite in a duplex stainless steel. Steel Res 67:253–256
Porcayo-Calderon J, Casales-Diaz M, Salinas-Bravo VM, Martinez-Gomez L (2015) Corrosion performance of Fe-Cr-Ni alloys in artificial saliva and mouthwash solution. Bioinorg Chem Appl 2015:1–14
Szalai Z, Kiss K, Jakab G, Sipos P, Belucz B, Németh T (2013) The use of UV–VIS–NIR reflectance spectroscopy to identify iron minerals. Astron Nachr 334:940–943
Le Calvar M, Lenglet M (1989) UV–Vis–NIR and FTIR reflectance studies of the initial stage of oxidation of 80 Ni–20 Cr Alloy. Stud Surf Sci Catal 48:575–580
Cornell RM, Schwertmann U (2003) The iron oxides: structure, properties, reactions, occurrences, and uses. Wiley-VCH, Weinheim
Liang S-T, Zhang H-L, Luo M-T, Liu H-X, Bai Y, Xu H-B, Zhang Y (2015) Preparation of Cr2O3-based pigments with high NIR reflectance via thermal decomposition of CrOOH. Trans Nonferrous Met Soc China 25:2646–2647
Leita L, Margon A, Pastrello A, Arcˇon I, Contin M, Mosetti D (2009) Soil humic acids may favour the persistence of hexavalent chromium in soil. Environ Pollut 157:1862–1866
Zhang N, Brugger J, Etschmann B, Ngothai Y, Zeng D (2015) Thermodynamic modeling of poorly complexing metals in concentrated electrolyte solutions: an X-ray absorption and UV–Vis spectroscopic study of Ni(II) in the NiCl2–MgCl2–H2O system. PLoS ONE 10:e0119805
Newman RC (1985) The dissolution and passivation kinetics of stainless alloys containing molybdenum—1. Coulometric studies of Fe–Cr and Fe–Cr–Mo alloys. Corros Sci 25:331–339
Marcus P (1994) On some fundamental factors in the effect of alloying elements on passivation of alloys. Corros Sci 36:2155–2158
Schneider A, Kuron D, Hofmann S, Kirchheim R (1990) AES analysis of pits and passive films formed on Fe–Cr Fe–Mo and Fe–Cr–Mo alloys. Corros Sci 31:191–196
Qi-Xun D, An-Dong W, Xiao-Nong C, Xin-Min L (2002) Stacking fault energy of cryogenic austenitic steels. Chin Phys 11:315
Rozenak P, Eliezer D (1988) Nature of the γ and γ* phases in austenitic stainless steels cathodically charged with hydrogen. Metall Trans A 19(3):723–730
Kerroum Y, Guenbour A, Bellaouchou A, Idrissi H, García-Antón J, Zarrouk A (2019) The corrosion of white cast iron in polluted phosphoric acid medium contains fluoride ions. Anal Bioanal Electrochem 11(4):497–509
Acknowledgements
Financial support of the Moroccan Ministry of Higher Education, Scientific Research and Training of managerial staff (MESRSFC), National Center for Scientific and Technical Research (CNRST) under the Project PPR/2015/4 (PPR1 Program), are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kerroum, Y., Guenbour, A., Bellaouchou, A. et al. Chemical and Physical Effects of Fluoride on the Corrosion of Austenitic Stainless Steel in Polluted Phosphoric Acid. J Bio Tribo Corros 5, 68 (2019). https://doi.org/10.1007/s40735-019-0261-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40735-019-0261-5